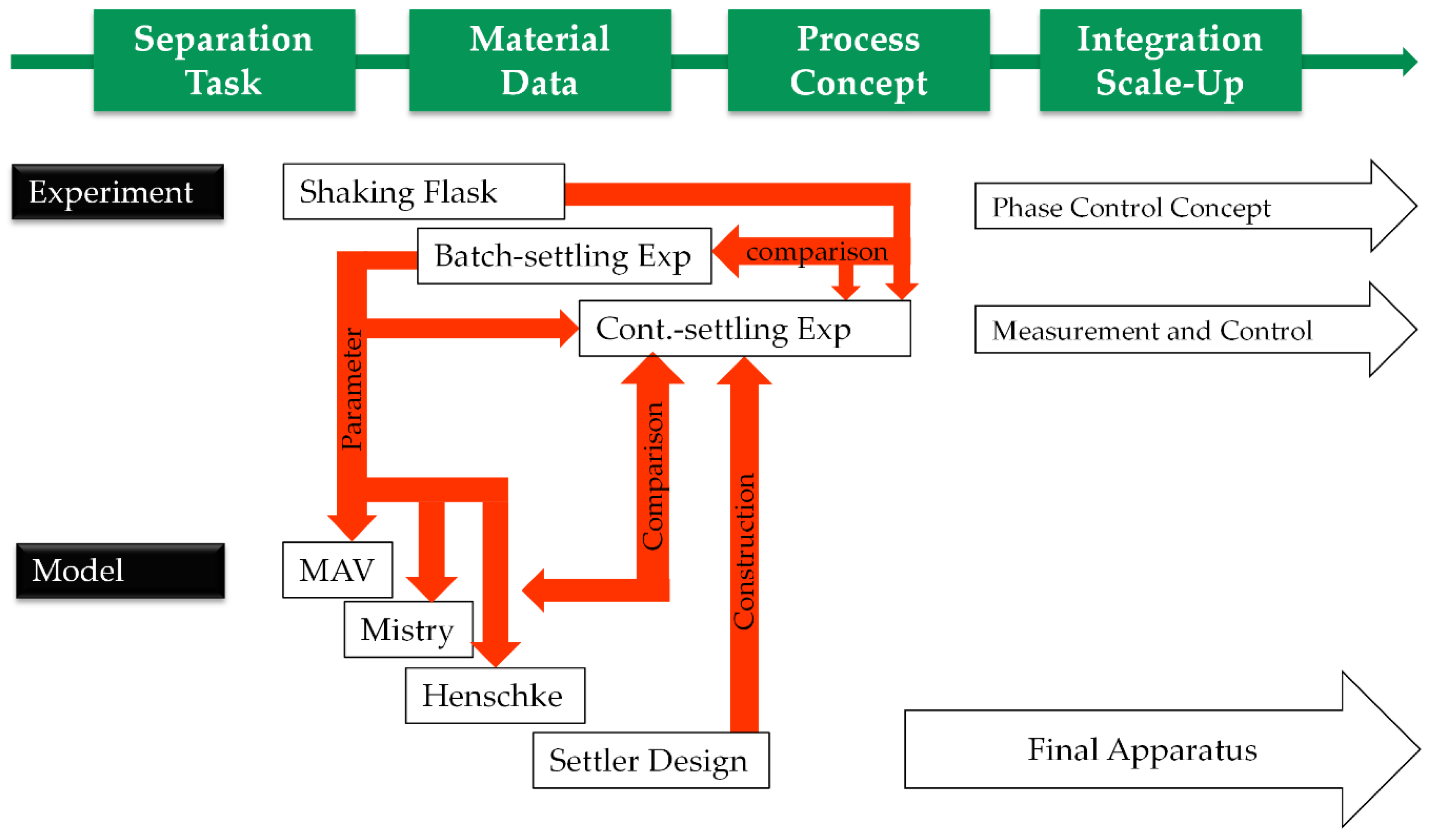
2.1. General Considerations
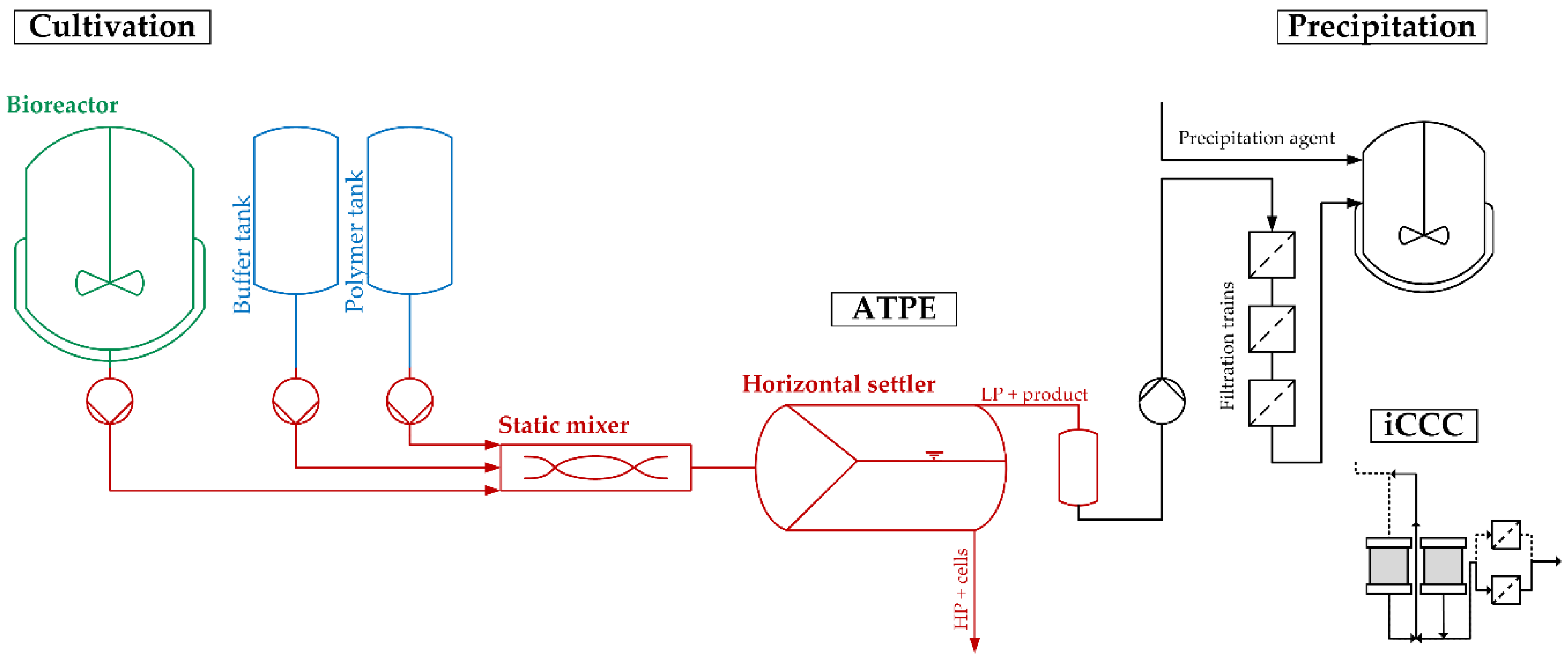
Unlike in the conventional production process of mAb, centrifugation and microfiltration as harvest and clearance operations are replaced with the outlined ATPE process. After the filtration trains, the product stream can, depending on the future process strategy, be further purified by precipitation or by integrated counter-current chromatography (iCCC) for continuous capture, replacing traditional protein A chromatography, as wells as ion-exchange and hydrophobic interaction chromatography for further purification and polishing [
9,
10,
11,
12,
13,
14].
