Synthesis of Chiral Cyclic Carbonates via Kinetic Resolution of Racemic Epoxides and Carbon Dioxide
Abstract
:1. Introduction
2. Enantioselective Synthesis of Cyclic Carbonates via Kinetic Resolution of Epoxides

2.1. Cobalt(III) Salen Complexes
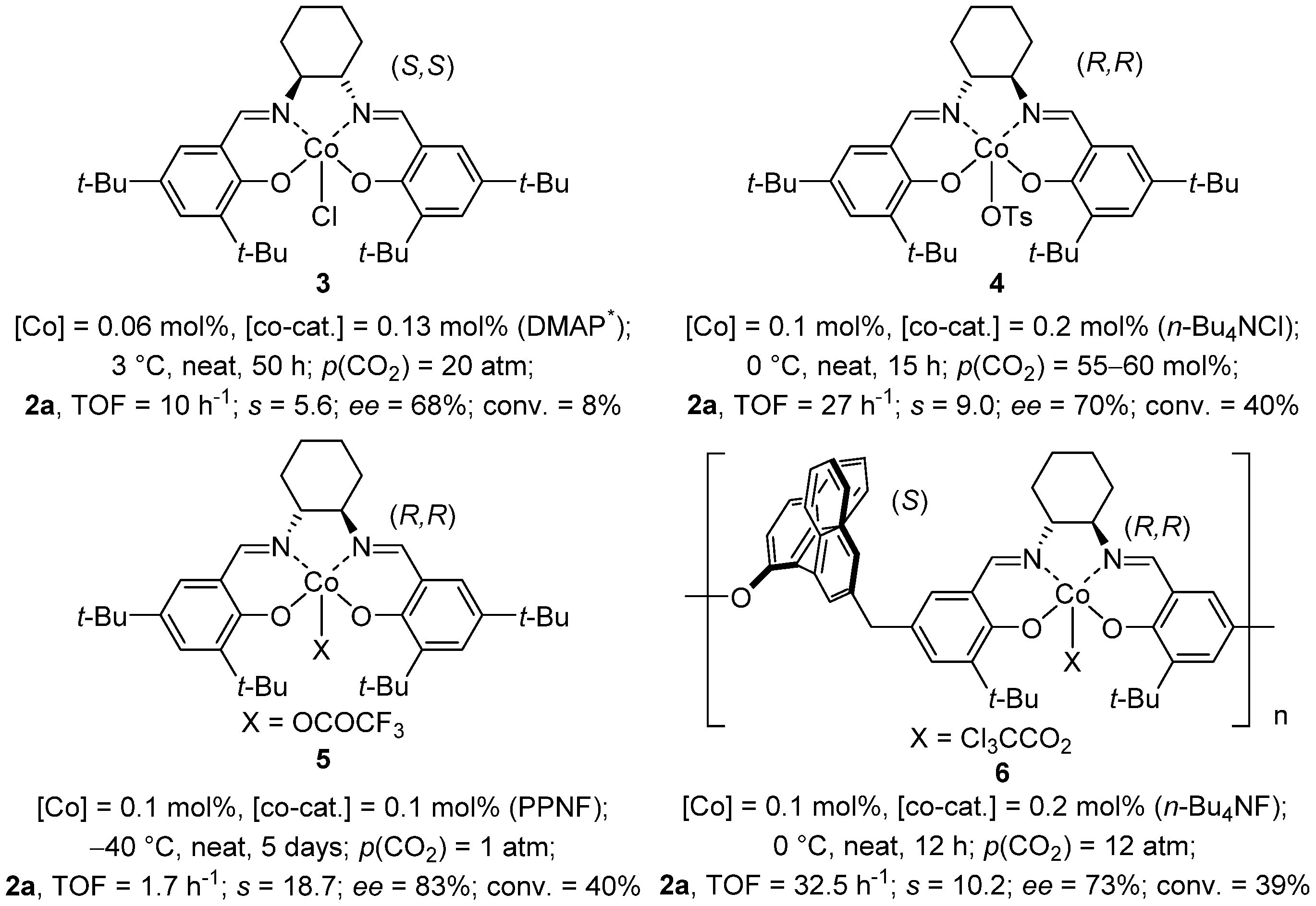
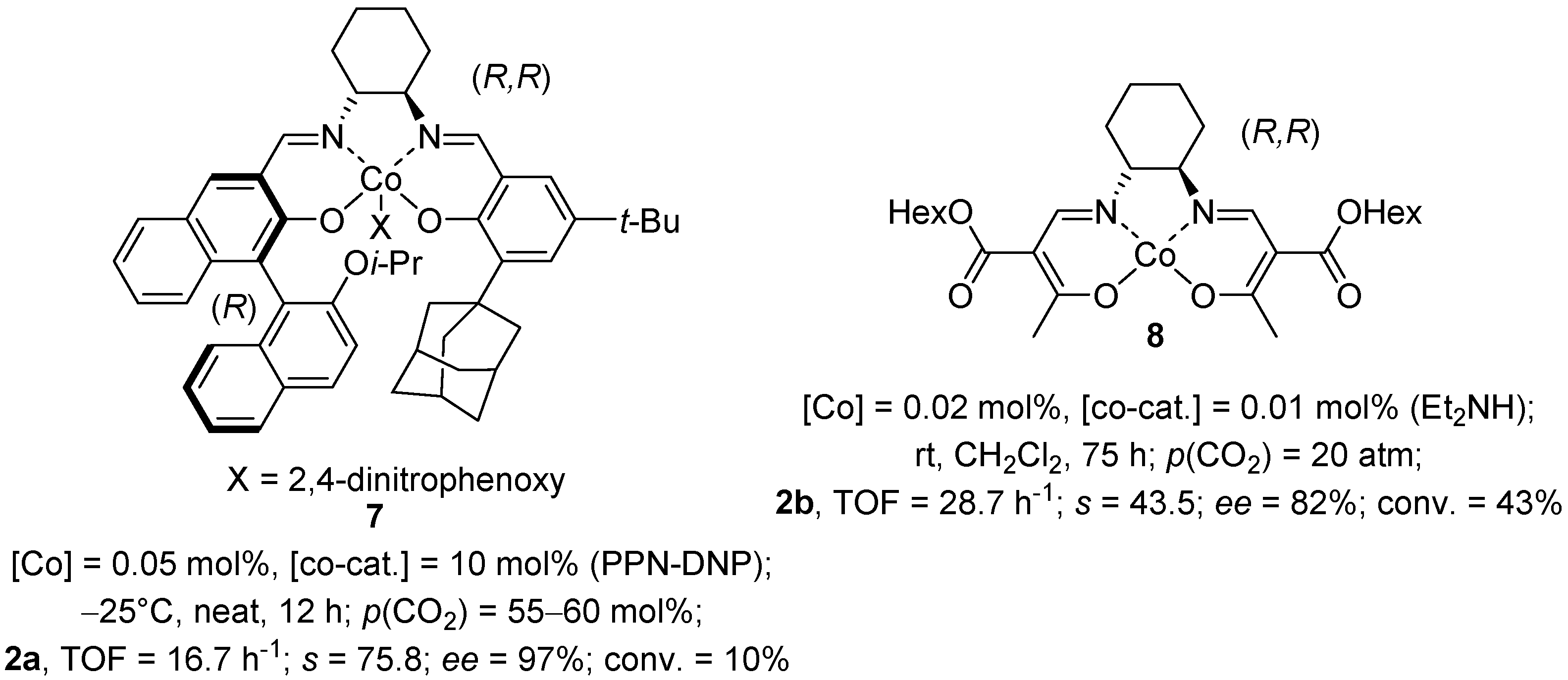
2.2. Cobalt(II) Salen Complexes
2.3. Other Metal Salen Complexes
