Spatial Distribution, Adsorption/Release Characteristics, and Environment Influence of Phosphorus on Sediment in Reservoir
Abstract
:1. Introduction
2. Materials and Methods
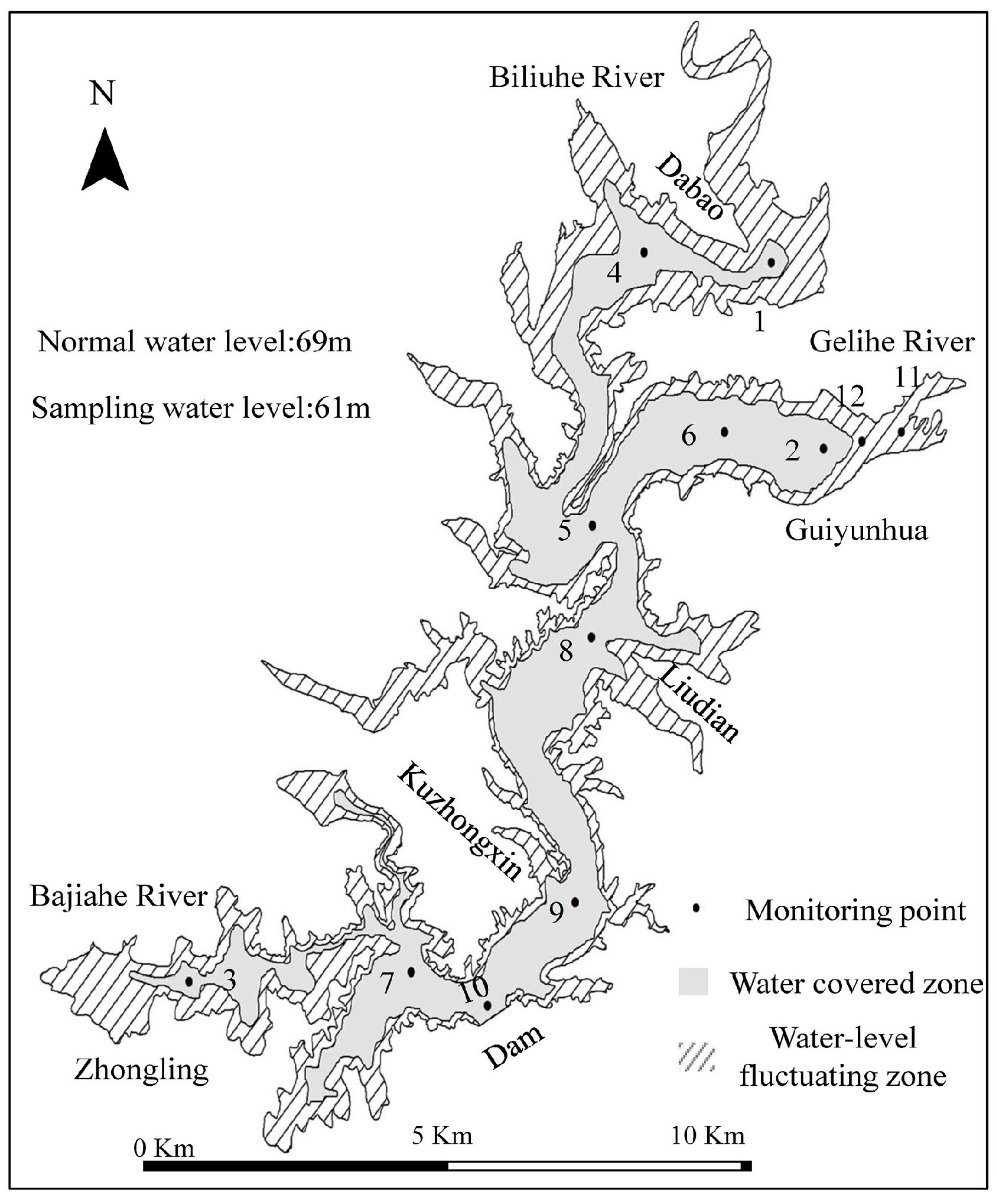
2.1. Study Area and Sediment Sampling
2.2. Experiments of PAdsorption/Releasein Sediment
2.3. Samples Detection and Analysis
3. Results
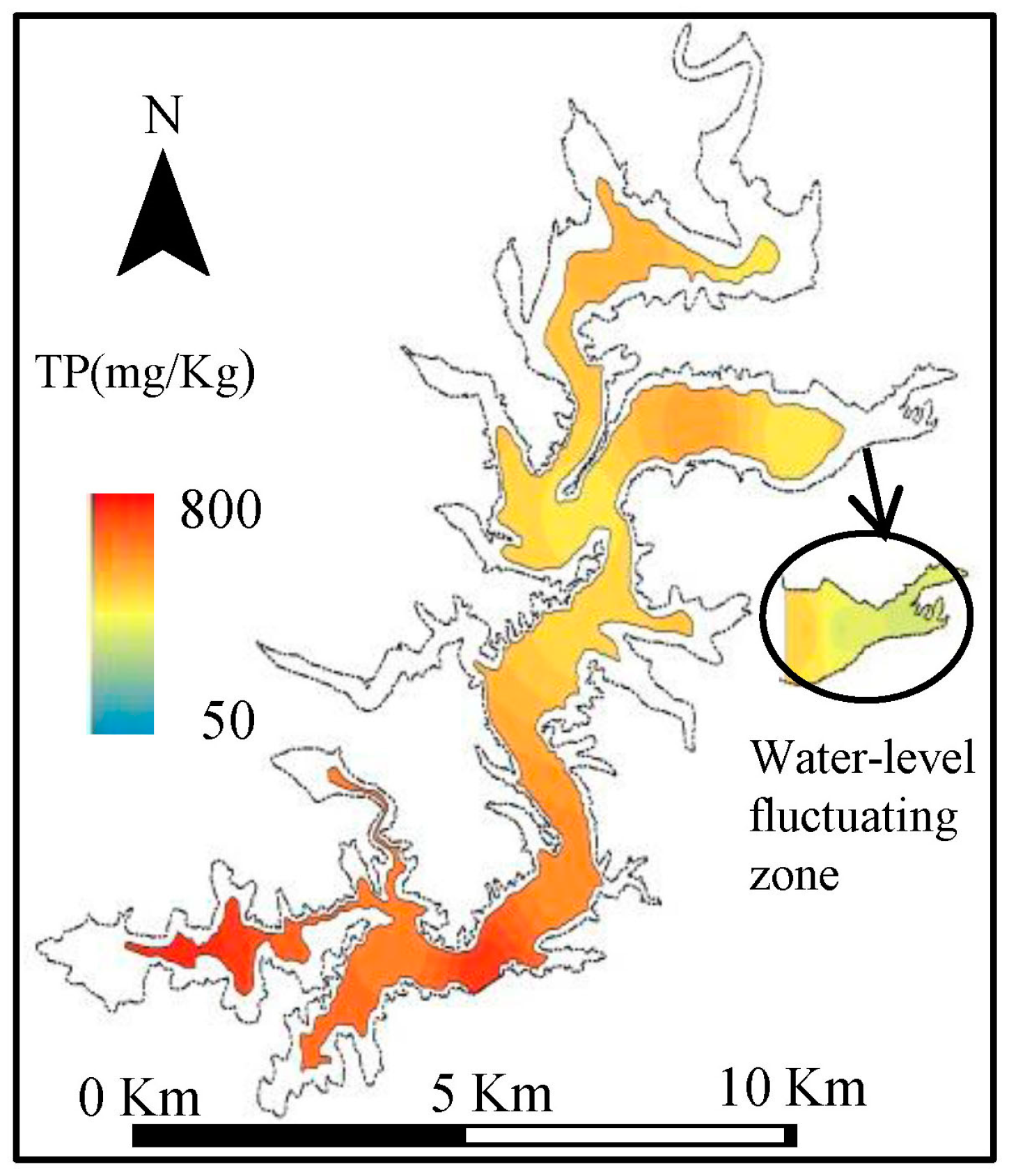
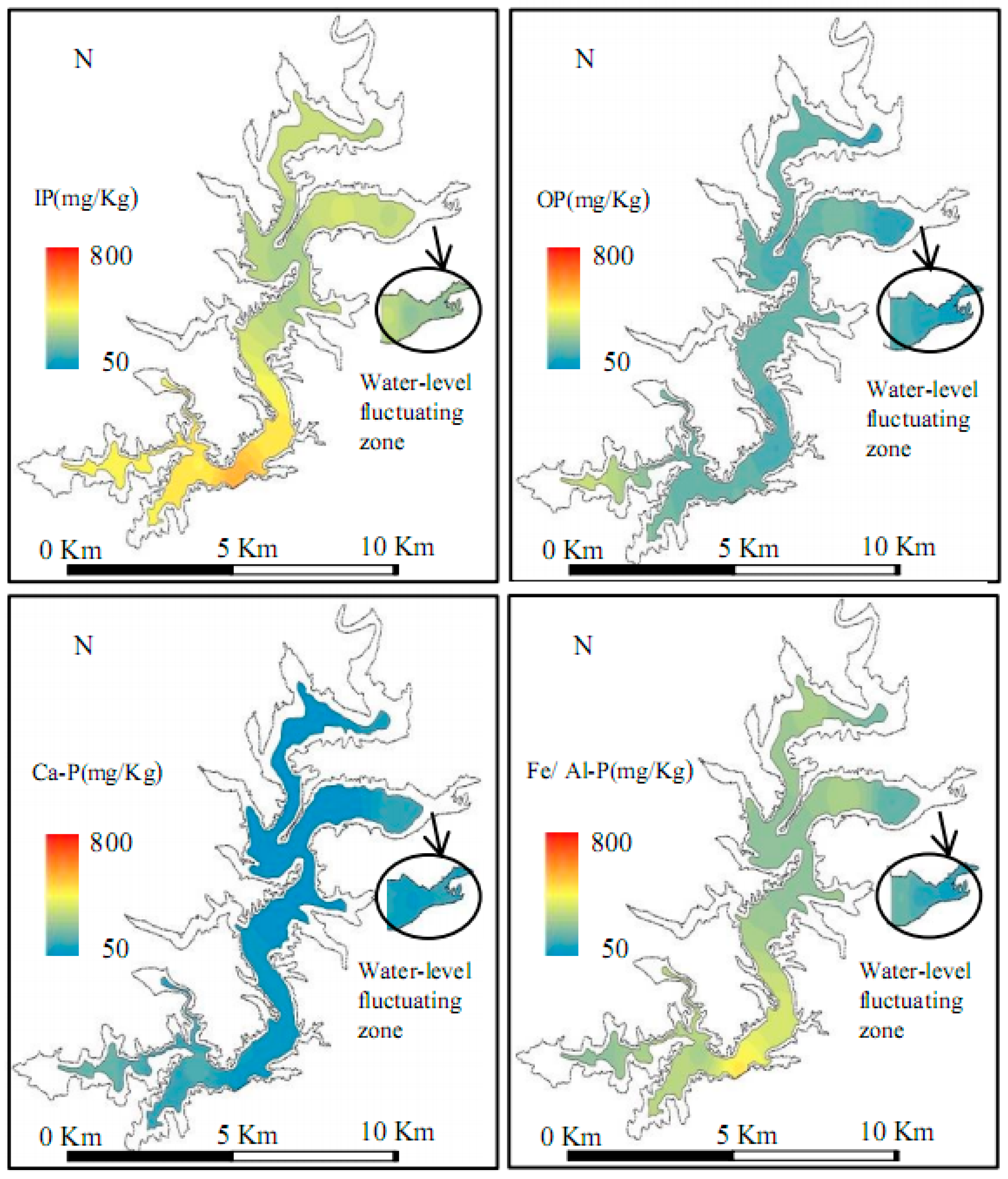
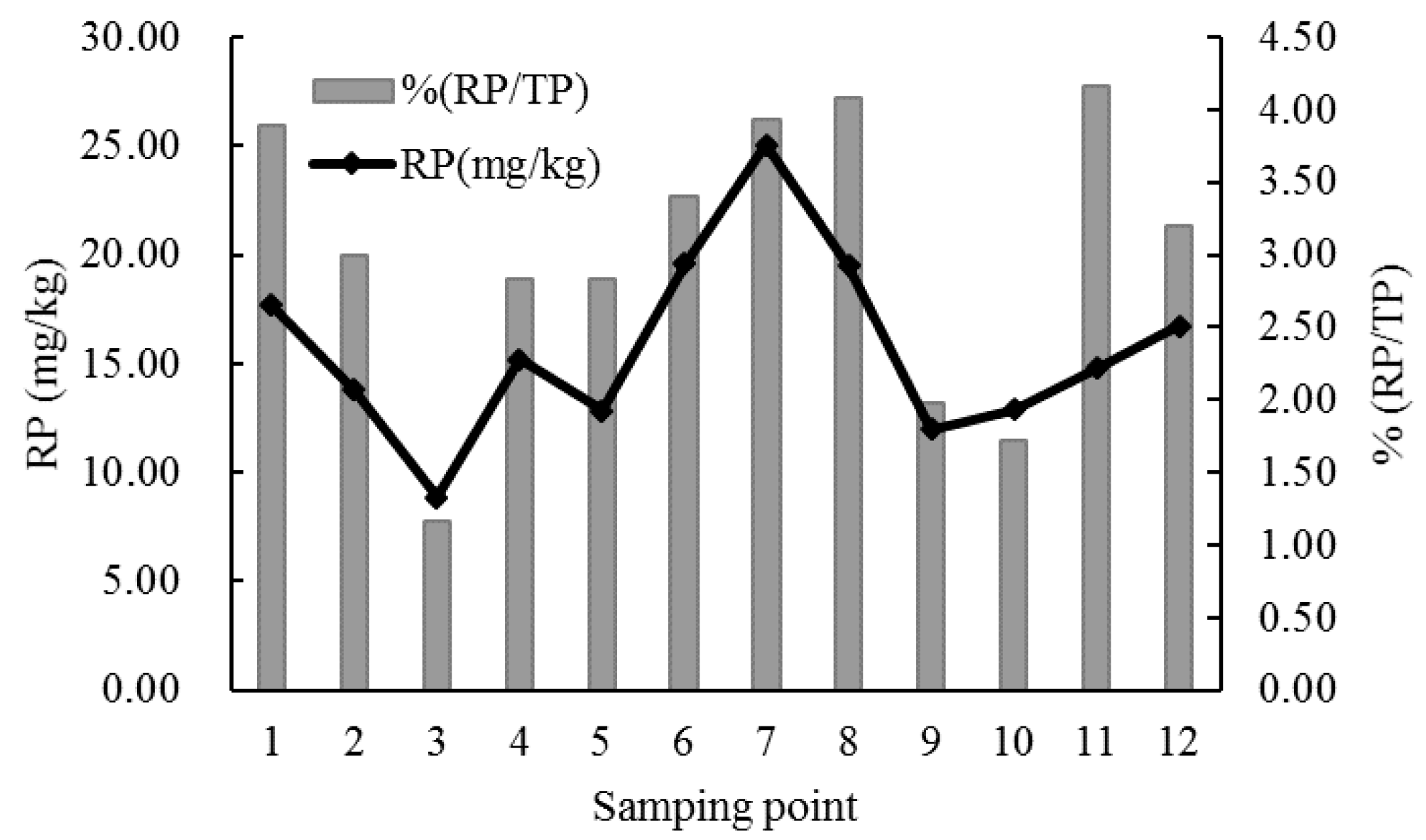
3.1. Results of Field Surveys
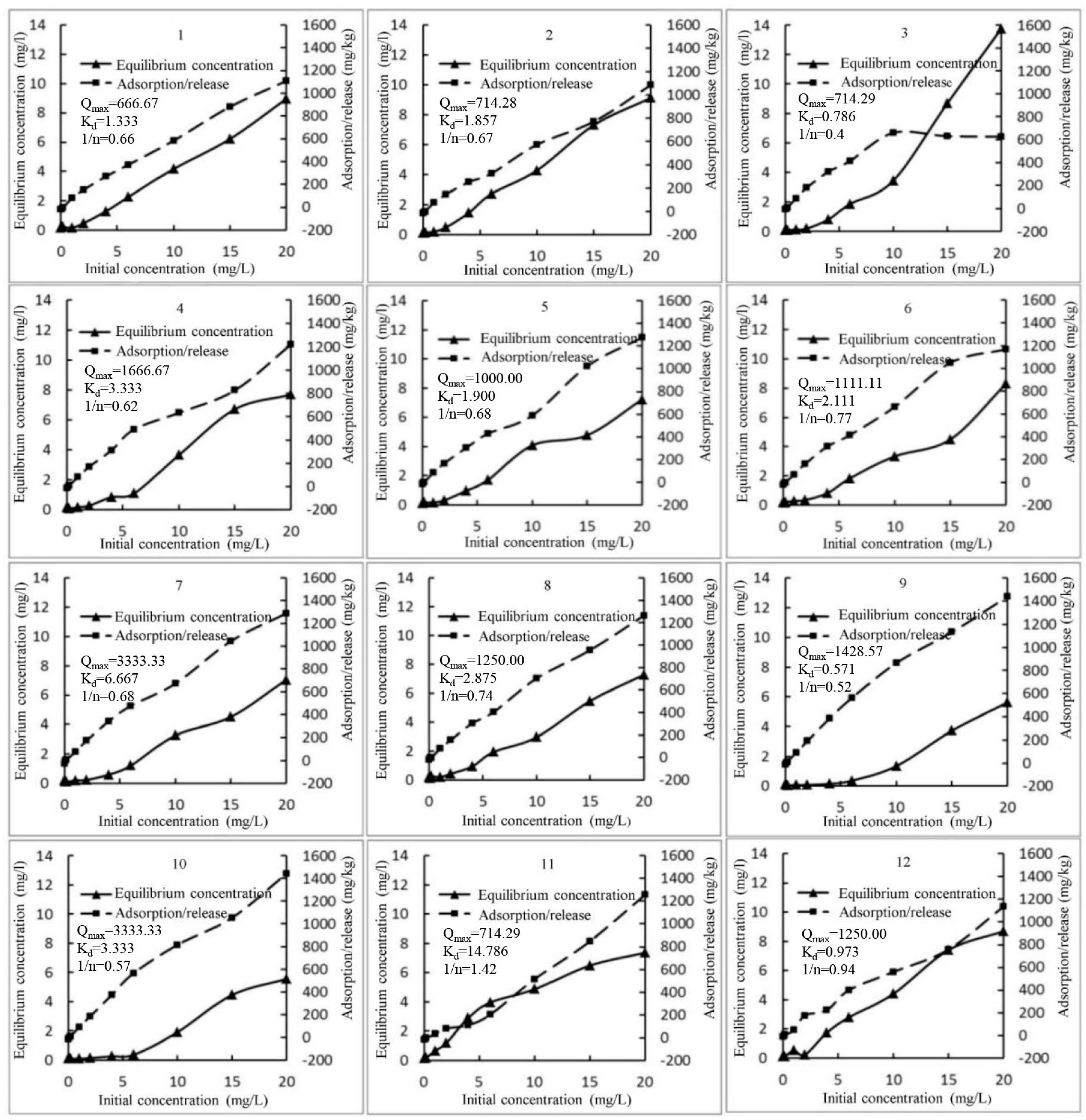
3.2. Results of Simulation Experiments