The flowsheet of the discussed process, including the two purification and polishing operations, is shown in
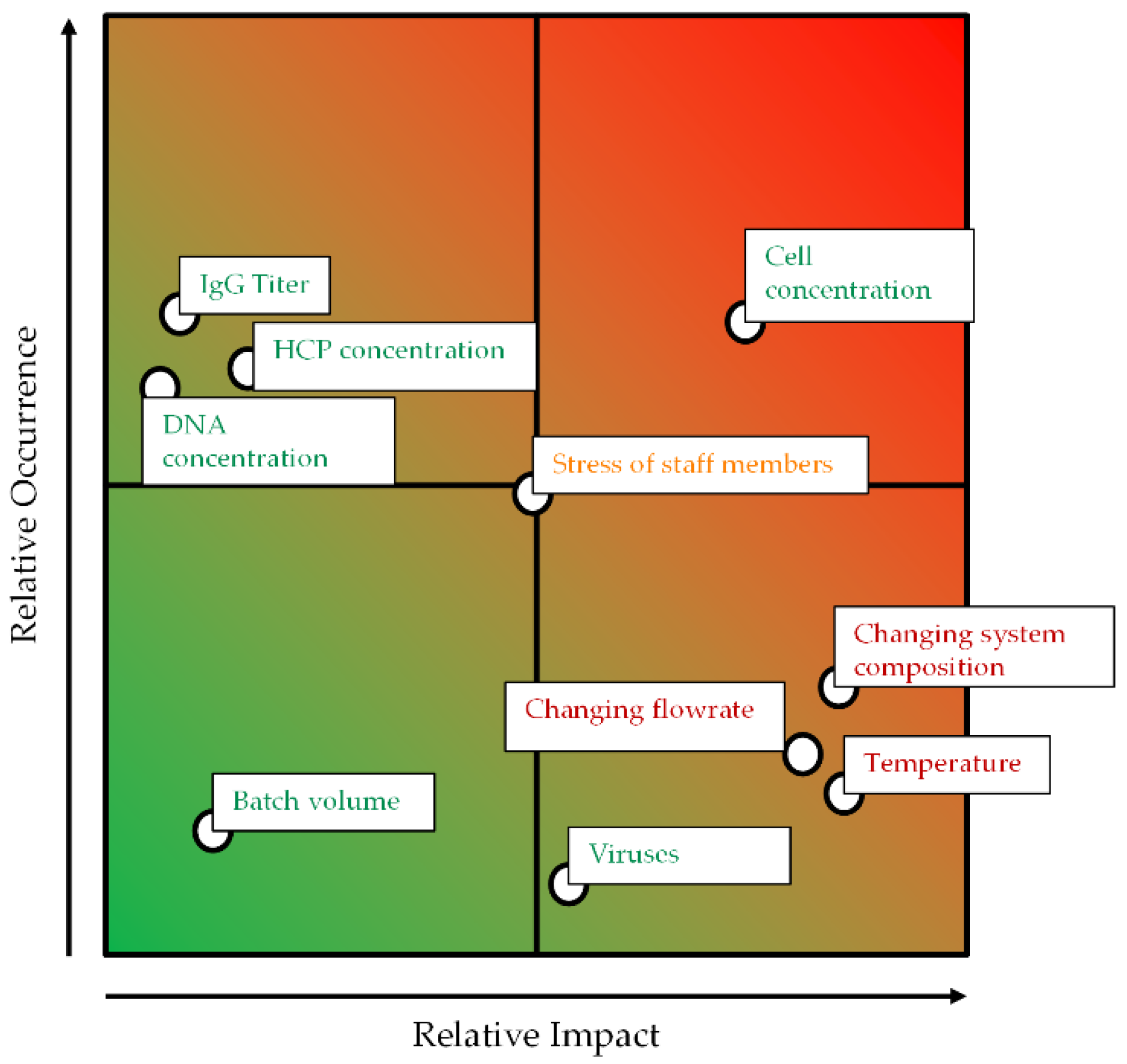
Figure 2. It provides a precise and straightforward view of how ATPE is integrated into the overall purification strategy. To quickly get an overview of all process-relevant effects, it is helpful to construct a cause-and-effect diagram, also known as an Ishikawa or fish bone diagram. It is meant to illustrate the different possible sources of reduced or insufficient process performance. The diagram is constructed of primary branches, which organize specific groups of effects. They can be fanned out further into major branches, representing major causes. Minor branches can be integrated as well to show the relationship between cause and effect in even more detail. Most diagrams have environment, people, materials, equipment, measurement systems, and methods as the primary branches. Though the diagram can be constructed like this, the causes and effects specific to the outlined ATPE targets is shown in
Figure 3.