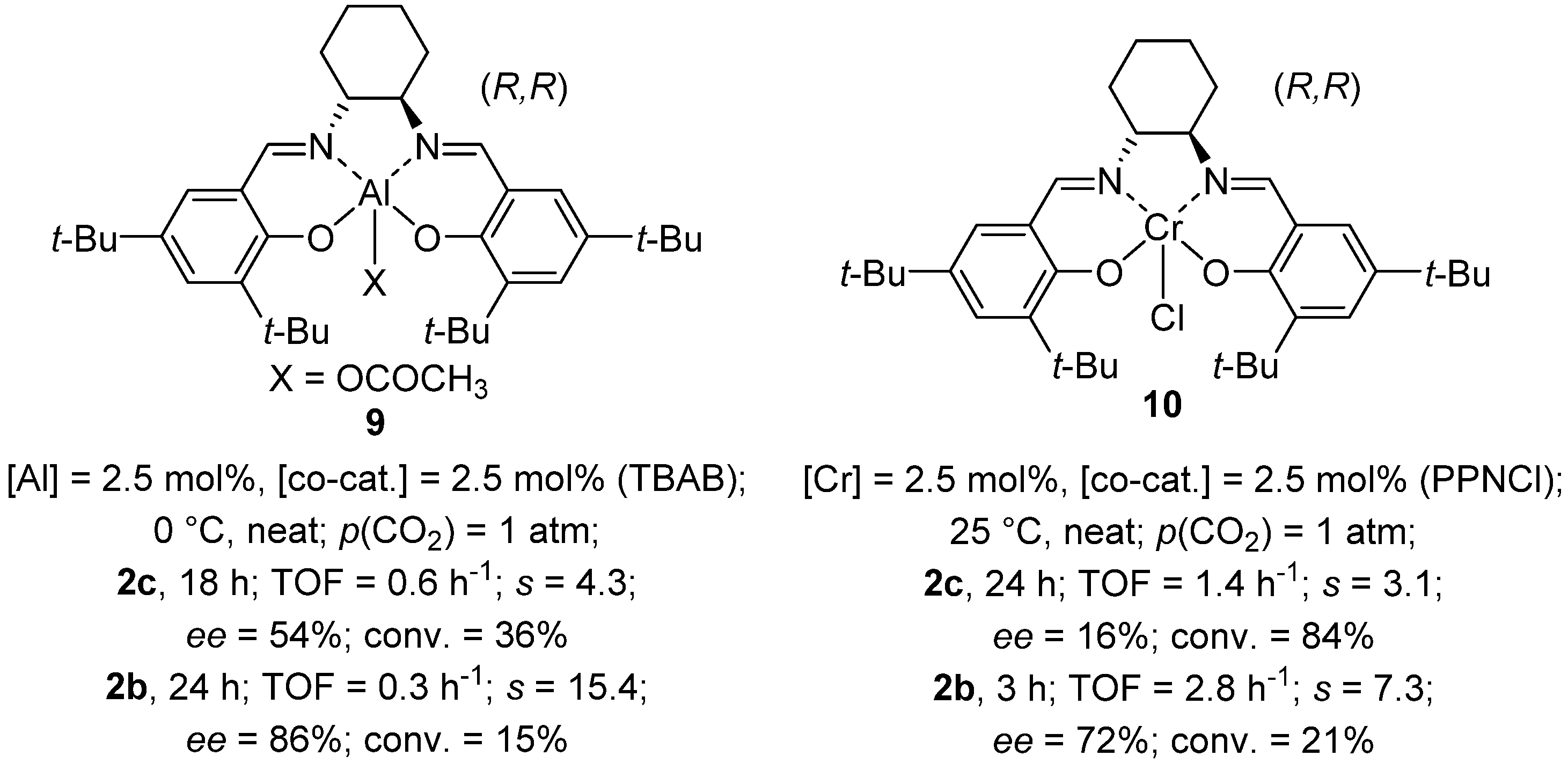
2.4. Other Metal Complexes
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Carbon Dioxide as Chemical Feedstock; Aresta, M. (Ed.) Wiley-VCH: Weinheim, Germany, 2010; pp. 1–375.