4. Discussion
4.1. Distribution and Fractions of Sediments P in Biliuhe Reservoir
4.2. Adsorption and Release Characteristics of Sediments P in Biliuhe Reservoir
4.3. Environment Influence of Sediment P
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, D.M.; Wang, W.L.; Jiang, G.Y.; Peng, X.D.; Yu, Y.L.; Li, Y.X.; Ding, W.B. Effects of disturbed landforms on the soil water retention function during urbanization process in the Three Gorges Reservoir Region, China. Catena 2016, 144, 84–93. [Google Scholar] [CrossRef]
- Xu, S.G.; Wang, T.X.; Hu, S.D. Dynamic assessment of water quality based on a Variable Fuzzy Pattern Recognition model. Int. J. Environ. Res. Public Health 2015, 12, 2230–2248. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.G.; Wang, T.X. Review of research on accumulation process and effect of internal pollution of reservoir. Adv. Sci. Technol. Water Resour. 2015, 35, 162–167. [Google Scholar] [CrossRef]
- Skordas, K.; Kelepertzis, E.; Kosmidis, D.; Panagiotaki, P.; Vafidis, D. Assessment of nutrients and heavy metals in the surface sediments of the artificially lake water reservoir Karla, Thessaly, Greece. Environ. Earth Sci. 2015, 73, 4483–4493. [Google Scholar] [CrossRef]
- Dhanakumar, S.; Solaraj, G.; Mohanraj, R. Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotox. Environ. Saf. 2015, 113, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Liu, J.J.; Wang, C.; Qian, J.; Hou, J.; Ren, L.X. Seasonal, Spatial Distribution and Ecological Risk Assessment of Heavy Metals in Surface Sediments from a Watershed Area in Gonghu Bay in Taihu Lake, China. Terr. Atmos. Ocean. Sci. 2014, 25, 605–616. [Google Scholar] [CrossRef]
- Wang, T.Y.; Tan, B.; Lu, Y.L. HCHs and DDTs in Soils around Guanting Reservoir in Beijing, China: Spatial-Temporal Variation and Countermeasures. Sci. World J. 2012, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Fu, Q.; Li, T.X.; Ma, W.L.; Liu, D.; Wang, M. Sediment-Water Exchange, Spatial Variations, and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in the Songhua River, China. Water 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of CHINA. Chinese Water Environment Bulletin; Ministry of Environmental Protection of the People’s Republic of CHINA: Beijing, China, 2015.
