Effects of the Food-to-Microorganism (F/M) Ratio on N2O Emissions in Aerobic Granular Sludge Sequencing Batch Airlift Reactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aerobic Granular Sludge Bioreactor Setup and Operation
2.2. DNA Extraction and qPCR
2.3. EPS Extraction and Chemical Analysis
2.4. Analytical and Statistic Analysis Methods
3. Results
3.1. Characterization of Aerobic Granular Sludge under Different F/M Ratios
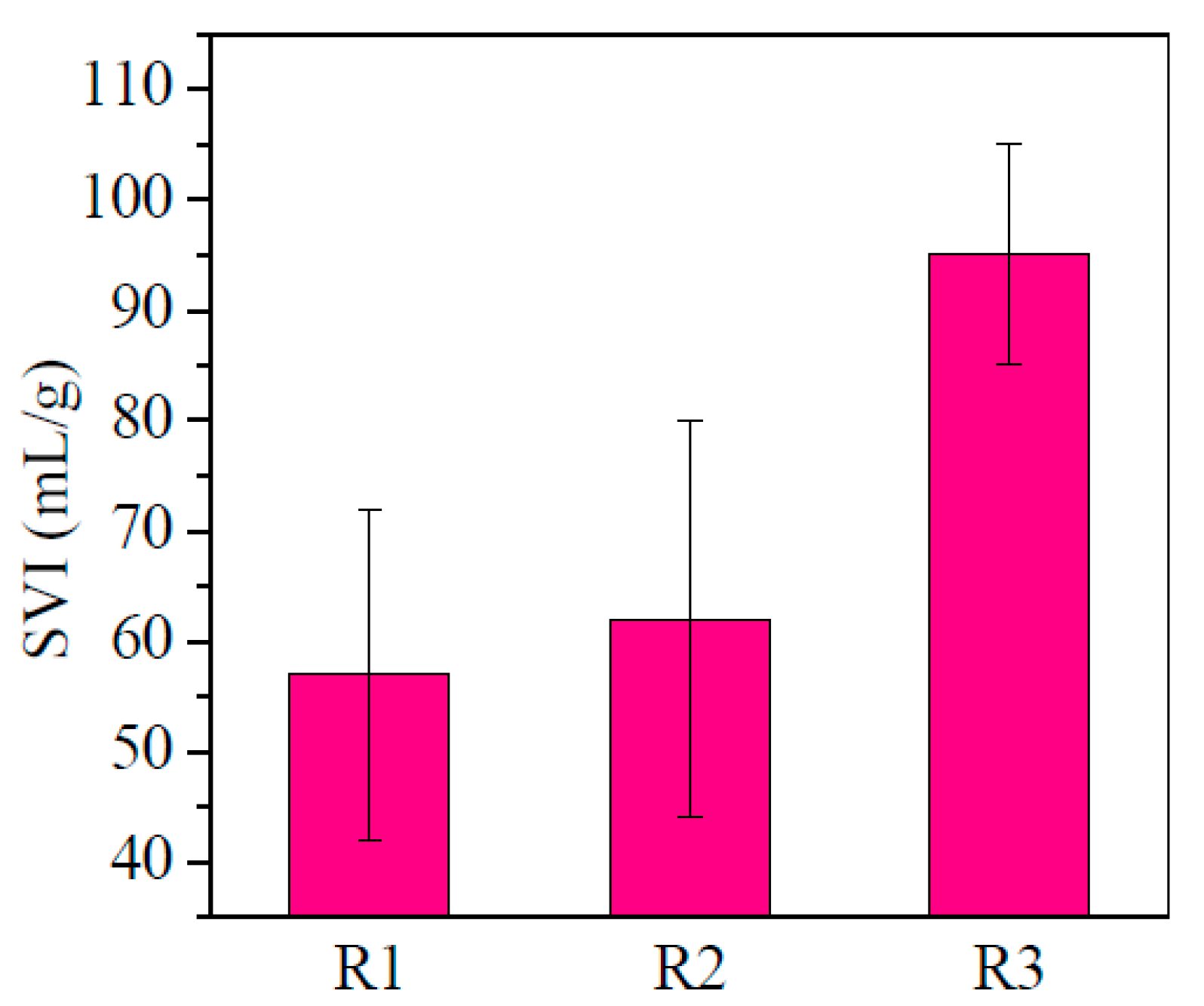
3.1.1. The Settling Ability
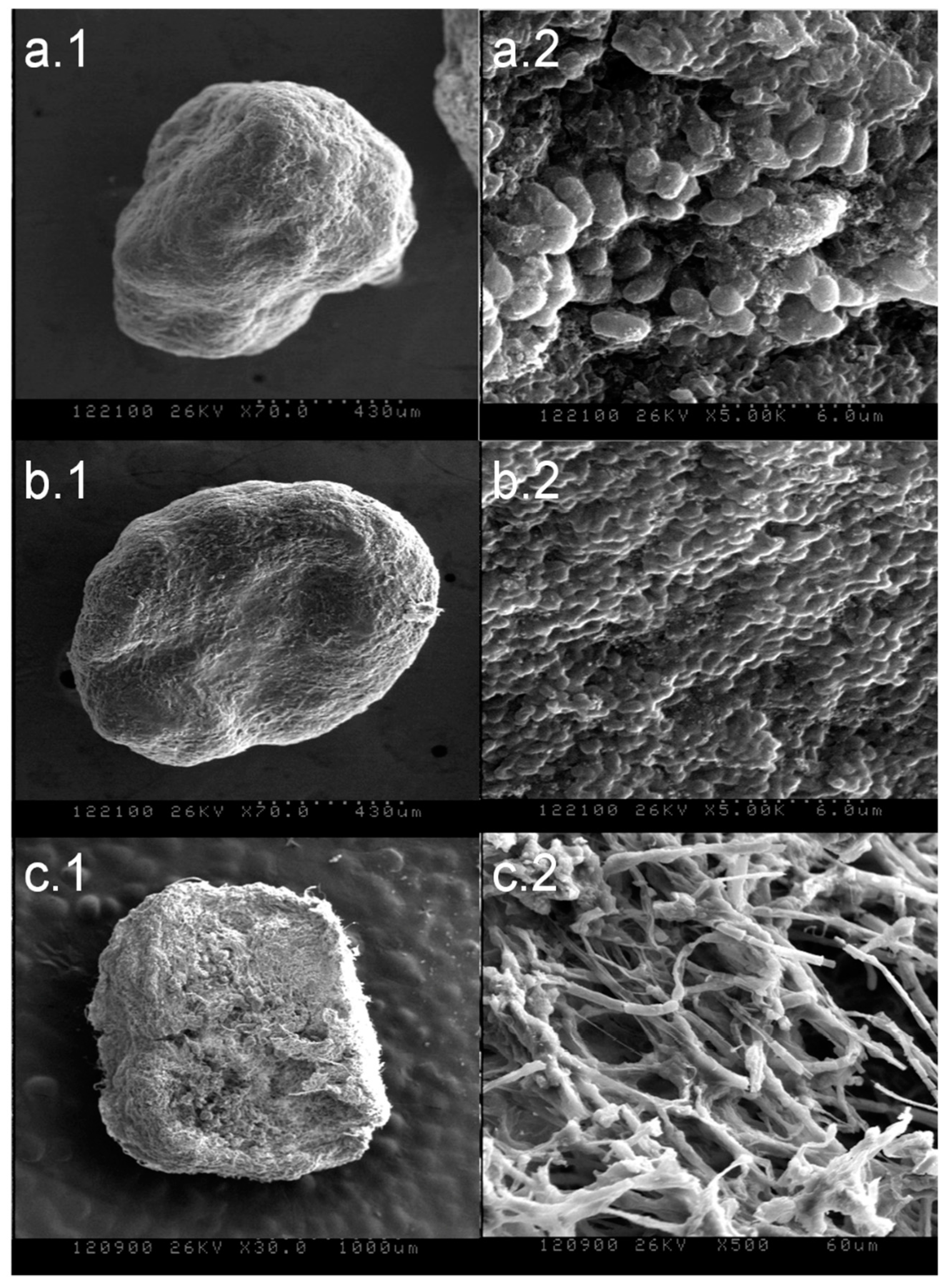
3.1.2. SEM Analysis
3.1.3. The Characteristics of EPS
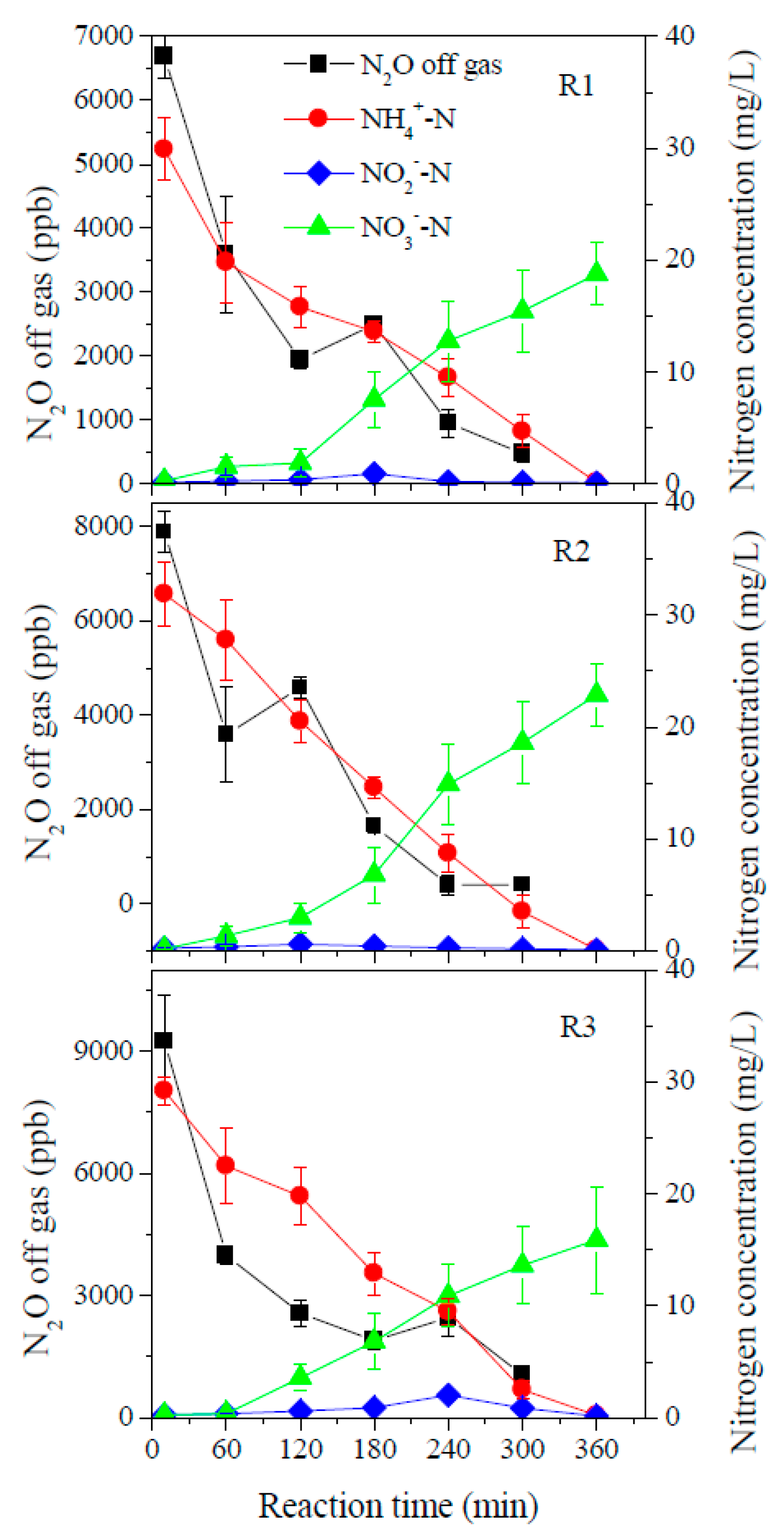
3.2. Performance of Aerobic Granular Sludge SBARs under Different F/M Ratios
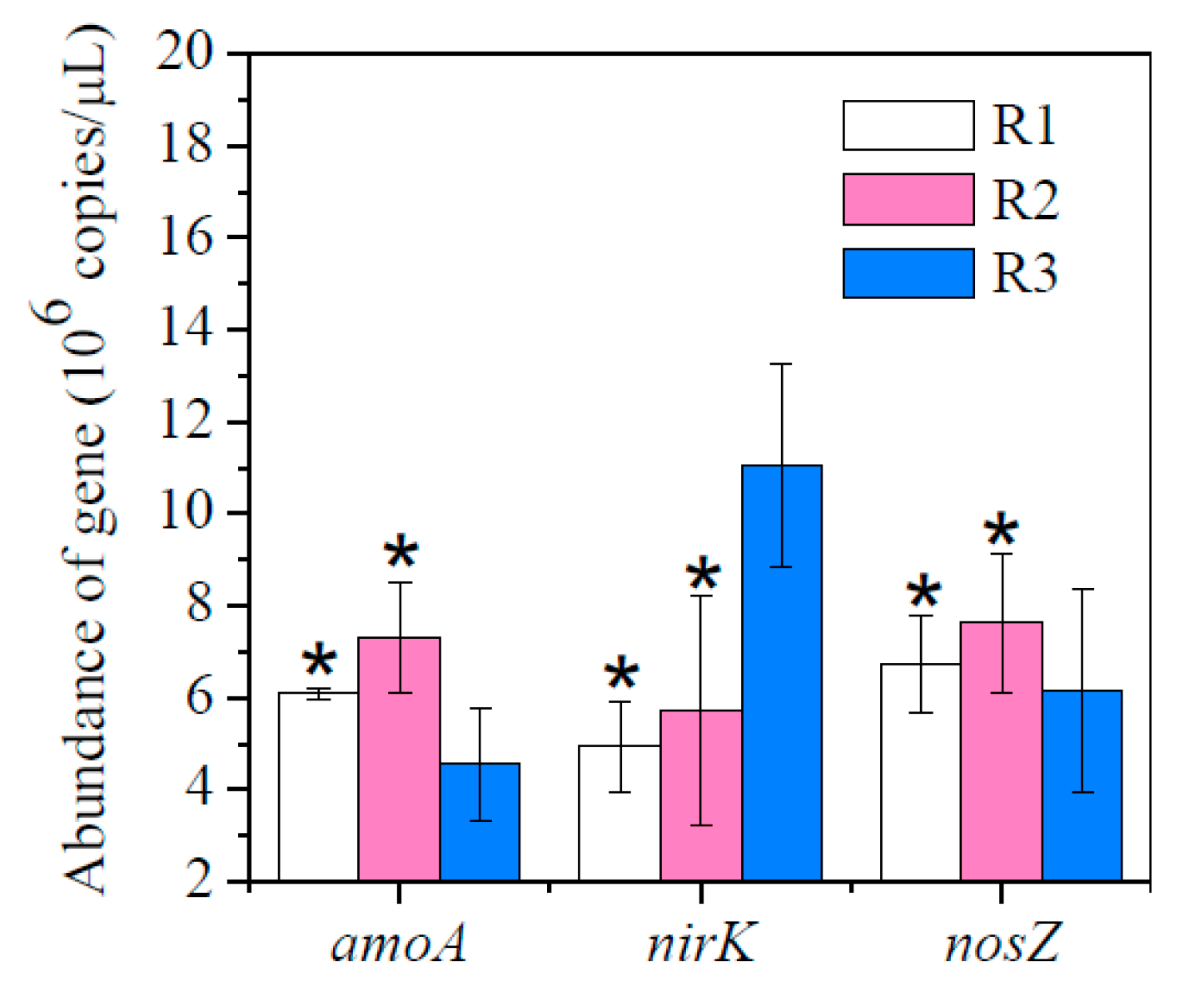
3.3. The Abundance of Functional Genes under Different F/M Ratios
4. Discussion
4.1. Characterization of Aerobic Granular Sludge
4.1.1. The Stability of Aerobic Granular Sludge
4.1.2. The Characteristics of EPS
4.2. N2O Emission of Aerobic Granular Sludge
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, Y.-J.; Wang, X.-H.; Yu, H.-B.; Xie, H.-J.; Teng, S.-X.; Sun, X.-F.; Tian, B.-H.; Wang, S.-G. Aerobic granulation for nitrogen removal via nitrite in a sequencing batch reactor and the emission of nitrous oxide. Bioresour. Technol. 2011, 102, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, D.P.; Belia, E. Nitrogen and phosphorus removal from an abattoir wastewater in a SBR with aerobic granular sludge. Water Res. 2005, 39, 4817–4823. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D.-J.; Show, K.-Y.; Tay, J.