- New and Future Developments in Catalysis, Activation of Carbon Dioxide; Suib, S.L. (Ed.) Elsevier B.V.: Amsterdam, The Netherlands, 2013.
- Carbon Dioxide Utilization: Closing the Carbon Cycle; Styring, P. (Ed.) Elsevier B.V.: Amsterdam, The Netherlands, 2014.
- Peters, M.; Kchler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 14, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Kasai, N.; Suzuki, T.; Furukawa, Y. Chiral C3 epoxides and halohydrins: Their preparation and synthetic application. J. Mol. Catal. B Enzym. 1998, 4, 237–252. [Google Scholar] [CrossRef]
- Shaikh, A.A.G.; Sivaram, S. Organic carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Couladouros, E.A.; Nantermet, P.G.; Renaud, J.; Guy, R.K.; Wrasidlo, W. Synthesis of C-2 taxol analogues. Angew. Chem. Int. Ed. Engl. 1994, 33, 1581–1583. [Google Scholar] [CrossRef]
- Chiral Salen Complexes. In Privileged Chiral Ligands and Catalysts; Zhou, Q.-L. (Ed.) Wiley-VCH: Weinheim, Germany, 2011; pp. 269–273.
- Paddock, R.L.; Nguyen, S.T. Chiral (salen)CoIII catalyst for the synthesis of cyclic carbonates. Chem. Commun. 2004, 1622–1623. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-B.; Liang, B.; Zhang, Y.-J.; Tian, Y.-Z.; Wang, Y.-M.; Bai, C.-X.; Wang, H.; Zhang, R. Asymmetric catalysis with CO2: Direct synthesis of optically active propylene carbonate from racemic epoxides. J. Am. Chem. Soc. 2004, 126, 3732–3733. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Brandenburg, M. Catalytic asymmetric addition of carbon dioxide to propylene oxide with unprecedented enantoselectivity. Org. Lett. 2006, 8, 4401–4404. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Jing, H.-W.; Jin, L.-L.; Qiu, W.-Y. Quaternary onium tribromide catalysed cyclic carbonate synthesis from carbon dioxide and epoxides. J. Mol. Catal. A 2007, 264, 241–247. [Google Scholar] [CrossRef]
- Chen, S.-W.; Kawthekar, R.B.; Kim, G.-J. Efficient catalytic synthesis of optically active cyclic carbonates via coupling reaction of epoxides and carbon dioxide. Tetrahedron Lett. 2007, 48, 297–300. [Google Scholar] [CrossRef]
- Jin, L.-L.; Huang, Y.-Z.; Jing, H.-W.; Chang, T.; Yan, P. Chiral catalysts for the asymmetric cycloaddition of carbon dioxide with epoxides. Tetrahedron Asymmetry 2008, 19, 1947–1953. [Google Scholar] [CrossRef]
- Kawthekar, R.B.; Kim, G.-J. Asymmetric ring opening of epoxides catalysed by novel heterobimetallic Schiff-bases containing transition metal salts. Bull. Korean Chem. Soc. 2008, 29, 313–318. [Google Scholar]
- Zhang, S.-L.; Song, Y.-Y.; Jing, H.-W.; Yan, P.; Cai, Q. Cinchona-derived quaternary ammonium salts-improved asymmetric cycloaddition of CO2 to epoxides. Chin. J. Catal. 2009, 30, 1255–1260. [Google Scholar] [CrossRef]
- Chang, T.; Jin, L.; Jing, H. Bifunctional chiral catalysts for the synthesis of cyclic carbonates from carbon dixide and epoxides. ChemCatChem 2009, 1, 379–383. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Jing, H.; Yao, W.; Yan, P. Chiral ionic liquids improved the asymmetric cycloaddition of CO2 to epoxides. Green Chem. 2009, 1, 935–938. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Jin, Q.-R.; Zhang, S.-L.; Jing, H.-W.; Zhu, Q.-Q. Chiral metal-containing ionic liquid: Sunthesis and applications in the enantioselective cycloaddition of carbon dioxide to epoxides. Sci. China Chem. 2011, 7, 1044–1050. [Google Scholar] [CrossRef]
