Design, Construction, and Application of an Inexpensive, High-Resolution Water Sampler
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of the Water Sampler
2.2. Specifications and Assembly of the Pump Apparatus
2.3. Water Sample Collection and Analyses
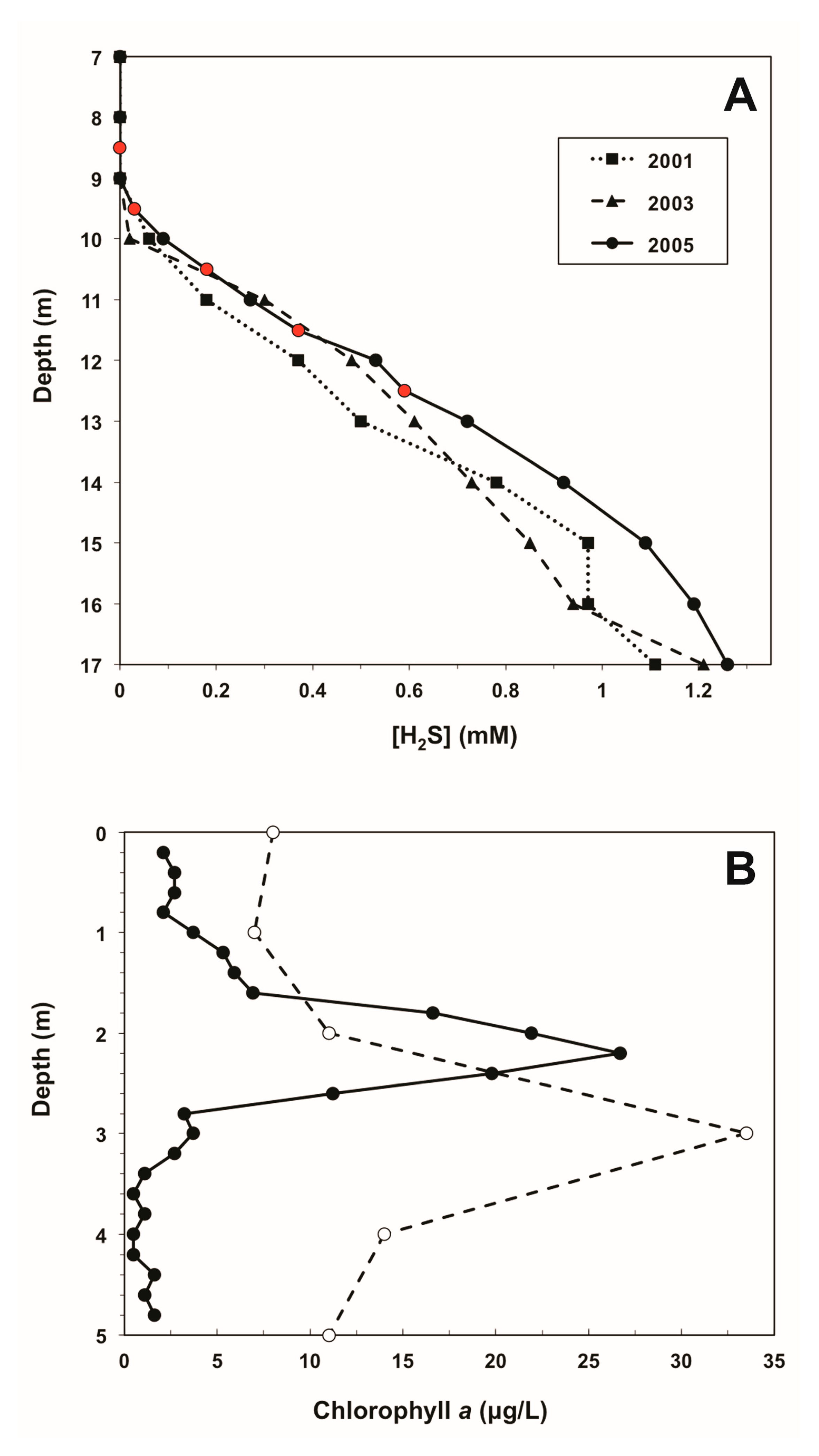
3. Results and Discussion
3.1. Performance of the Water Sampler
3.2. Field Results Verification: Chlorophyll a and Sulfide Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biological Examination (part B, Sample Collection). Standard Methods for the Examination of Water and Wastewater, 20th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; Copyright 1999 by American Public Health Association, American Water Works Association, Water Environment Federation; United Book Press, Inc.: Baltimore, MD, USA, 1998; pp. 10-2–10-9. ISBN 0-87553-235-7. [Google Scholar]
