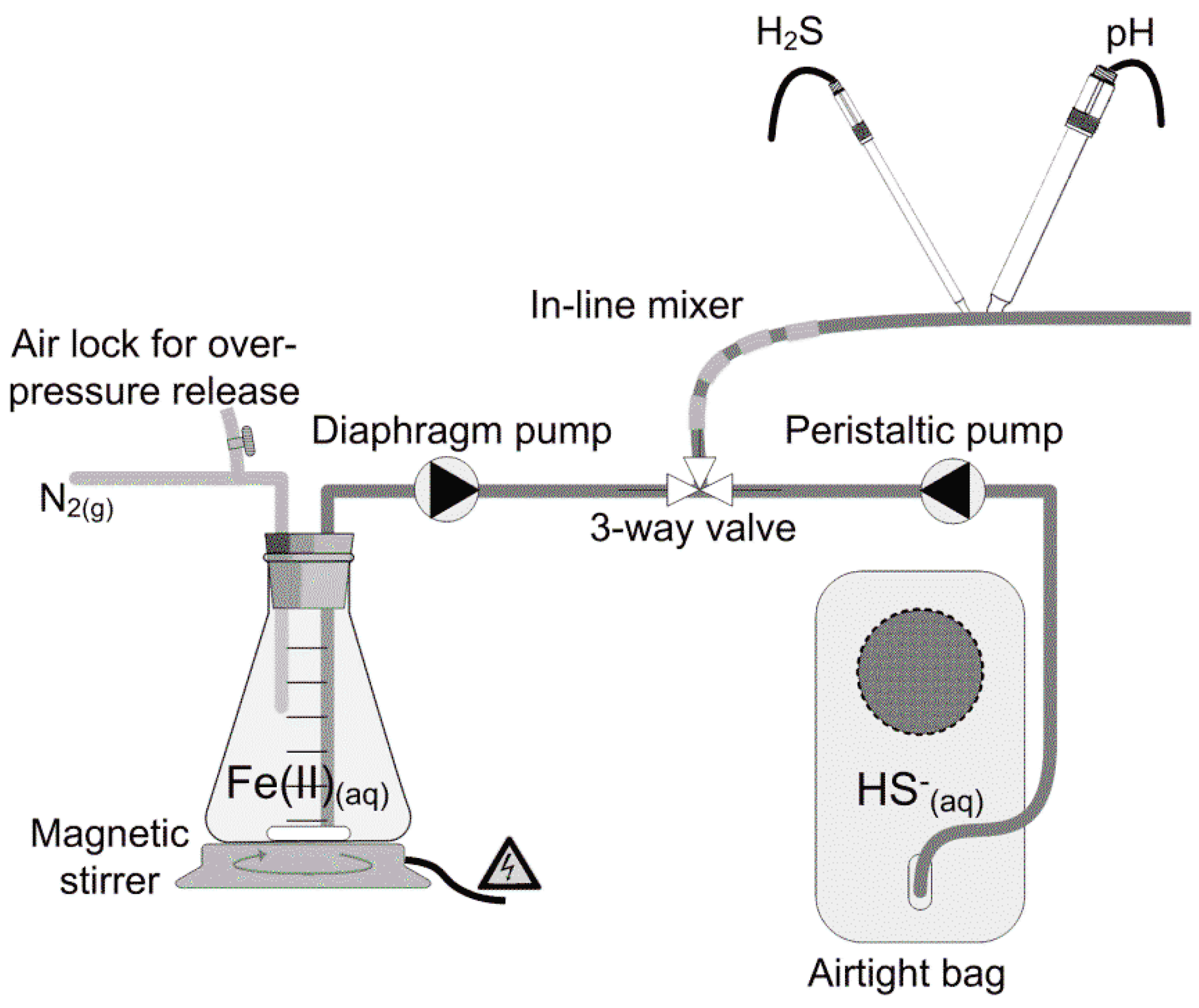
The buffered water was prepared to have a calcium carbonate alkalinity reflecting that of typical wastewater, as reported in the literature. Its pH was adjusted to approx. 6.5, 7.0 and 7.5 (
Table 2), which is typical for wastewater that has been subject to anaerobic transformation and hence sulfide formation [
2]. The three wastewaters had calcium carbonate alkalinities that were 3–4.5 times higher than the buffered water. This variation from typical values was due to the public water supply of the region being based on groundwater extracted from limestone aquifers of high carbonate content. pH of the wastewaters was adjusted to approx. 7 prior to experiments, in order to allow comparable precipitation conditions. The sulfide solution and the iron stock were mixed in the three-way valve (
Figure 1), achieving initial molar ratios ranging from 1.75–2.84 mol Fe (mol·S)
−1 (
Table 2).