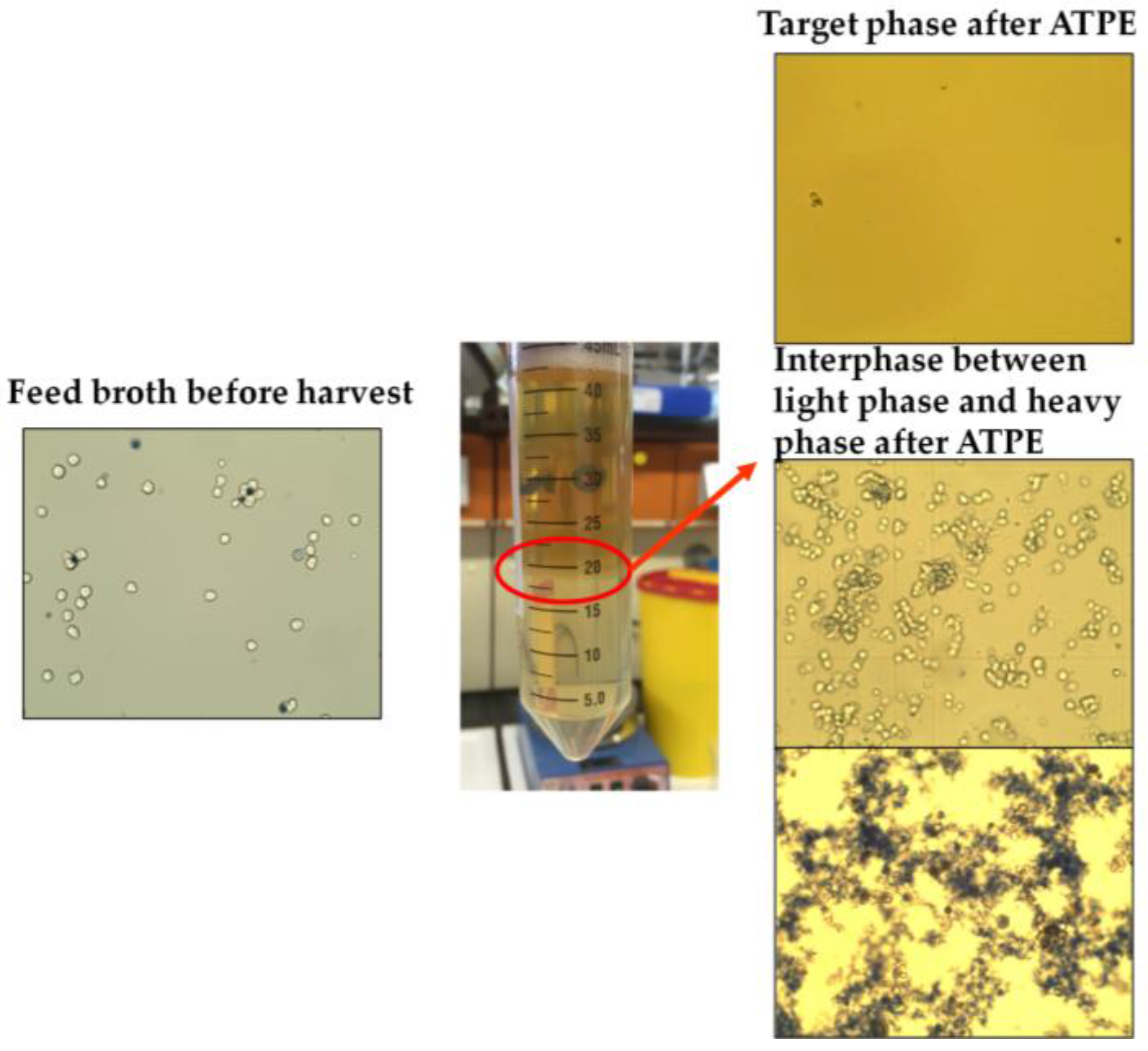
The groups of causes in the diagram are set up by considering so-called prior knowledge. The green branch summarizes the factors that arise from upstream processing (USP) during cultivation and harvesting of the target parameters in this process step. There are several factors that can be optimized during upstream process development, like cultivation time, feed media, and supplement compositions, etc. An extensive overview and methodology for USP optimization has been published by Gronemeyer et al. [
15]. The overall cell number or cell concentration is very important to consider, since ATPE as a harvest operation aims to remove most of the particles to lower the burden for the subsequent filtration trains. In addition to that, higher cell numbers decrease the settling velocity inside the apparatus as during interfacial partitioning the cells adsorb on the dispersed phase and hinder the coalescence of the droplets [
3,
16,
17]. The effect of overall protein content, which is, for the most part, the sum of the mAb and the host cell proteins (HCP), needs to be considered as well. In most cases, protein partitioning between the specific light phase and heavy phase is a function of protein concentration, where total partitioning is only observable for low protein concentrations [
18,
19]. However, by increasing the polarity of the heavy phase, protein solubility can be significantly lowered due to salting-out effects, resulting in very high partitioning coefficients for the target component towards the light phase [
18,
20,
21]. There are two main side-effects of this: firstly, salting-out very often affects HCP in the same way as mAb, which means that achieving high yield comes at the cost of low protein-based purity. Secondly, total partitioning of all proteins, in combination with purposely lowering the volume of the light phase in order to concentrate the target component, can lead to protein saturation and precipitation [
22,
23].
The considered effects are also included in the second primary branch, which organizes the effects of ATPS composition and phase properties (marked blue in
Figure 3). The effects of ATPS type, composition, and phase properties are discussed widely in literature [
21,
24,
25,
26,
27,
28]. The identification of a potential operation space should, at this point, already have occurred in a system optimization study [
29].
The third primary branch organizes the influences of the applied equipment (marked red in
Figure 3). There are several potential devices available for LLE in general, and ATPE specifically. An extensive overview was published by Espitia-Saloma et al. [
30]. In the outlined ATPE process, the combination of a static mixing pipe and a horizontal settler is applied. Although gravity settling is a relatively robust process, even slight changes to inlet geometry or the installation of a baffle have been shown to have a noticeable impact on the settling behavior [
31].