- Karr, E.A.; Ng, J.M.; Belchik, S.M.; Sattley, W.M.; Madigan, M.T.; Achenbach, L.A. Biodiversity of methanogenic and other Archaea in the permanently frozen Lake Fryxell, Antarctica. Appl. Environ. Microbiol. 2006, 72, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Karr, E.A.; Sattley, W.M.; Jung, D.O.; Madigan, M.T.; Achenbach, L.A. Remarkable diversity of phototrophic purple bacteria in a permanently frozen Antarctic lake. Appl. Environ. Microbiol. 2003, 69, 4910–4914. [Google Scholar] [CrossRef] [PubMed]
- Karr, E.A.; Sattley, W.M.; Rice, M.R.; Jung, D.O.; Madigan, M.T.; Achenbach, L.A. Diversity and distribution of sulfate-reducing bacteria in permanently frozen Lake Fryxell, McMurdo Dry Valleys, Antarctica. Appl. Environ. Microbiol. 2005, 71, 6353–6359. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Mikucki, J.A.; Foreman, C.M.; Priscu, J.C.; DiTullio, G.R.; Riseman, S.F.; de Mora, S.J.; Wolf, C.F.; Kester, L. Thermodynamic constraints on microbially mediated processes in lakes of the McMurdo Dry Valleys, Antarctica. Geomicrobiol. J. 2004, 21, 221–237. [Google Scholar] [CrossRef]