3.1. Precipitation Stoichiometry Versus pH in Buffered Water
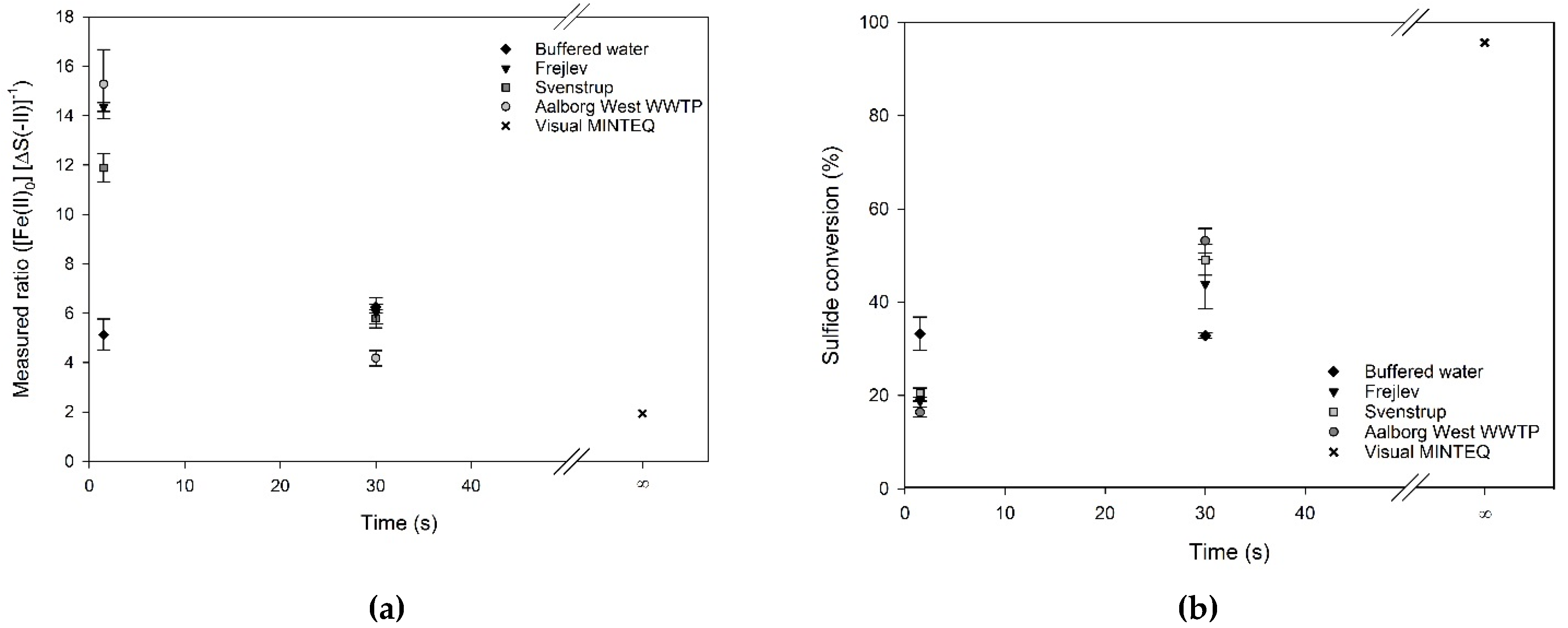
Figure 3 shows the precipitation ratios and the sulfide conversion in buffered water (
Table 1) at 1.5 and 30 s of reaction time. A comparison with the ratio of a fully equilibrated reaction is included, which has been calculated from a simulation hereof using Visual MINTEQ. It is evident from the figure that the stoichiometric ratios and sulfide conversions depended on pH, where a lower pH resulted in higher stoichiometric ratio and lower conversion. For the buffered water, the stoichiometric ratios were not greatly affected by the reaction time. The difference in the ratio between 1.5 and 30 s reaction time at pH 6.5 was believed to be due to a difference in pH between the two runs. Although the pH only differed by 0.05 pH-units, the trend of the data indicates that this slight variation was likely to have caused the difference in stoichiometric ratio. Similarly, [
1] stated that below pH 6.5, addition of iron will only have little effect, and a slight change in pH might consequently be expected to result in much higher demands for ferrous iron and thus a higher stoichiometric ratio. An increase in ratio when lowering the pH was also observed for the modelled equilibrium conditions, albeit less distinct.
The stoichiometric ratios at the lowest pH-values were up to 30 times higher than the stoichiometric requirement (1 mol Fe (mol·S)
−1) and 15 times higher than the equilibrium condition modelled by Visual MINTEQ. At pH 7.5, the obtained stoichiometric ratios were around 3.5 times higher than the stoichiometric requirement and two-fold higher than the modeled ratio. Even though the stoichiometric ratio came closer to the modeled equilibrium ratio as pH increased, it was still higher than what has been reported in literature (e.g. [
3,
11,
15,
21]). In the analysis performed by Visual MINTEQ, the concentrations of the different chemical species were kept equal to the buffered water experiments. This implies that the inorganic ligands for ferrous iron in the buffered water were also considered during modelling of equilibrium conditions. The 2–15 fold differences between measured and modeled ratios were consequently expected to account for the fact that the ferrous sulfide reaction had not fully equilibrated. The validity of this statement was indirectly supported by a study by [