-H. Aerobic granular sludge: Recent advances. Biotechnol. Adv. 2008, 26, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; de Haas, D.; Yuan, Z.; Lant, P. Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res. 2010, 44, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Sabba, F.; Picioreanu, C.; Perez, J.; Nerenberg, R. Hydroxylamine diffusion can enhance N2O emissions in nitrifying biofilms: A modeling study. Environ. Sci. Technol. 2015, 49, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Trussell, R.S.; Merlo, R.P.; Hermanowicz, S.W.; Jenkins, D. The effect of organic loading on process performance and membrane fouling in a submerged membrane bioreactor treating municipal wastewater. Water Res. 2006, 40, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, G. Cross effects of organic loadings rates and substrate COD/N/P ratios on aerobic granulation. In Proceedings of the 4th International Conference on Bio-informaticsand Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar]
- Tay, J.H.; Yan, Y.-G. Influence of substrate concentration on microbial selection and granulation during start-up of upflow anaerobic sludge blanket reactor. Water Environ. Res. 1996, 68, 1140–1150. [Google Scholar] [CrossRef]
- Moy, B.Y.; Tay, J.-H.; Toh, S.-K.; Liu, Y.; Tay, S.T. High organic loading influences the physical characteristics of aerobic sludge granules. Lett. Appl. Microbiol. 2002, 34, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D.J.; Lai, J.Y. Potential cause of aerobic granular sludge breakdown at high organic loading rates. Appl. Microbiol. Biotechnol. 