- Read, E.K.; Ivancic, M.; Hanson, P.; Cade-Menun, B.J.; Mcmahon, K.D. Phosphorus speciation in a eutrophic lake by 31P NMR spectroscopy. Water 2014, 62, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.W.; Wang, F.; Gao, G.; Zhang, Y.L. Variability of phosphorus concentration in large, shallow and eutrophic Lake Taihu, China. Water Environ. Res. 2008, 80, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Liang, T. Distribution Characteristics of Phosphorus in the Sediments and Overlying Water of Poyang Lake. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.T.; Liber, K.; Doig, L.E. Spatial and temporal in reservoir physicochemistry and phosphorus speciation within lake Diefenbaker, a great plains reservoir, as inferred form depositional sediments. J. Great Lakes Res. 2015, 41, 67–80. [Google Scholar] [CrossRef]
- Ruban, V.; López-Sánchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Fresenius J. Anal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fang, F.; Guo, J.S.; Chen, Y.P.; Guo, S.S. Phosphorus fractions and phosphate sorption-release characteristics relevant tothe soil composition of water-level-fluctuating zone of Three Gorges Reservoir. Ecol. Eng. 2012, 40, 153–159. [Google Scholar] [CrossRef]
- Dong, L.M.; Yang, Z.F.; Liu, X.H. Phosphorus fractions, sorption characteristics, and its release in the sediments of Baiyangdian Lake, China. Environ. Monit. Assess. 2011, 179, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Shi, A.B.; Li, M.Y.; Zhang, X.R. Effects of pH, temperature, dissolved oxygen, and flow rate of overlying water on heavy metals release from storm sewer sediments. J. Chem. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Bai, Z.L.; Liu, W.X.; Kong, X.Z.; Yang, B.; Yang, C.; Jorgensen, S.E.; Xu, F.L. Occurrence, spatial distribution, sources, and risks of polychlorinated biphenyls and heavy metals in surface sediments from a large eutrophic Chinese lake (Lake Chaohu). Environ. Sci. Pollut. Res. 2016, 23, 10335–10348. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Luo, X.X.; Zhang, N.M.; Wu, Y.H. Phosphorus released from sediment of Dianchi Lake and its effect on growth of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2016, 23, 16321–16328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Fan, J.; Zhang, X.P. Effects of simulated wind followed by rain on runoff and sediment yield from a sandy loessial soil with rills. J. Soil. Sediment. 2016, 16, 2306–2315. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wen, Y.J.; Zhou, J.X.; Wu, Y.Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE J. Civ. Eng. 2014, 18, 323–329. [Google Scholar] [CrossRef]
- Kleeberg, A.; Freidank, A.; Joehnk, K. Effects of ice cover on sediment resuspension and phosphorus entrainment in shallow lakes: Combining in situ experiments and wind-wave modeling. Limnol. Oceanorgr. 2013, 58, 1819–1833. [Google Scholar] [CrossRef]
- Wasserman, J.C.; Wasserman, M.A.V.; Barrocas, P.R.G.; Almeida, A.M. Predicting pollutant concentrations in the water column during dredging operations: Implications for sediment quality criteria. Mar. Pollut. Bull. 2016, 108, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, R. Hydraulic performance of sewer pipes with deposited sediments. Water Sci. Technol. 2008, 11, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Belmont, M.A.; White, J.R.; Reddy, K.R. Phosphorus sorption and potential phosphorus storage in sediments of Lake Istokpoga and the upper chain of lakes, Florida, USA[J]. J. Environ. Qual. 2009, 38, 987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zeng, C.S.; Tong, C.; Zhai, S.J.; Lin, X.; Gao, D.Z. Spatial distribution of phosphorus speciation in marsh sediments along a hydrologic gradient in a subtropical estuarine wetland, China. Estuar. Coast. Shelf. Sci. 2015, 154, 30–38. [Google Scholar] [CrossRef]