22] where reaction times of 5–7 minutes agreed with the analysis performed in Visual MINTEQ.
The fact that the difference between modeled and measured ratios became less as pH increased, might be related to speciation of sulfide and iron. Such a phenomenon was observed by [
14,
23] which showed that the precipitation mechanism at ambient temperature depends on pH and sulfide concentration, and thus indirectly also on the speciation of sulfide. It was reported that at sulfide concentrations below 10
−3 M, a H
2S-pathway of FeS formation dominated in environments up to pH 8, with FeS forming directly, and that the rate of FeS formation was greater in neutral and acidic environments. At concentrations above 10
−3 M, the rate was instead greater at neutral to alkaline conditions where a bisulfide pathway dominated. For this concentration range, Fe(HS)
2 formed as an intermediate before further transformation to FeS. However, even in the concentration range above 10
−3 M, the H
2S-pathway took over in acidic environments to yield FeS directly.
The findings of the present study, where sulfide was added to concentrations of 10
−6 M, are somewhat contradictory to those of [
14,
23]. In contrast here, a higher conversion was observed at increasing pH even though sulfide in the present study was added to concentrations < 10
−3 M. Thereby it seems that the bisulfide pathway, with an intermediate forming as suggested by [
14], could be dominant even at sulfide concentrations as low as 10
−6 M.
Similarly, [
24] suggested that an intermediate species in the reaction pathway is present. By stopped-flow spectrophotometry they found that within the first few seconds of the reaction, an intermediate formed and a subsequent conversion of this intermediate took place. They suggested that this initial product of the reaction between Fe
2+ and HS
− was Fe(HS)
+. Also, [
25] found that different types of FeS form at neutral pH compared to slightly acidic conditions, indicating that different reaction pathways are followed.