2010, 85, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.L.; Zeng, R.J.; Giugliano, V.; Blackall, L.L. Challenges for simultaneous nitrification, denitrification, and phosphorus removal in microbial aggregates: Mass transfer limitation and nitrous oxide production. FEMS Microbiol. Ecol. 2005, 52, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.L.; Liang, S.; Zhang, J.; Ngo, H.H.; Guo, W.S.; Yan, Y.J.; Zou, Y.N. Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: Sources and mechanisms. Bioresour. Technol. 2013, 136, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, M.; Tora, J.; Rodriguez-Caballero, A.; Cesar, E.; Carrera, J.; Perez, J. Effect of process parameters and operational mode on nitrous oxide emissions from a nitritation reactor treating reject wastewater. Water Res. 2014, 49, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Zhang, J.; Ngo, H.H.; Ni, S.Q.; Fu, R.S.; Guo, W.S.; Guo, N.; Tian, L. Nitrous oxide emission in an aerobic granulation sequencing batch airlift reactor at ambient temperatures. Int. Biodeterior. Biodegrad. 2013, 85, 533–538. [Google Scholar] [CrossRef]
- Guo, N.; Wang, Y.; Yan, L.; Wang, X.; Wang, M.; Xu, H.; Wang, S. Effect of bio-electrochemical system on the fate and proliferation of chloramphenicol resistance genes during the treatment of chloramphenicol wastewater. Water Res. 2017, 117, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ligi, T.; Truu, M.; Truu, J.; Nõlvak, H.; Kaasik, A.; Mitsch, W.J.; Mander, Ü. Effects of soil chemical characteristics and water regime on denitrification genes (nirS, nirK, and nosZ) abundances in a created riverine wetland complex. Ecol. Eng. 2014, 72, 47–55. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Walter, W.G. Standard methods for the examination of water and wastewater (11th ed.). Am. J. Public Health Nations Health 1961, 51, 940. [Google Scholar] [CrossRef]