- Baastrup-Spohr, L.; Moller, C.L.; Sand-Jensen, K. Water-level fluctuations affect sediment properties, carbon flux and growth of the isoetidLittorellauniflora in oligotrophic lakes. Freshw. Biol. 2016, 61, 301–315. [Google Scholar] [CrossRef]
- Brdgeman, T.B.; Chaffin, J.D.; Kane, D.D.; Conroy, J.D.; Panek, S.E.; Armenio, P.M. From river to lake: Phosphorus partitioning and algal community compositional changes in Western lake Erie. J. Great Lakes Res. 2012, 38, 90–97. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, G.; Li, W.; Zhang, Y.; Zhao, L.; Gu, Z. Estimation of the algal-available phosphorus pool in sediments of a large, shallow eutrophic lake (Taihu, China) using profiled SMT fractional analysis. Environ. Pollut. 2013, 173, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.D.; He, Y.L.; Kirumba, G. Phosphorus fractions and phosphate sorption-release characteristics of the sediment in the Yangtze River estuary reservoir. Ecol. Eng. 2013, 55, 62–66. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Y.Q.; Yu, L.; Hua, Z.L. Distribution behavior of superparamagnetic carbon nanotubes in an aqueous system. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tayo, O.O.; John, O.O.; Georges-Ivo, E.E. Fluoride Sorption Efficiency of Vermiculite Functionalised with Cationic Surfactant: Isotherm and Kinetics. Appl. Sci. 2016, 6, 277. [Google Scholar] [CrossRef]
- Qiu, H.; Vijver, M.G.; He, E.K.; Peijnenburg, W.J.G.M. Predicting Copper Toxicity to Different Earthworm Species Using a Multicomponent Freundlich Model. Environ. Sci. Technol. 2013, 47, 4796–4803. [Google Scholar] [CrossRef] [PubMed]
- Hafeznezami, S.; Zimmer-Faust, A.G.; Dunne, A.; Tran, T.; Yang, C.; Lam, J.R.; Reynolds, M.D.; Davis, J.A.; Jay, J.A. Adsorption and desorption of arsenate on sandy sediments from contaminated and uncontaminated saturated zones: Kinetic and equilibrium modelling. Environ. Pollut. 2016, 215, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Deng, W.M.; Xu, X.M.; He, J.; Wang, S.R.; Jiao, L.X.; Zhang, Y. Phosphorus adsorption and release characteristics of surface sediment in Dianchi lake, China. Environ. Earth Sci. 2015, 74, 3689–3700. [Google Scholar] [CrossRef]
- Wang, S.R.; Jin, X.C.; Pang, Y.; Zhao, H.C.; Zhou, X.N.; Wu, F.C. Phosphorus fractions and phosphate sorption characteristics in relation to the sediment compositions of shallow lakes in the middle and lower reaches of Yangtze River region China. J. Colloid Interface Sci. 2005, 289, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, X.L.; Wang, P.F.; Hou, J.; Ao, Y.H.; Miao, L.Z. Adsorption behavior of lead on aquatic sediments contaminated with cerium dioxide nanoparticles. Environ. Pollut. 2016, 219, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, B.; Aydin, F.; Aydin, I.; Hamamci, C. Study of phosphorus distribution in coastal surface sediment by sequential extraction procedure (NE Mediterranean Sea, Antalys-Turkey). Microchem. J. 2011, 98, 72–76. [Google Scholar] [CrossRef]
- GikumaNjuru, P.; Hecky, R.E.; Guildford, S.J. Surficial sediment phosphorus fractions along a biogeochemical gradient in Nyanza Gulf, northeastern lake Victoria and their possible role in phosphorus recycling and internal loading. Biogeochemistry 2010, 97, 247–261. [Google Scholar] [CrossRef]
- Ye, H.X. A Study on the Spatial Differention and Potential Ecological Risk of Nutrients and Heavy Metals in the Sediments of Zhalong Wetland in China; Harbin Normal University Press: Harbin, China, 2014. [Google Scholar]