As reported in literature, it is not completely agreed upon whether the FeS that forms under the different pH conditions of these earlier studies is in the form of an intermediate reaction product, amorphous FeS, nanocrystallineor microcrystalline mackinawite, or greigite (e.g. [
23,
25,
26]). This discrepancy might be because ferrous sulfide salts appear in nature in various different forms and crystal structures, and the mechanisms leading to the different forms are complicated [
25]. The specific form of FeS generated, and hence also the resulting reaction kinetics, might thus vary with e.g. pH, redox conditions, ionic strength, and available ligands.
FeS readily precipitates, and hence plays a role in controlling the concentrations of aqueous ferrous and sulfide concentrations [
27,
28]. The equilibrium simulations in Visual MINTEQ indicate that there might have been free sulfide at low pH, as the removal of sulfide was not complete even though ferrous iron was in excess (
Figure 3). The concentration of free sulfide decreased at higher pH, indicating that more FeS was formed (
Figure 3). Depending on the resulting structure of the FeS complex the values of the solid/liquid-partitioning coefficient, pKs, are reported to be in the range of 2.95–5.25, with the crystalline forms having the highest values [
27]. This agrees well with the values used in Visual MINTEQ for the simulations with pKs values of 2.95 for amorphous FeS and 3.60 for mackinawite.
The crystalline forms of ferrous sulfide, such as mackinawite and greigite [
26], are probably not the first to be formed in the reaction. In shallow and deep natural water bodies, [
29] found that aging of the FeS precipitate might play a role in the solubility and that a metastable phase of FeS is transformed on aging to crystalline and less soluble forms. Also, [
30] found that amorphous FeS at room temperature transformed into mackinawite and greigite; however, this was found to occur on a timescale of days and months. Whether the process of aging is pH-dependent is not reported in these works, but the pH-values tested in the present study are within the same range as those of natural waters. The tested reaction times of 1.5 and 30 s were very different from those addressed by [
29] and would not induce an aging effect, where amorphous FeS transforms into its crystalline forms. This further supports that the precipitation of sulfide did not reach equilibrium at the reaction times tested.
The overall trend of sulfide conversion in the buffered water at different pH showed that higher conversions could be obtained at higher pH (
Figure 3). The highest conversions were around 60% at pH 7.5, and the sulfide conversion decreased almost linearly with decreasing pH and reached around 10% at pH 6.5. This trend in decreasing conversion level with decreasing pH is in line with findings of [
11], who in the range of pH 5–10.5 obtained sulfide conversions of 10% and 90%, respectively. At reaction times of 1.5 and 30 s, no significant differences in the sulfide conversion were observed for the samples. Even though the absolute level of sulfide conversion was much lower in the experiments compared to the model results, this tendency was still in line with the overall trend for conversion as predicted by Visual MINTEQ, where a higher sulfide conversion was obtained at higher pH values.
Nevertheless, using buffered water for the reaction is a simplification of the real wastewater system, where the actual precipitation must take place during abatement of sulfide. In the wastewater matrix, organic or inorganic ligands could also be of importance to the reaction, complexing with sulfide or iron and impeding the reaction.
3.2. Precipitation Stoichiometry versus Water Type
The impact of water type on the precipitation was studied for three wastewaters and the buffered water previously discussed (
Table 1). The pH was kept close to 7 and the reaction times were 1.5 and 30 s. MINTEQ simulations were done to estimate equilibrium conditions corresponding to an infinite reaction time, also at pH 7 (
Figure 4). The wastewaters had an average initial molar ratio of ferrous iron to sulfide of 2.54 ± 0.11 and 2.55 ± 0.26 mol Fe (mol·S)
−1 for reaction times of 1.5 and 30 s, respectively. The buffered water samples had initial molar ratios of 1.68 and 2.05 mol Fe (mol·S)
−1 for the two reaction times (
Table 2).