As a further step, a risk assessment considering the relative impact and the relative occurrence of the different branches is applied. This assessment considers factors like temperature, which influences the settling behavior, and changing flow rates, which influences the hydrodynamic residence time and the specific power input.
Both parameters—temperature and changing flow rates—can potentially negatively impact the yield, purity, and cell reduction performance of the ATPE as illustrated in
Figure 4. Increasing process temperatures can alter the intrinsic material properties of the phase-forming components which, like reducing the viscosities, could result in the creation of smaller droplets and, in the presence of cells, cell debris, and other solids, very large separation times [
31]. However, coalescence kinetics in general are accelerated at higher temperatures, such that particle-free ATPS separation times can be reduced. Furthermore, the phase equilibrium itself is dependent on the temperature, since it affects the composition of the specific light and heavy phases, and therefore also changes the partitioning behavior of the target and side components [
32,
33,
34]. The total flow rate determines the hydrodynamic residence time within the apparatus, but can result in an unnecessary firm dispersion. It is even possible to change the point of phase inversion of the system, which drastically affects the settling rates [
35,
36].
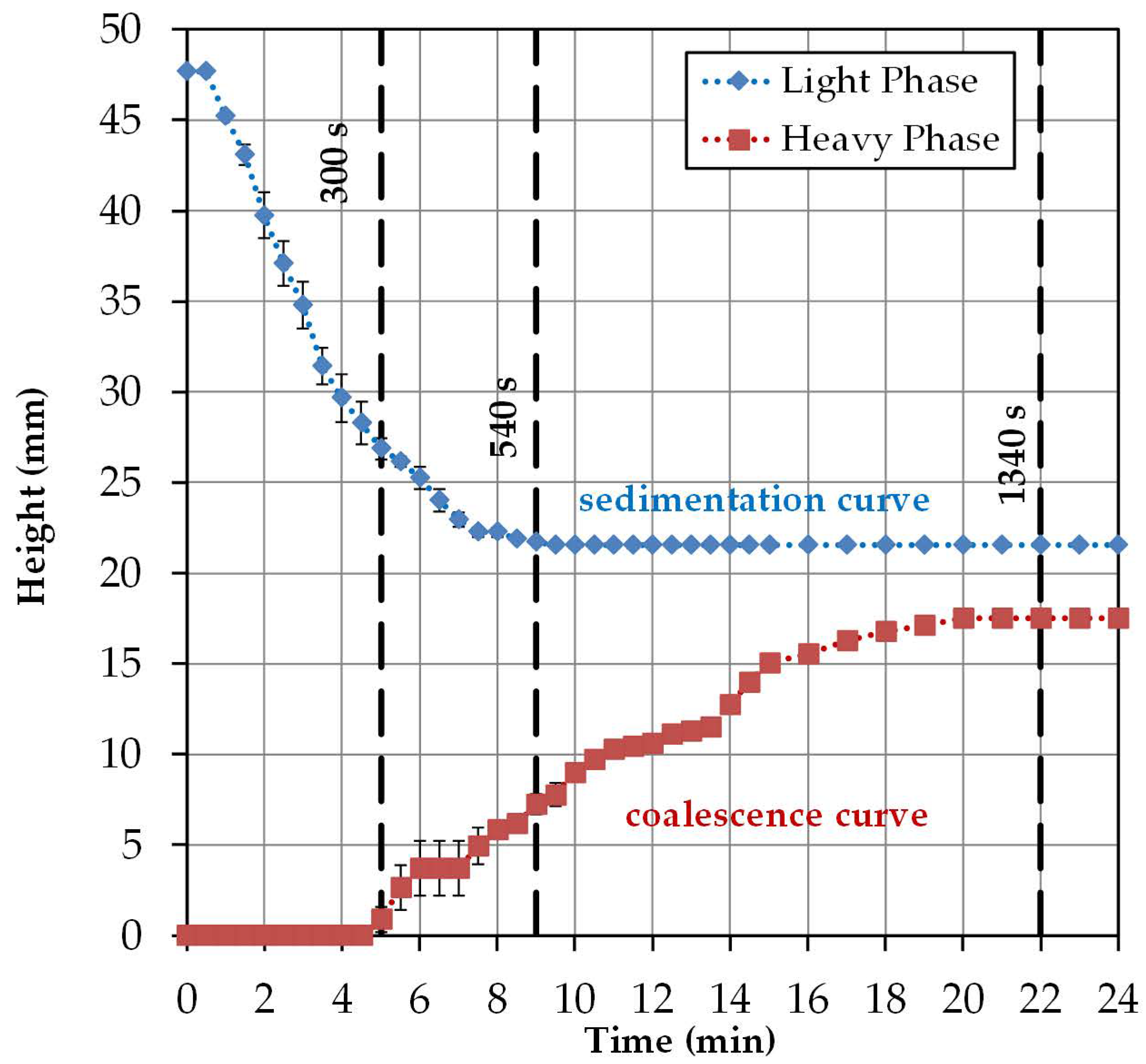
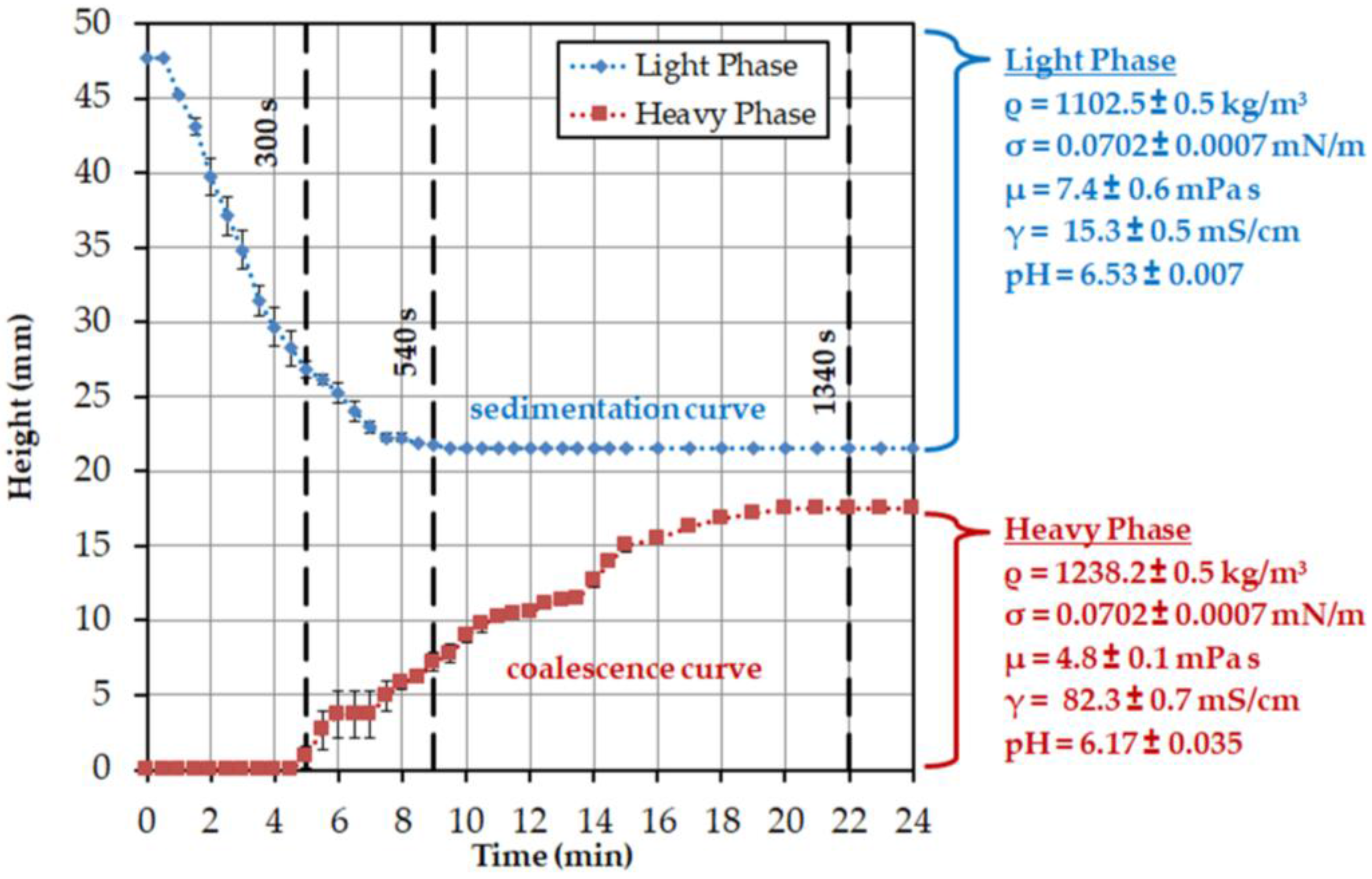
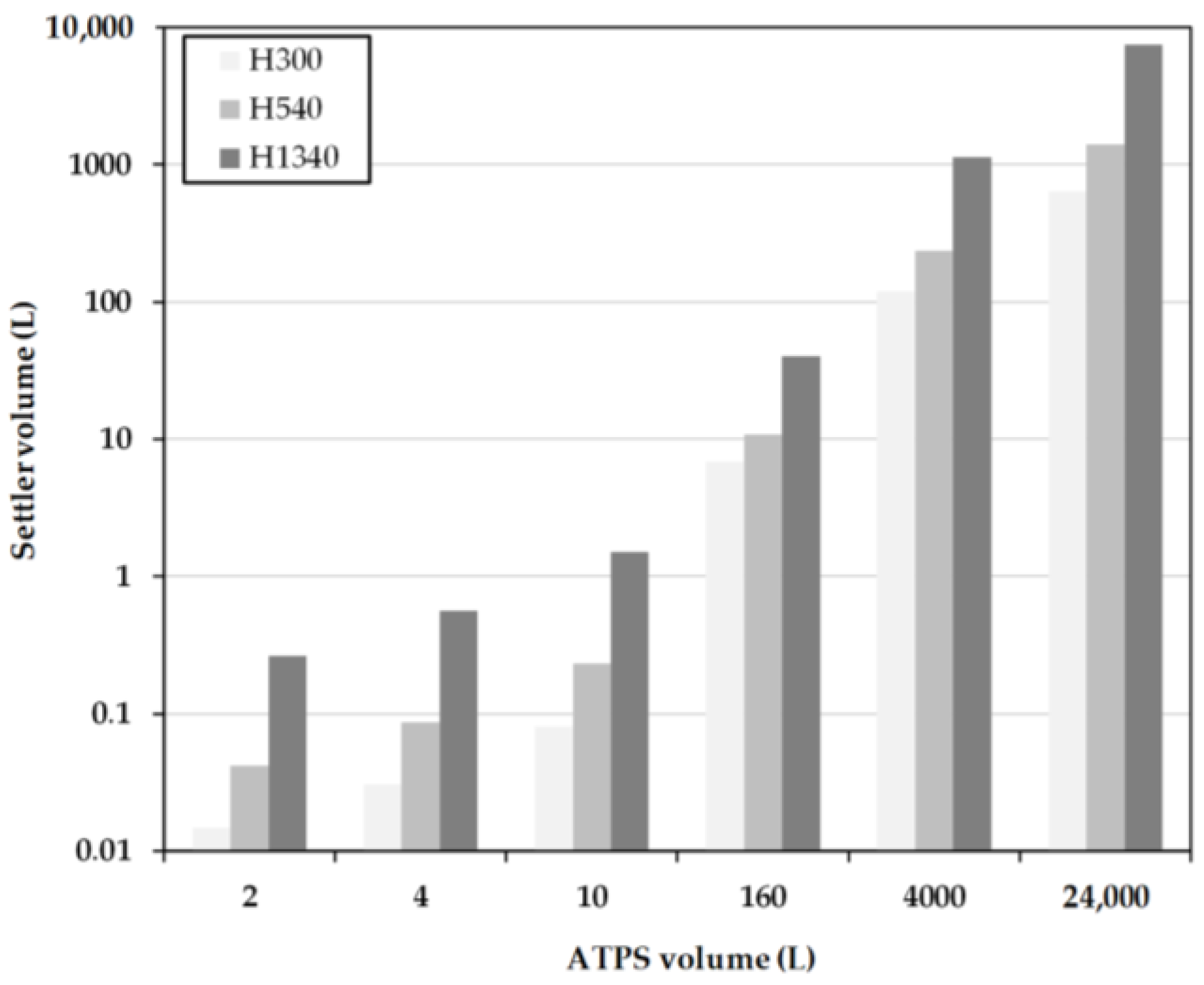
2.3. Batch-Settling Behavior
To enable the early prediction of settler dimensions, other than physical properties, settling and coalescence rates must also be determined.