- Jang, D.Y.; Jang, H.G.; Kim, G.R.; Kim, G.-J. Synthesis of chiral propylene carbonate via asymmetric ring opening of racemic propylene oxide by carbon dioxide on immobilised cobalt salen catalyst. Catal. Today 2012, 185, 306–312. [Google Scholar] [CrossRef]
- Roy, T.; Kureshy, R.I.; Khan, N.H.; Abdi, S.H.R.; Bajaj, H.C. Asymmetric cycloaddition of CO2 and an epoxide using recyclable bifunctional polymeric Co(III) salen complexes under mild conditions. Catal. Sci. Technol. 2013, 3, 2661–2667. [Google Scholar] [CrossRef]
- Yan, P.; Jing, H. Catalytic asymmetric cycloaddition of carbon dioxide and propylene oxide using novel chiral polymers of BINOL-salen cobalt(III) salts. Adv. Synth. Catal. 2009, 351, 1325–1332. [Google Scholar] [CrossRef]
- Ren, W.-M.; Wu, G.-P.; Lin, F.; Jiang, J.-Y.; Liu, C.; Luo, Y.; Lu, X.-B. Role of the co-catalyst in the asymmetric coupling of racemic epoxides with CO2 using multichiral Co(III) complexes: Product selectivity and enantioselectivity. Chem. Sci. 2012, 3, 2094–2102. [Google Scholar] [CrossRef]
- Tanaka, H.; Kitaichi, Y.; Sato, M.; Ikeno, T.; Yamada, T. Enantioselective CO2 fixation catalysed by optically active cobalt complexes. Chem. Lett. 2004, 6, 767–677. [Google Scholar]
- Yamada, W.; Kitaichi, Y.; Tanaka, H.; Kojima, T.; Sato, M.; Ikeno, T.; Yamada, T. Enantioselective incorporation of carbon dioxide into epoxides catalysed by optically active cobalt complexes. Bull. Chem. Soc. Jpn. 2007, 80, 1391–1401. [Google Scholar] [CrossRef]
- North, M.; Quek, S.C.Z.; Pridmore, N.E.; Whitwood, A.C.; Wu, X. Aluminum(salen) complexes as catalysts for the kinetic resolution of terminal epoxides via CO2 coupling. ACS Catal. 2015, 5, 3398–3402. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Ren, Y.; Cheng, X.; Yang, S.; Qi, C.; Jiang, H.; Mao, Q. A chiral mixed metal-organic framework based on a Ni(saldpen) metalloligand: Synthesis, characterization and catalytic performances. Dalton Trans. 2013, 42, 9930–9937. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Mußmann, L.; Vogt, D. Kinetic resolution of oxiranes by use of chiral Lewis acid catalysts. Synlett 1993, 1993, 893–894. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Gianfrate, L.; Pastore, C. Enantioselective synthesis of organic carbonates promoted by Nb(IV) and Nb(V) catalysts. Appl. Catal. A Gen. 2003, 255, 5–11. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Castro-Osma, J.A.; North, M. Synthesis of Chiral Cyclic Carbonates via Kinetic Resolution of Racemic Epoxides and Carbon Dioxide. Symmetry 2016, 8, 4. https://doi.org/10.3390/sym8010004
Wu X, Castro-Osma JA, North M. Synthesis of Chiral Cyclic Carbonates via Kinetic Resolution of Racemic Epoxides and Carbon Dioxide. Symmetry. 2016; 8(1):4. https://doi.org/10.3390/sym8010004
Chicago/Turabian StyleWu, Xiao, José A. Castro-Osma, and Michael North. 2016. "Synthesis of Chiral Cyclic Carbonates via Kinetic Resolution of Racemic Epoxides and Carbon Dioxide" Symmetry 8, no. 1: 4. https://doi.org/10.3390/sym8010004