It is evident from
Figure 4 that the stoichiometric ratios and sulfide conversions of the three wastewaters depended on reaction time, with a longer reaction time resulting in a lower stoichiometric ratio and thereby a higher conversion. This trend was not observed for the buffered water, where no time-dependency could be documented (two-sided t-test, α = 5%). However, this lack of time-dependency might be caused by the slight dissimilarities in pH and initial molar ratios between the experimental runs. Nevertheless, the ratio in buffered water did not reach the values predicted by equilibrium simulations, indicating that the reaction might continue at a slow rate for longer time.
The stoichiometric ratios for wastewater at a reaction time of 1.5 s were 2.5–3 times higher than those for the buffered water. However, at a reaction time of 30 s, this difference disappeared and the ratios showed values in the same range as for the buffered water. Compared to the 1 mol Fe (mol·S)−1 theoretically needed according to Equation (1), and the 1.94 mol Fe (mol·S)−1 predicted by the equilibrium modeling, the obtained ratios at 1.5 and 30 s reaction times were 5–15 and 2.5–7.5 times higher, respectively.
The results for the three wastewaters showed comparable stoichiometric ratios at the two reaction times, despite the fact that wastewater is a heterogeneous medium and the variation in COD between the wastewaters was large (
Table 1). A one-way analysis of variance (ANOVA) test (α = 5%) revealed that the mean of the samples, including the buffered water, were not equal and a multiple comparison procedure using the Holm-Sidiak method showed that at 1.5 s the stoichiometric ratio of the buffered water and Svenstrup wastewater differed from the Aalborg and Frejlev wastewaters. However, at 30 s, the Aalborg wastewater differed as the only one from the three other samples.
Previous studies have reported the stoichiometric ratios for iron sulfide precipitation in wastewater to vary between a better than stoichiometric ratio and up to a ratio of 5.7 (e.g. [
3,
11,
15,
21]). This variation could be due to the fact that many studies are site- and wastewater-specific, as both pH, initial sulfide concentration, and other ligands for ferrous iron are known to influence the stoichiometric ratios [
3,
6,
11]. Furthermore [
3,
6] reported that the stoichiometric ratio depends on the initial sulfide concentration. A near stoichiometric ratio was achieved by [
3] at high initial sulfide concentrations, and it was observed that the ratio increased drastically at lower initial concentrations. The initial sulfide concentrations in the present study were in the high end of what is typical for septic wastewater [
2]; however, the stoichiometric ratios obtained in the present study were higher than what those studies led to expect. Also, compared to the ratios found by [
11], which used an almost equal initial sulfide concentration of around 0.3 mM, the obtained stoichiometric ratios were high. The above indicates that the reaction most likely had not run to completion within the 30 s of reaction time.
The amount of iron needed to precipitate sulfide in wastewater will exceed the stoichiometric amount of Equation (1) as stated by [
1,
11]. According to those studies, near stoichiometric ratios can be expected at pH around 8. For pH around 6.5, a ratio of around 4.6 mol Fe (mol·S)
−1 can be expected, and below this pH, addition of iron will only have little effect, thus the stoichiometric ratio will increase substantially. According to the above, the present experiments conducted at pH 7 should have had a stoichiometric ratio somewhat better than 4.6 mol Fe (mol·S)
−1. They were, however, on average 13.8 ± 1.43 for reaction times of 1.5 s and of 5.3 ± 0.82 for reaction times of 30 s, indicating that reaction times on this timescale are important for the achieved stoichiometric ratio.
The difference in stoichiometric ratios observed for wastewater samples (
Figure 4) at the two reaction times might be ascribed to the fact that ferrous iron initially reacted with other inorganic or organic constituents in the wastewater before it subsequently precipitated dissolved sulfides as previously discussed by [
11,
12]. This implies that the reaction between ferrous iron and sulfide was inhibited, and thus the stoichiometric ratio observed at low reaction times became higher than that for longer reaction times. However, the stoichiometric ratios observed for buffered water showed time-independent behavior, and thus the carbonate and phosphate content by themselves did not seem to influence the precipitation reaction to any significant extent, and thus explained the difference observed for wastewater at the two reaction times. It hence seems reasonable to assume that the retardation in precipitation was caused by organic wastewater constituents.