- Diao, M.; Li, X.Y.; Gu, J.D.; Shi, H.C.; Xie, Z.M. Electron microscopic investigation of the bactericidal action of electrochemical disinfection in comparison with chlorination, ozonation and Fenton reaction. Process. Biochem. 2004, 39, 1421–1426. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Xie, H.; Li, S.; Zhang, T.; Wang, J. Identifying sources of nitrous oxide emission in anoxic/aerobic sequencing batch reactors (A/O SBRs) acclimated in different aeration rates. Enzyme Microb. Technol. 2011, 49, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D.J.; Tay, J.H. Extracellular polymeric substances and structural stability of aerobic granule. Water Res. 2008, 42, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.B.; De Kreuk, M.K.; Picioreanu, C.; Van Loosdrecht, M.C.M. Multi-scale individual-based model of microbial and bioconversion dynamics in aerobic granular sludge. Environ. Sci. Technol. 2007, 41, 6410–6417. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Shirotani, K.; Yoshie, S.; Tsuneda, S. Effects of carbon source on denitrification efficiency and microbial community structure in a saline wastewater treatment process. Water Res. 2008, 42, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.-S. Causes and control of filamentous growth in aerobic granular sludge sequencing batch reactors. Biotechnol. Adv. 2006, 24, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.P.; Heijnen, J.J.; van Loosdrecht, M.C.M. Effect of dissolved oxygen concentration on sludge settleability. Appl. Microbiol. Biotechnol. 