- Sattley, W.M. Microbiology of Sulfur Cycling and Other Biogeochemical Processes in Dry Valleys Lakes of Antarctica. Ph.D. Dissertation, Southern Illinois University, Carbondale, IL, USA, 2006. [Google Scholar]
- Sattley, W.M.; Madigan, M.T. Isolation, characterization, and ecology of cold-active, chemolithotrophic, sulfur-oxidizing bacteria from perennially ice-covered Lake Fryxell, Antarctica. Appl. Environ. Microbiol. 2006, 72, 5562–5568. [Google Scholar] [CrossRef] [PubMed]
- Sattley, W.M.; Madigan, M.T. Temperature and nutrient induced responses of Lake Fryxell sulfate-reducing prokaryotes and description of Desulfovibrio lacusfryxellense, sp. nov., a pervasive, cold-active, sulfate-reducing bacterium from Lake Fryxell, Antarctica. Extremophiles 2010, 14, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Takacs, C.D.; Priscu, J.C.; McKnight, D.M. Bacterial dissolved organic carbon demand in McMurdo Dry Valley lakes, Antarctica. Limnol. Oceanogr. 2001, 46, 1189–1194. [Google Scholar] [CrossRef]
- Trüper, H.G.; Schlegel, H.G. Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Van Leeuwenhoek 1964, 30, 225–238. [Google Scholar] [CrossRef]
- Biological Examination (part H, Chlorophyll). Standard Methods for the Examination of Water and Wastewater, 20th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; Copyright 1999 by American Public Health Association, American Water Works Association, Water Environment Federation; United Book Press, Inc.: Baltimore, MD, USA, 1998; pp. 10-18–10-20. ISBN 0-87553-235-7. [Google Scholar]
- Roberts, E.C.; Laybourn-Parry, J.; McKnight, D.M.; Novarinos, G. Stratification and dynamics of microbial loop communities in Lake Fryxell, Antarctica. Freshw. Biol. 2000, 44, 649–661. [Google Scholar] [CrossRef]
- Vincent, W.F. Production strategies in Antarctic inland waters: Phytoplankton eco-physiology in a permanently ice-covered lake. Ecology 1981, 62, 1215–1224. [Google Scholar] [CrossRef]