| Type | Item | Maximum (mg/kg) | Minimum (mg/kg) | Mean (mg/kg) | Standard Deviation | Coefficient of Variation |
|---|---|---|---|---|---|---|
| Water covered zone (n = 10) | TP | 764.57 | 452.73 | 571.56 | 117.85 | 0.21 |
| Fe/Al-P | 455.00 | 173.90 | 277.52 | 82.51 | 0.30 | |
| Ca-P | 194.14 | 53.20 | 102.76 | 49.39 | 0.48 | |
| IP | 549.73 | 284.40 | 380.28 | 86.66 | 0.23 | |
| OP | 336.05 | 135.68 | 191.28 | 57.93 | 0.30 | |
| Water-level fluctuating zone (n = 2) | TP | 523.96 | 355.46 | 439.71 | 119.15 | 0.27 |
| Fe/Al-P | 219.56 | 112.71 | 166.13 | 75.55 | 0.45 | |
| Ca-P | 130.10 | 115.23 | 122.67 | 10.51 | 0.09 | |
| IP | 334.79 | 242.81 | 288.80 | 65.04 | 0.23 | |
| OP | 189.18 | 112.65 | 150.91 | 54.11 | 0.36 |
| TP | Fe/Al-P | Ca-P | IP | OP | |
|---|---|---|---|---|---|
| TP | 1 | 0.778 ** | 0.316 | 0.922 ** | 0.770 ** |
| Fe/Al-P | 0.778 ** | 1 | −0.223 | 0.888 ** | 0.320 |
| Ca-P | 0.316 | −0.223 | 1 | 0.250 | 0.312 |
| IP | 0.922 ** | 0.888 ** | 0.250 | 1 | 0.465 |
| OP | 0.770 ** | 0.320 | 0.312 | 0.465 | 1 |
| Sampling Site | Equation | Qmax | Kd | R2 |
|---|---|---|---|---|
| 1 | 1/q = 0.002/C + 0.0015 | 666.67 | 1.333 | 0.970 |
| 2 | 1/q = 0.0026/C + 0.0014 | 714.28 | 1.857 | 0.981 |
| 3 | 1/q = 0.0011/C + 0.0014 | 714.29 | 0.786 | 0.960 |
| 4 | 1/q = 0.002/C + 0.0006 | 1666.67 | 3.333 | 0.959 |
| 5 | 1/q = 0.0019/C + 0.001 | 1000.00 | 1.900 | 0.986 |
| 6 | 1/q = 0.0019/C + 0.0009 | 1111.11 | 2.111 | 0.977 |
| 7 | 1/q = 0.002/C + 0.0003 | 3333.33 | 6.667 | 0.864 |
| 8 | 1/q = 0.0023/C + 0.0008 | 1250.00 | 2.875 | 0.996 |
| 9 | 1/q = 0.0004/C + 0.0007 | 1428.57 | 0.571 | 0.985 |
| 10 | 1/q = 0.001/C + 0.0003 | 3333.33 | 3.333 | 0.949 |
| 11 | 1/q = 0.0207/C + 0.0014 | 714.29 | 14.786 | 0.960 |
| 12 | 1/q = 0.0035/C + 0.0008 | 1250.00 | 4.375 | 0.973 |
| Sampling Site | Equation | 1/n | R2 |
|---|---|---|---|
| 1 | Log(q) = 0.6589Log(C) + 2.3855 | 0.66 | 0.993 |
| 2 | Log(q) = 0.6707Log(C) + 2.3193 | 0.67 | 0.981 |
| 3 | Log(q) = 0.3977Log(C) + 2.4667 | 0.40 | 0.888 |
| 4 | Log(q) = 0.6159Log(C) + 2.5049 | 0.62 | 0.935 |
| 5 | Log(q) = 0.6821Log(C) + 2.4805 | 0.68 | 0.972 |
| 6 | Log(q) = 0.7654Log(C) + 2.4433 | 0.77 | 0.935 |
| 7 | Log(q) = 0.6771Log(C) + 2.5598 | 0.68 | 0.935 |
| 8 | Log(q) = 0.7439Log(C) + 2.4545 | 0.74 | 0.992 |
| 9 | Log(q) = 0.5189Log(C) + 2.825 | 0.52 | 0.879 |
| 10 | Log(q) = 0.5663Log(C) + 2.7532 | 0.57 | 0.872 |
| 11 | Log(q) = 1.4172Log(C) + 1.6828 | 1.42 | 0.900 |
| 12 | Log(q) = 0.9388Log(C) + 2.1797 | 0.94 | 0.899 |
| Samping Point | Critical Concentration (mg/L) | Sampling Point | Critical Concentration (mg/L) |
|---|---|---|---|
| 1 | 0.2–1.0 | 7 | 0.1–0.2 |
| 2 | 0.1–0.2 | 8 | 0.2–1.0 |
| 3 | 0.1–0.2 | 9 | 0–0.1 |
| 4 | 0.1–0.2 | 10 | 0.1–0.2 |
| 5 | 0.1–0.2 | 11 | 0.2–1.0 |
| 6 | 0.2–1.0 | 12 | 0.1–0.2 |
| Level/Index | TN (mg/kg) | TP (mg/kg) |
|---|---|---|
| Security level | - | - |
| Lowest level | 550 | 600 |
| Serious level | 4800 | 2000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, J.; Xu, S.; Qin, G.; Sun, Y.; Wang, F. Spatial Distribution, Adsorption/Release Characteristics, and Environment Influence of Phosphorus on Sediment in Reservoir. Water 2017, 9, 724. https://doi.org/10.3390/w9090724
Wang T, Liu J, Xu S, Qin G, Sun Y, Wang F. Spatial Distribution, Adsorption/Release Characteristics, and Environment Influence of Phosphorus on Sediment in Reservoir. Water. 2017; 9(9):724. https://doi.org/10.3390/w9090724
Chicago/Turabian StyleWang, Tianxiang, Jianwei Liu, Shiguo Xu, Guoshuai Qin, Ya Sun, and Fuqiang Wang. 2017. "Spatial Distribution, Adsorption/Release Characteristics, and Environment Influence of Phosphorus on Sediment in Reservoir" Water 9, no. 9: 724. https://doi.org/10.3390/w9090724