2003, 62, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Liu, Q.S.; Liu, Y. The role of cellular polysaccharides in the formation and stability of aerobic granules. Lett. Appl. Microbiol. 2001, 33, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Al-Halbouni, D.; Traber, J.; Lyko, S.; Wintgens, T.; Melin, T.; Tacke, D.; Janot, A.; Dott, W.; Hollender, J. Correlation of EPS content in activated sludge at different sludge retention times with membrane fouling phenomena. Water Res. 2008, 42, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Colliver, B.B.; Stephenson, T. Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol. Adv. 2000, 18, 219–232. [Google Scholar] [CrossRef]
- Martin, G.K.; Lisa, Y.S. Nitrifier genomics and evolution of the nitrogen cycle. FEMS Microbio. Lett. 2008, 278, 146–156. [Google Scholar]
- Meng, Q.; Yang, F.; Liu, L.; Meng, F. Effects of COD/N ratio and DO concentration on simultaneous nitrifcation and denitrifcation in an airlift internal circulation membrane bioreactor. J. Environ. Sci. 2008, 20, 933–939. [Google Scholar] [CrossRef]
- Pai, T.-Y.; Wan, T.-J.; Tsai, Y.-P.; Tzeng, C.-J.; Chu, H.-H.; Tsai, Y.-S.; Lin, C.-Y. Effect of Sludge Retention Time on Nitrifiers’ Biomass and Kinetics in an Anaerobic/Oxic Process. CLEAN Soil Air Water 2010, 38, 167–172. [Google Scholar] [CrossRef]

| Target Primers | Primers | Primer Sequence | Annealing (°C) |
|---|---|---|---|
| 16S | F338/R518 | ACTCCTACGGGAGGCAGCAGATTACCGCGGCTGCTGG | 55 |
| amoA | amoA-1F/amoA-2R | GGGGTTTCTACTGGTGGTCCCCTCKGSAAAGCCTTCTTC | 53 |
| nirK | FlaCu/R3Cu | ATCATGGTSCTGCCGCGGCCTCGATCAGRTTGTGGTT | 55 |