3.3. Conversion of Sulfide in Different Water Types
The mean of conversions (
Figure 4) was tested statistically using a one-way ANOVA (α = 5%) and a subsequent multiple comparison with the Holm-Sidiak method to find statistical differences. This showed that there was no difference in conversion between the three wastewaters, and that they all differed significantly from the conversion mean of the buffered water at both 1.5 and 30 s of reaction time. The conversion at 1.5 s was found to be greater in buffered water compared to the wastewater samples, and reversed at 30 s, where conversion was greater in the wastewaters. This might again be due to the interaction between iron and organic matter in the samples, and thus ultimately the differences in matrices between buffered water and wastewater.
The sulfide conversion observed in this study differed considerably from previously reported numbers where [
31] in real wastewater installations in Florida at a stoichiometric ratio of 1.43–2.86 mol Fe (mol·S)
−1 attained a conversion of more than 80%. For a trunk sewer in California, [
32] showed that a 95% reduction of initial sulfide levels could be attained with a stoichiometric ratio of around 1.4 mol Fe (mol·S)
−1 when adding a mix of ferrous and ferric iron. The pH range of the precipitation in these full-scale installations was not reported. However, under laboratory conditions in a setup using wastewater at pH around 8, [
11] showed an 80% conversion of sulfide. But when increasing the ratio from 0.8 to 1.3 mol Fe (mol·S)
−1, thus adding iron in excess, they only experienced a 90% conversion of sulfide using wastewater. They moreover observed that when lowering pH below 7, conversion in some cases decreased and even attained values below 40%. This trend of decreasing conversion level is in line with both what was observed in this study and predicted theoretically by Visual MINTEQ (
Figure 3).
Overall, the differences in sulfide conversion in wastewater between the two reaction times, as well as the differences found comparing to literature values and theoretical equilibrium conversions, most likely was caused by the reaction not having reached completion within the maximum reaction time tested.
3.4. Influence of Organic Matter
The organic content of the wastewaters seemed to influence the precipitation reaction. This influence was most pronounced at a reaction time of 1.5 s where a clear difference in stoichiometric ratios as well as conversions was observed (
Figure 4). Between the two different criteria evaluated for the precipitation reaction (stoichiometric ratio and conversion), a consistent picture of a certain type of wastewater differing statistically significant from the others, either due to wastewater age or a specific reaction time, could not be established. This indicates that gross wastewater characteristics such as COD or age were poor indicators for the precipitation reaction.
The observed differences in stoichiometry between the three wastewaters must hence have been caused by specific organic and inorganic substances of the waters. This hypothesis is supported by, for example, [
33], who showed that ferrous and ferric iron in aqueous solution at neutral pH and under oxic conditions interacted with organic matter and that both iron species were held in solution in concentrations in excess of what would be theoretically expected. Furthermore, [
34] discovered that under reduced conditions, ferrous iron in the form of ferrous hydroxide can interact with organic matter, resulting in co-precipitation of the species. They showed that it is primarily the proteinaceous fraction of the organic matter which participates in these interactions. Those findings substantiate the conclusion of the present study that a gross parameter like COD is a poor indicator for the precipitation reaction in itself, and that a more detailed analysis of both organic and inorganic substances, as well as their interrelations, is needed to allow the prediction of stoichiometric requirements and reactions rates. Which specific substances are of importance and how their importance and interrelations depend on conditions like pH and redox, is still an unsolved issue.
The finding of this study implies that practitioners should take reaction time into account when managing H2S issues in sewer networks and at wastewater treatment plants. Depending on the actual wastewater characteristics, several minutes of reaction time might be needed to achieve optimal precipitation. Such reaction times can though be difficult to achieve in practice. The issue of sufficient reaction time must hence be considered when choosing the best suited H2S management strategies.