- Lovell, C.R.; Konopka, A. primary and bacterial production in two dimictic Indiana lakes. Appl. Environ. Microbiol. 1985, 49, 485–491. [Google Scholar] [PubMed]
- Konopka, A. Metalimnetic cyanobacteria in hard-water lakes: Buoyancy regulation and physiological state. Limnol. Oceanogr. 1989, 34, 1174–1184. [Google Scholar] [CrossRef]
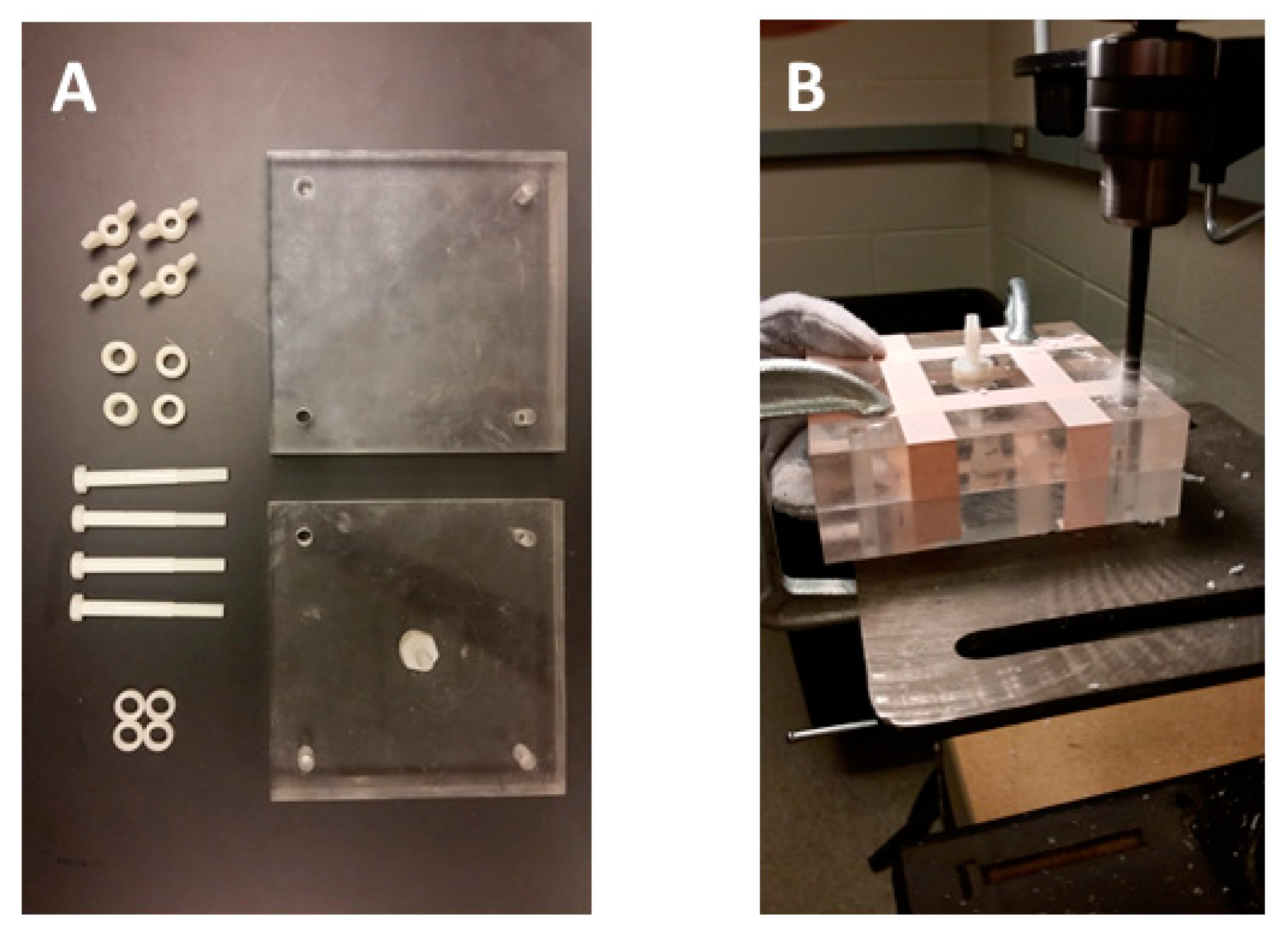
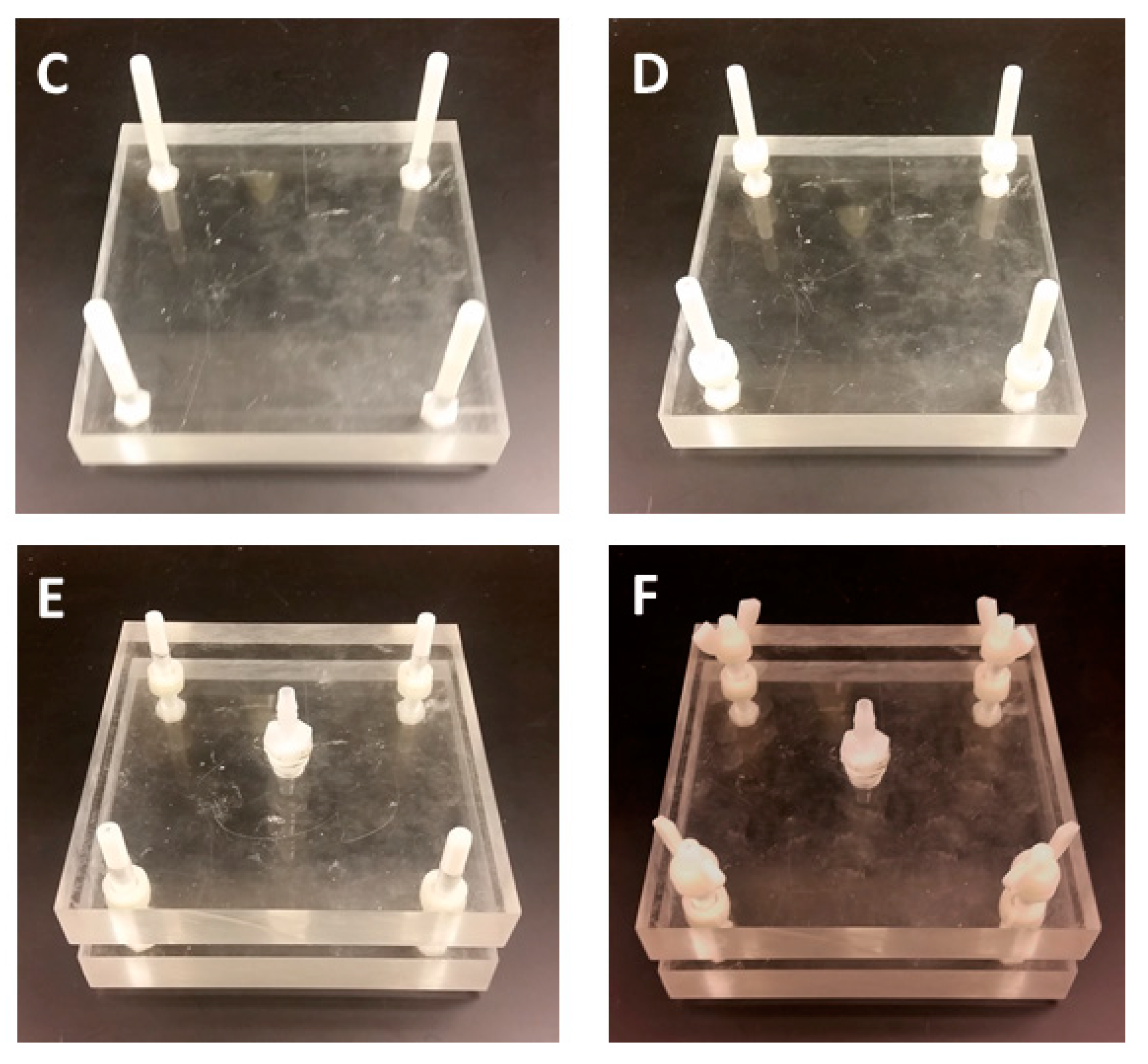

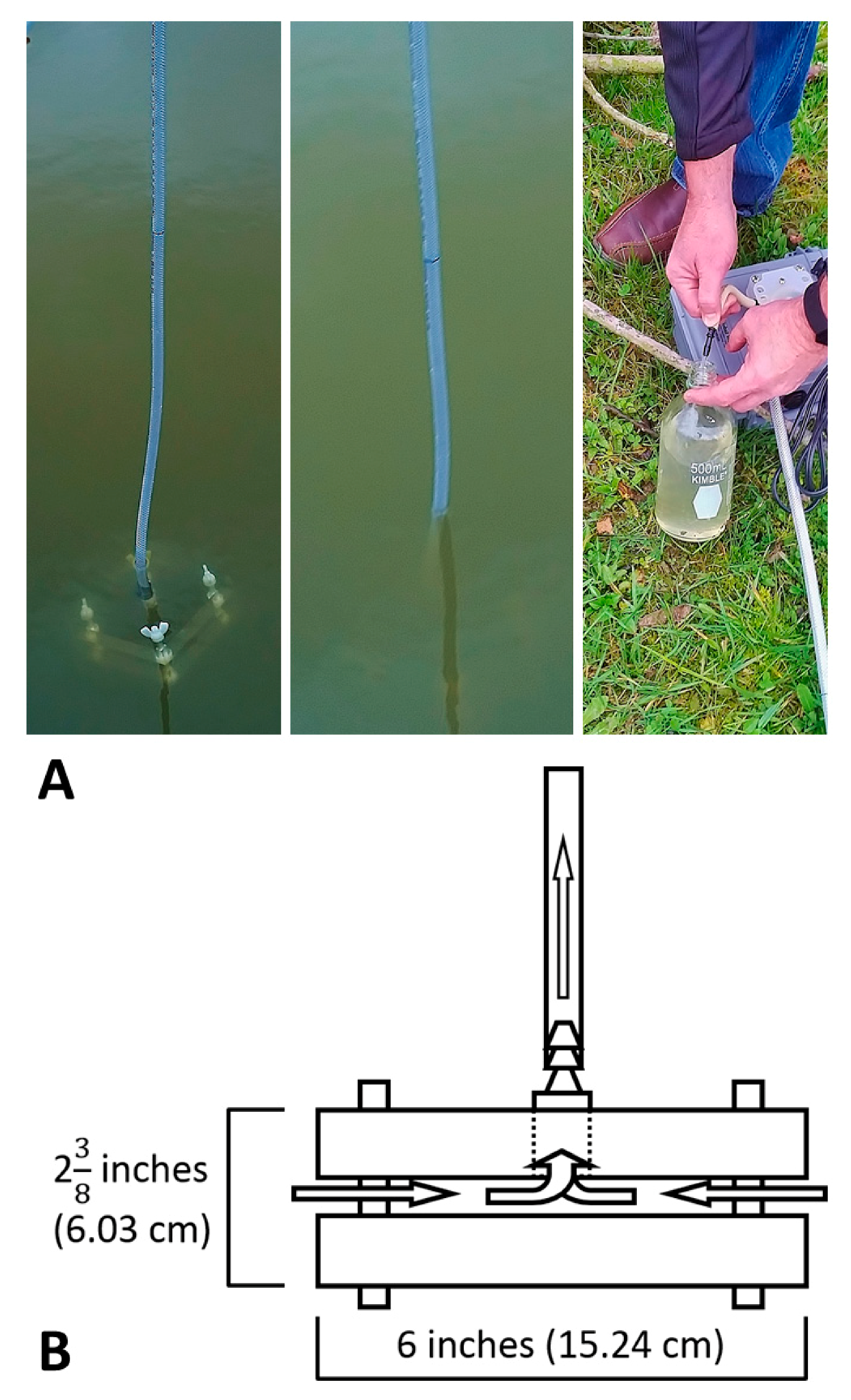
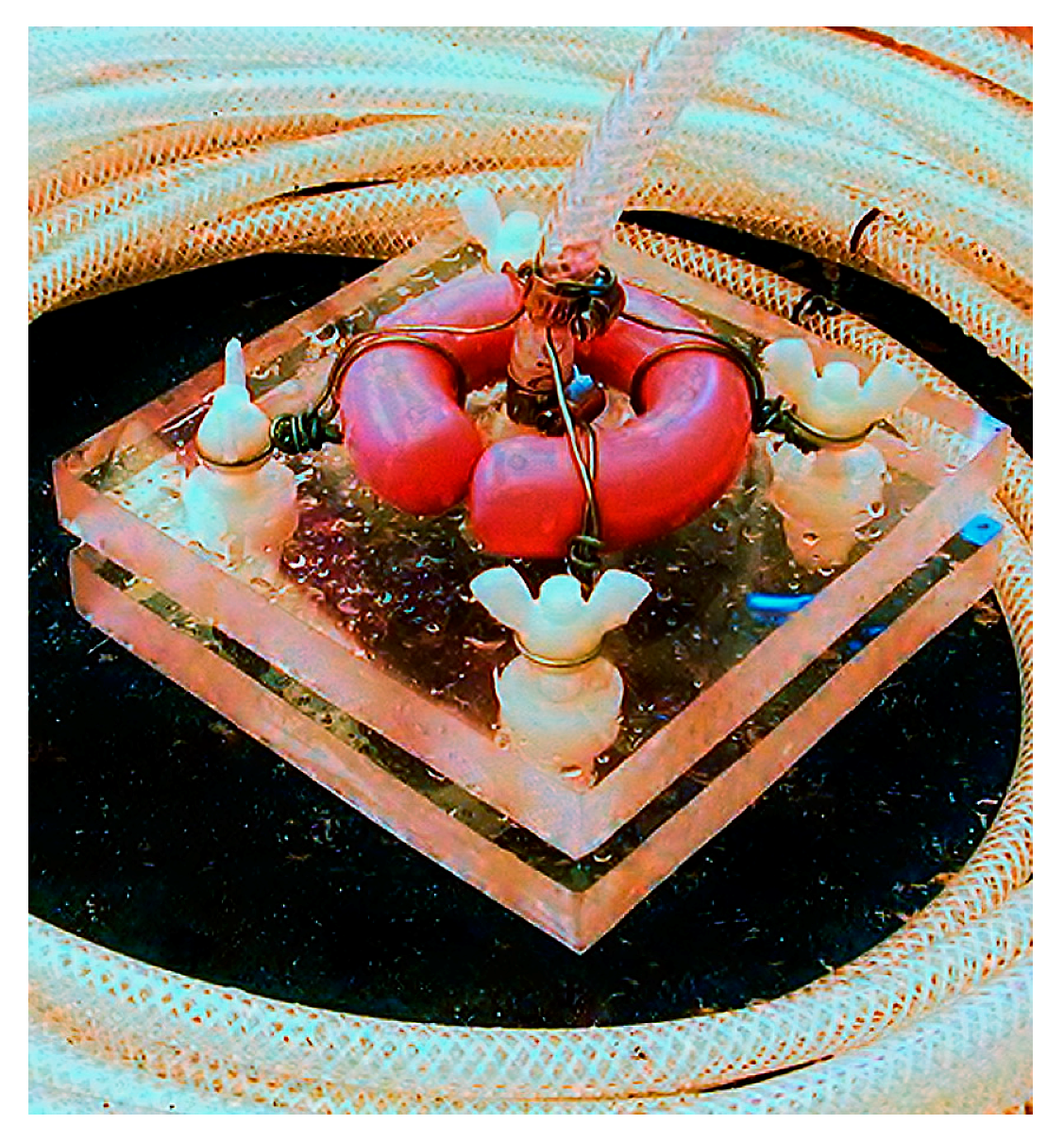
| Item | Quantity | Pricing (USD) |
|---|---|---|
| Acrylic squares—approximately 15 cm × 15 cm × 2.5 cm | 2 | $80 |
| Peristaltic pump with tubing (Global Water SP200) * | 1 | $325 |
| 12V lawnmower battery with charger | 1 | $80 |
| Nylon washers—5/16-inch I.D. (inner diameter) × 1/8-inch thickness | 8 | $3 |
| Nylon hex bolts—5/16-inch T.D. (thread diameter) | 4 | $5 |
| Nylon wing nuts—5/16-inch T.D. | 4 | $6 |
| Nylon fitting (3/8-inch male pipe thread × 5/16-inch hose base) | 1 | $1 |
| Total Cost | $500 | |
| Parameter | Measurement |
|---|---|
| Total length of tubing | 4.9 m |
| Inner diameter of tubing | 6.4 mm |
| Total volume of tubing and pump | 160 mL |
| Time to first drop in collection vessel | 19 s |
| Time required to fill 500-mL collection vessel | 60 s |
| Resolution of sampling device (space between the two acrylic squares) | 10 mm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattley, W.M.; Burchell, B.M.; Conrad, S.D.; Madigan, M.T. Design, Construction, and Application of an Inexpensive, High-Resolution Water Sampler. Water 2017, 9, 578. https://doi.org/10.3390/w9080578
Sattley WM, Burchell BM, Conrad SD, Madigan MT. Design, Construction, and Application of an Inexpensive, High-Resolution Water Sampler. Water. 2017; 9(8):578. https://doi.org/10.3390/w9080578
Chicago/Turabian StyleSattley, W. Matthew, Brad M. Burchell, Stephen D. Conrad, and Michael T. Madigan. 2017. "Design, Construction, and Application of an Inexpensive, High-Resolution Water Sampler" Water 9, no. 8: 578. https://doi.org/10.3390/w9080578