| nosZ | nosZ-F/nosZ-1622R | CGYTGTTCMTCGACAGCCAGCGSACCTTSTTGCCSTYGCG | 56 |
| EPS Compostions (mg/g MLSS) | R1 | R2 | R3 |
|---|---|---|---|
| Protein content | 3.86 ± 0.23 * | 5.23 ± 0.28 * | 7.81 ± 0.97 |
| Humic acid content | 1.57 ± 0.16 * | 1.55 ± 0.19 * | 4.27 ± 0.62 |
| Polysaccharide content | 9.21 ± 0.33 * | 11.83 ± 0.46 * | 8.27 ± 0.48 |
| EPS content | 14.64 ± 0.74 * | 18.6 ± 0.96 * | 20.34 ± 1.24 |
| Removal Efficiency and N2O Emission | R1 | R2 | R3 |
|---|---|---|---|
| N2O emission (mg/d); N2O-N conversion (%) | 3.32 ± 0.86 *; 2.77 ± 0.56 * | 3.64 ± 0.38 *; 2.6 ± 0.69 * | 4.4 ± 0.94; 3.79 ± 0.48 |
| NH4+-N removal (%) | 95.20 ± 3.13 | 96.30 ± 2.98 | 99.30 ± 2.79 |
| COD removal (%) | 93.50 ± 3.4 | 94.5 ± 4.5 | 90.3 ± 6.91 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, N.; Zhang, J.; Xie, H.-J.; Tan, L.-R.; Luo, J.-N.; Tao, Z.-Y.; Wang, S.-G. Effects of the Food-to-Microorganism (F/M) Ratio on N2O Emissions in Aerobic Granular Sludge Sequencing Batch Airlift Reactors. Water 2017, 9, 477. https://doi.org/10.3390/w9070477
Guo N, Zhang J, Xie H-J, Tan L-R, Luo J-N, Tao Z-Y, Wang S-G. Effects of the Food-to-Microorganism (F/M) Ratio on N2O Emissions in Aerobic Granular Sludge Sequencing Batch Airlift Reactors. Water. 2017; 9(7):477. https://doi.org/10.3390/w9070477
Chicago/Turabian StyleGuo, Ning, Jian Zhang, Hui-Jun Xie, Lin-Rui Tan, Jie-Ning Luo, Ze-Yu Tao, and Shu-Guang Wang. 2017. "Effects of the Food-to-Microorganism (F/M) Ratio on N2O Emissions in Aerobic Granular Sludge Sequencing Batch Airlift Reactors" Water 9, no. 7: 477. https://doi.org/10.3390/w9070477