The settling behavior of an ATPS can be mathematically described by three model parameters. The aim is to describe the influence of different material parameters on the separation behavior. The system is separated when the majority of the phases have settled and an interphase has formed.
The settling time is dependent on the ratio of the interfacial tension between the phases (
σ) and the viscosity of the continuous phase (
μc). The values are composed of the dispersed (
μd) and continuous viscosities, the interfacial tension of water (
σw) and the two phases, as well as the density difference of the dispersed and continuous phases (Δ
ρ) and the density of the continuous phase (
ρc). The exponents
a,
b, and
c are calculated numerically based on experimentally-determined settling times [
37].
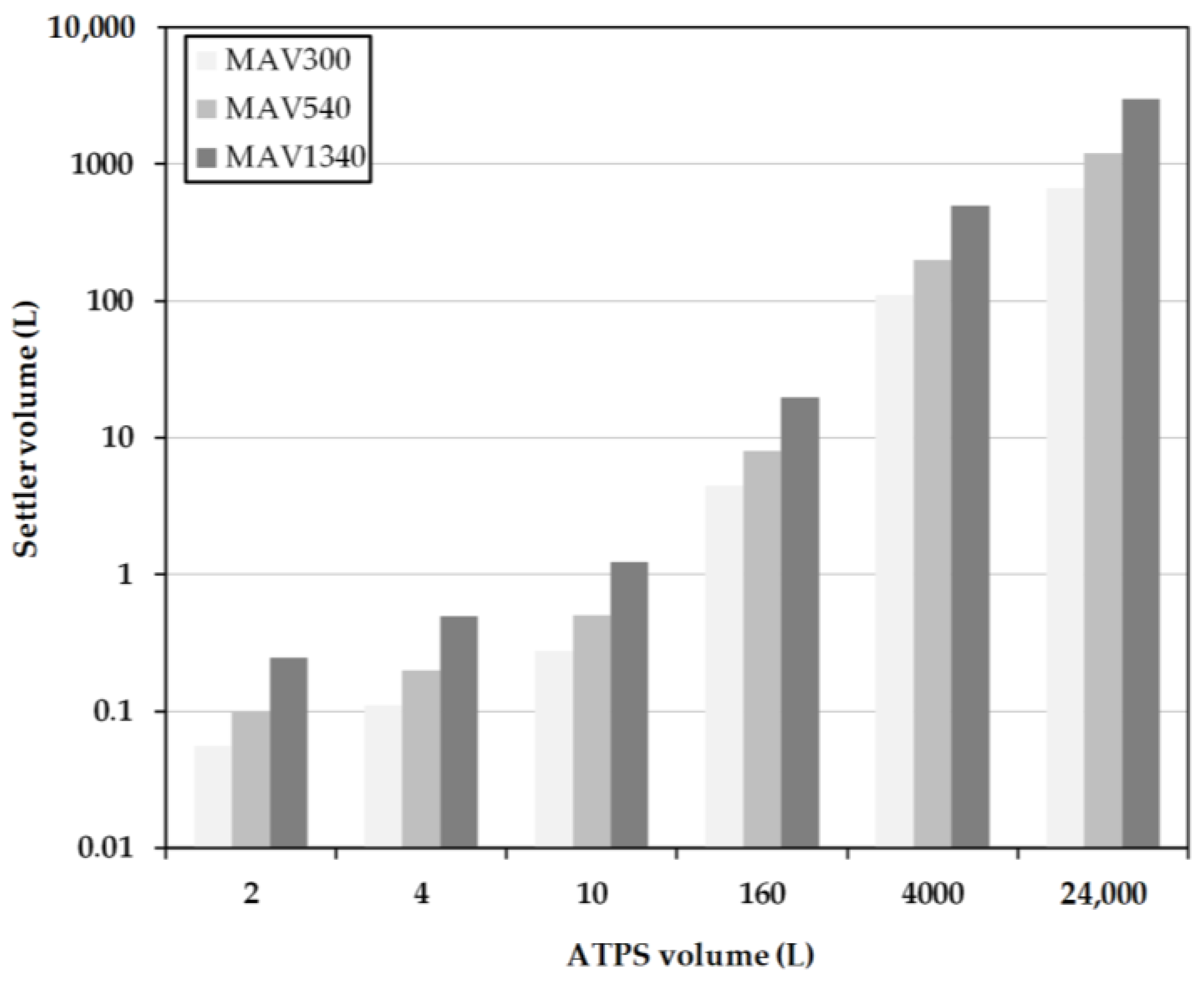
2.4. Early Prediction of Settler Dimensions
To get a rough estimation of the dimensions of the apparatus, volume flow (
) is calculated, which is dependent on the entire volume (
Vtotal) of the ATPS in relation to the predetermined process duration (
ttotal):
The length can then be calculated using volume flow and the predetermined residence time (
tRDT), as well as the cross-sectional area of the device (
AS):
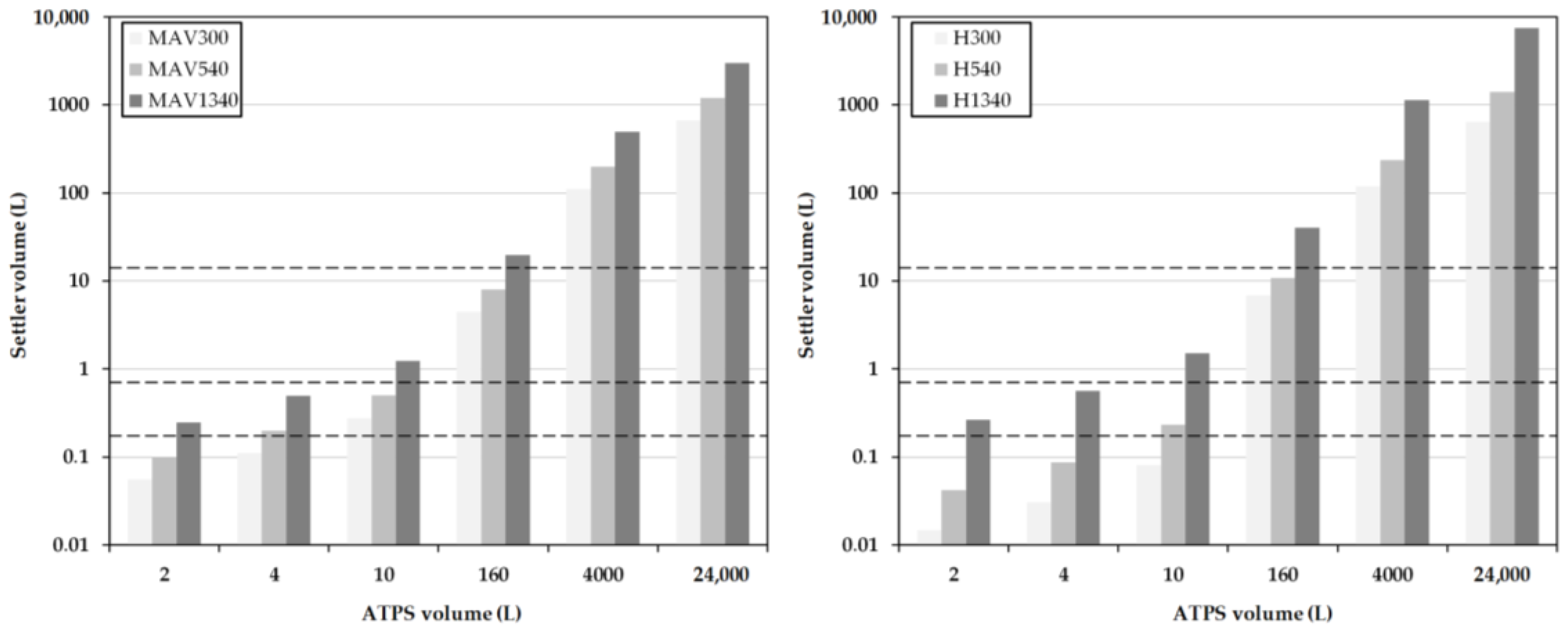
Further influencing variables, such as the drop size, sedimentation rates, or other parameters influencing coalescence, are not taken into account. This quick estimation is often referred to as minimum-apparatus-volume (MAV).
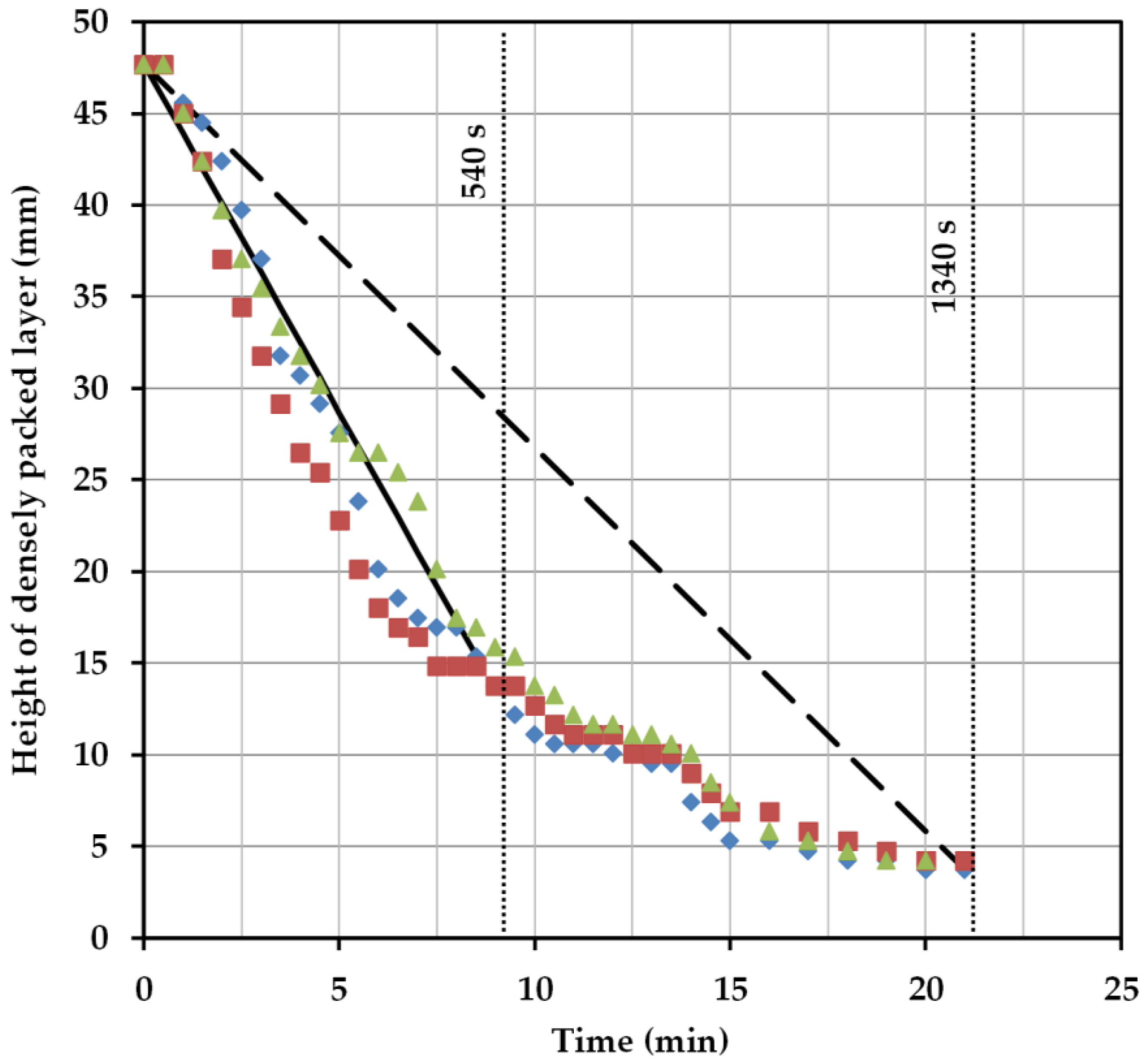
2.5. Henschke Method for Settler Dimensioning
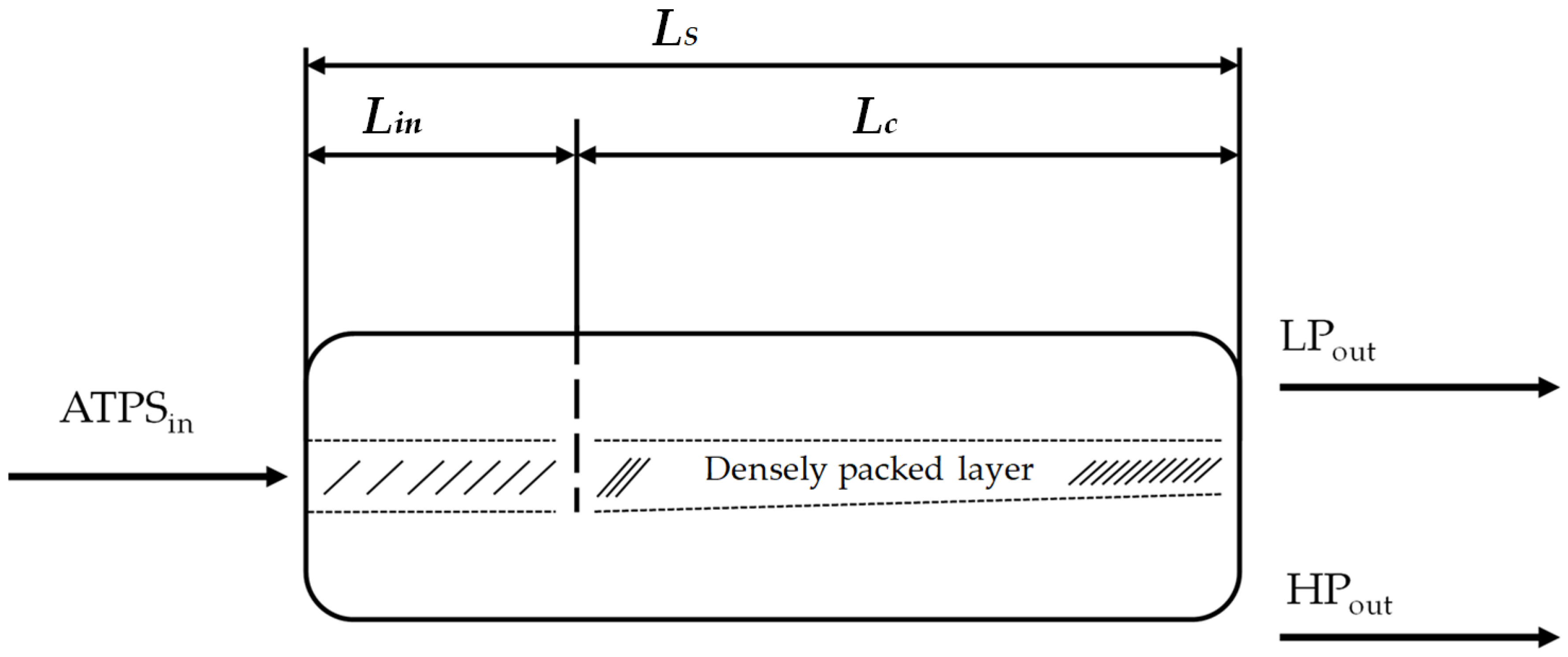
A procedure to calculate the dimensions of horizontal settler units was introduced in 1994 [
38]. It was derived from aqueous organic systems and is investigated here in terms of its suitability for bio-particle loaded aqueous two-phase systems. In this model, the power input, sedimentation rate, and coalescence behavior of the single droplets are taken into account. To examine these influencing factors more precisely, the length of the separator (
LS) is divided into two regions: the inlet area of the dispersion (
Lin) and the coalescence region (
Lc) (
Figure 5).
In the inlet area, occurring turbulences and the formation of a droplet layer are considered. Within the apparatus, drop coalescence and the formation of the densely packed layer (
hp) are of greater interest. The change in the densely packed height can be calculated as follows:
It is dependent on the dispersion volume flow (
), the dispersion viscosity (
), a slippage parameter (
s), the diameter of the settler (
DS) and the dispersed volume fraction in the densely packed layer (
). The settling time (
ts) is normalized to level out the influence of long coalescence times at the boundary surface. The compensation parameter is (
C*). With the aid of these two parameters, the coalescence length of the separator can be determined as follows:
It is dependent on the final height of dispersed phase in a batch-settling experiment (
Hd,0), the volume flow of dispersed phase (
) and the starting droplet diameter in a batch-settling experiment (
) and inside the settler (
). The height of the densely packed layer inside the settler (
Hp) should be predefined. The inlet length must also be determined. It is dependent on the velocity inside the settler (
vS) the volume of the inlet (
Vin), the diameter of the inlet (
Din), earth gravity (
g) and the dispersion band height inside the settler (
). In this case, the mean density (
) and the hold-up (ε
0), which corresponds to the volume fraction of the dispersed phase in the total volume, are determined:
The total length of the separator (
LS) is composed of the inlet length (
Lin) and the coalescence length (
Lc):
The geometry of the dispersion layer is not further considered in the simplified model. For further explanations, reference is made to Henschke et al. [
38].