Combined Impact of Acute Exposure to Ammonia and Temperature Stress on the Freshwater Mussel Unio pictorum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Mussel Exposure
2.3. Mussel Behavior Assessment
2.3.1. Visual Observation on Filtering Activity
2.3.2. Clearance Rate
2.3.3. Filtering Activity Based on Clearance Rate
2.4. Hemolymph Collection and Hemocyte Assessment
2.5. Glycogen Quantification
2.6. Data Analyses
3. Results
3.1. Mortality
3.2. Overall Results—Multivariate GLM
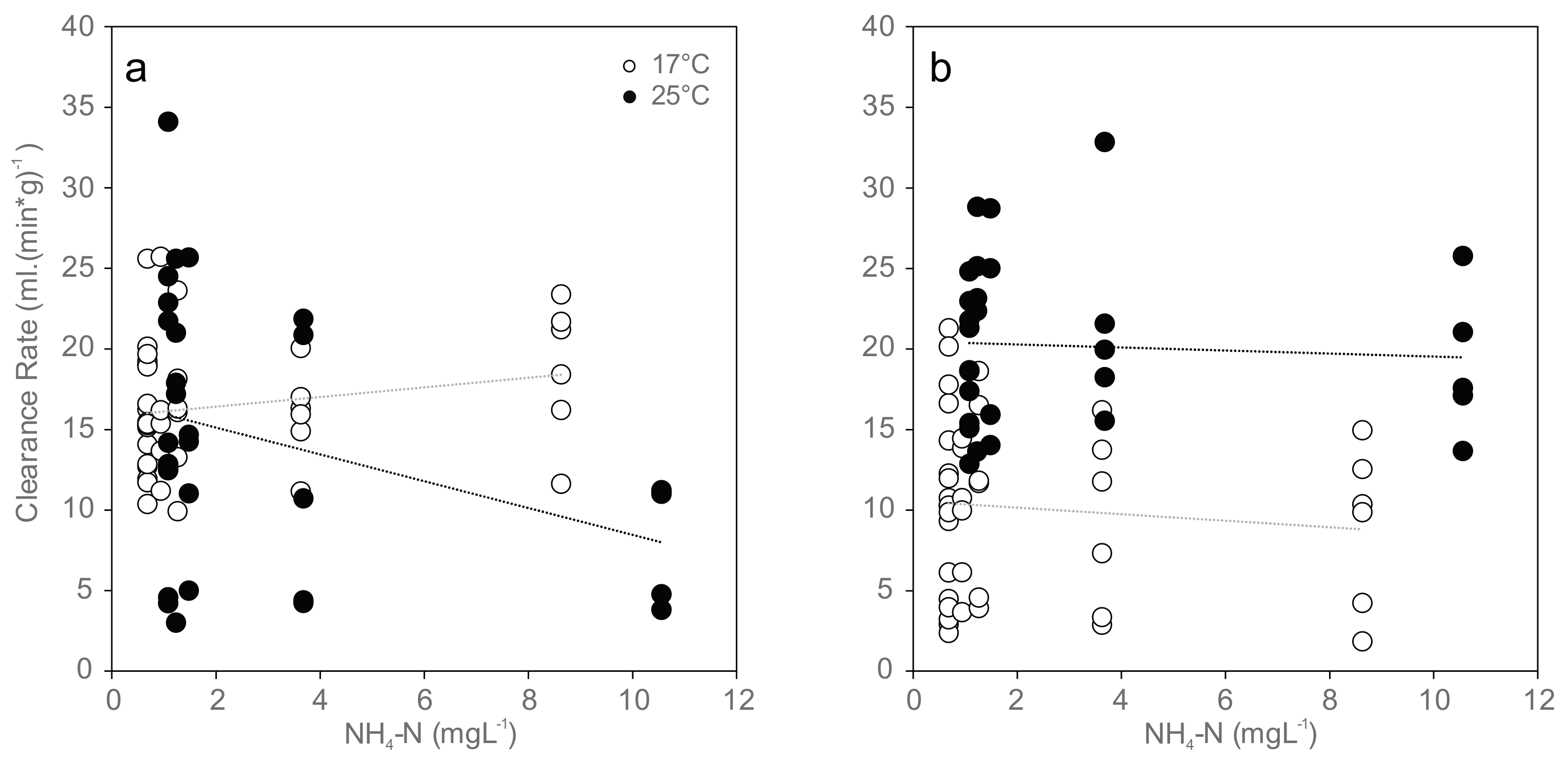
3.3. Clearance Rate and Filtration Activity
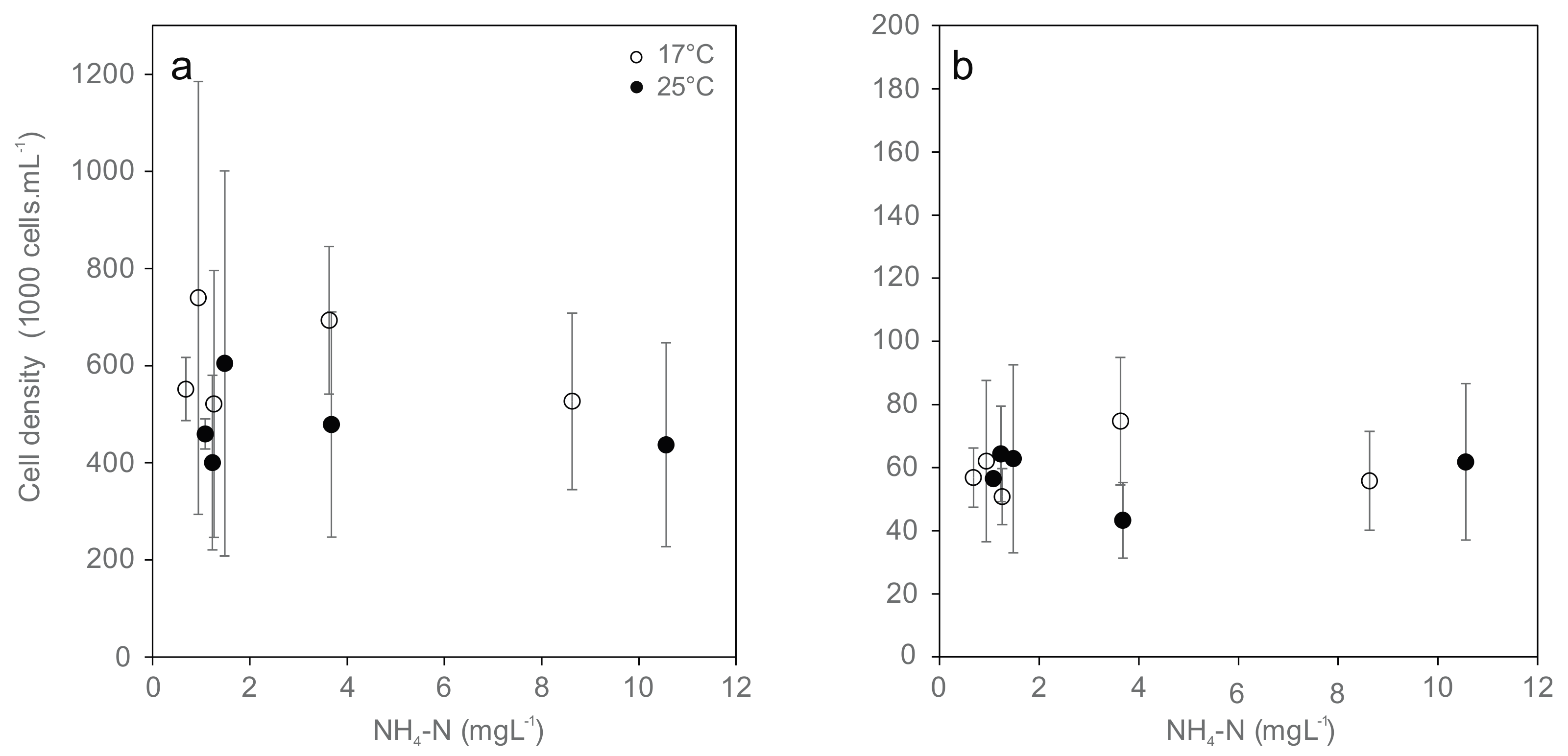
3.4. Hemocyte Density and Viability
3.5. Hemocyte Morphology
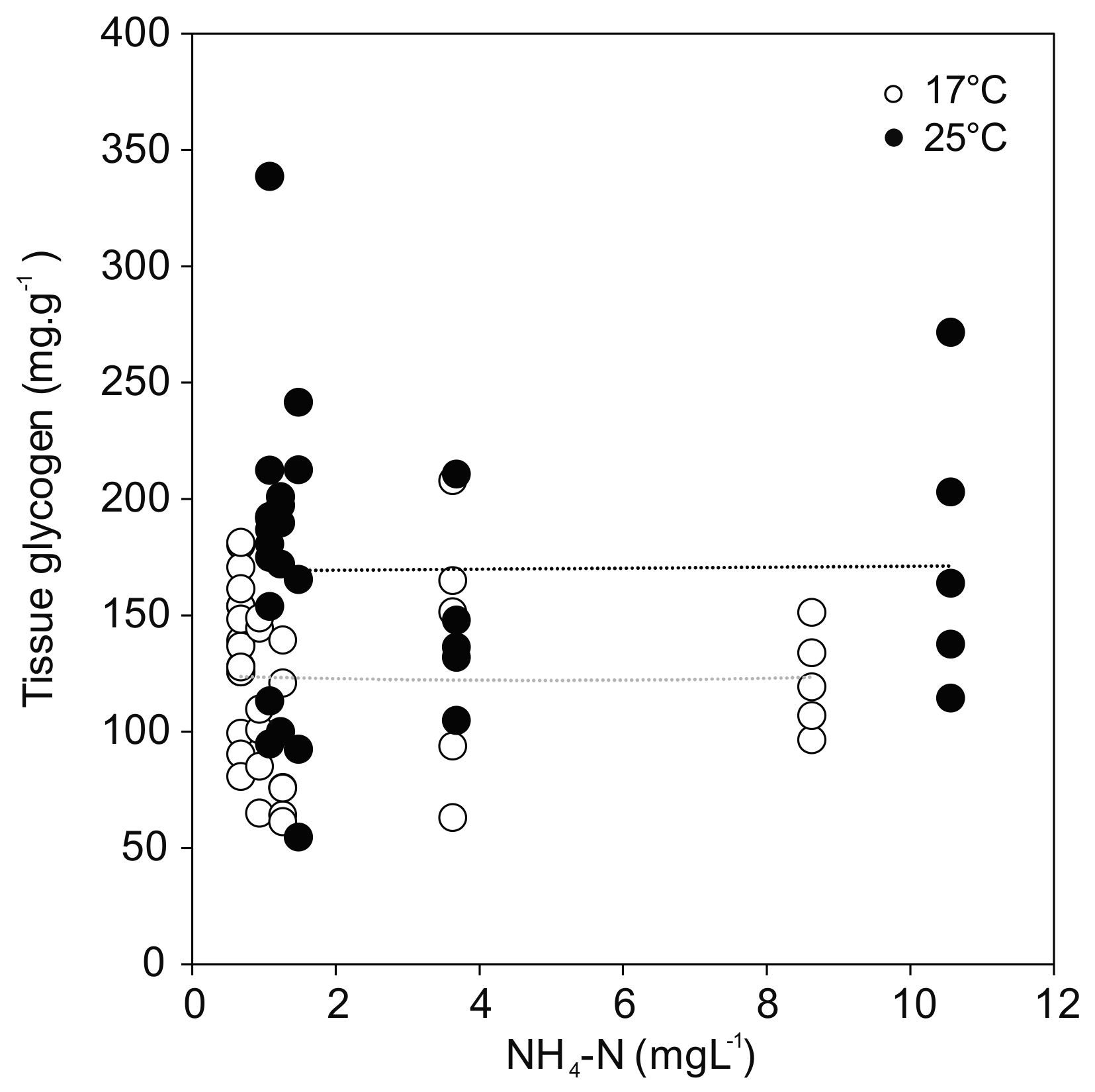
3.6. Energy Reserves
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bogan, A.E. Freshwater Bivalve Extinctions (Mollusca: Unionoida): A Search for Causes. Integr. Comp. Biol. 1993, 33, 599–609. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2016, 92, 572–607. [Google Scholar] [CrossRef] [PubMed]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The global decline of nonmarine mollusks. Bioscience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Naimo, T.J. A review of the effects of heavy metals on freshwater mussels. Ecotoxicology 1995, 4, 341–362. [Google Scholar] [CrossRef] [PubMed]
- Milam, C.D.; Farris, J.L.; Dwyer, F.J.; Hardesty, D.K. Acute toxicity of six freshwater mussel species (glochidia) to six chemicals: Implications for daphnids and Utterbackia imbecillis as surrogates for protection of freshwater mussels (Unionidae). Arch. Environ. Contam. Toxicol. 2005, 48, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Boeker, C.; Lueders, T.; Mueller, M.; Pander, J.; Geist, J. Alteration of physico-chemical and microbial properties in freshwater substrates by burrowing invertebrates. Limnologica 2016, 59, 131–139. [Google Scholar] [CrossRef]
- Lummer, E.M.; Auerswald, K.; Geist, J. Fine sediment as environmental stressor affecting freshwater mussel behavior and ecosystem services. Sci. Total Environ. 2016, 571, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Stoeckl, K.; Denic, M.; Geist, J. Association between the occurrence of the Thick-shelled River Mussel (Unio crassus) and macroinvertebrate, microbial, and diatom communities. Freshw. Sci. 2016, 35, 922–933. [Google Scholar] [CrossRef]
- Strayer, D.L. Challenges for freshwater invertebrate conservation. J. N. Am. Benthol. Soc. 2006, 25, 271–287. [Google Scholar] [CrossRef]
- Strayer, D.L. Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 2014, 735, 277–292. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity losses and ecosystem function in freshwaters: Emerging conclusions and research directions. Bioscience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): A synthesis of Conservation Genetics and Ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Geist, J. Seven steps towards improving freshwater conservation. Aquat. Conserv. 2015, 25, 447–453. [Google Scholar] [CrossRef]
- Hasenbein, M.; Werner, I.; Deanovic, L.A.; Geist, J.; Javidmehr, A.; Foe, C.; Fangue, N.A.; Connon, R.E. Transcriptomic profiling permits the identification of pollutant sources and effects in ambient water samples. Sci. Total Environ. 2014, 468–469, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Augspurger, T.; Keller, A.E.; Black, M.C.; Cope, W.G.; Dwyer, F.J. Water quality guidance for protection of freshwater mussels (Unionidae) from ammonia exposure. Environ. Toxicol. Chem. 2003, 22, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Geist, J.; Auerswald, K. Physicochemical streambed characteristics and recruitment of the freshwater pearl mussel (Margaritifera margaritifera). Freshw. Biol. 2007, 52, 2299–2316. [Google Scholar] [CrossRef]
- Mummert, A.K.; Neves, R.J.; Newcomb, T.J.; Cherry, D.S. Sensitivity of juvenile freshwater mussels (Lampsilis fasciola, Villosa iris) to total and un-ionized ammonia. Environ. Toxicol. Chem. 2003, 22, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Newton, T.J.; Allran, J.W.; O’Donell, J.A.; Bartsch, M.R.; Richardson, W.B. Effects of ammonia on juvenile unionid mussels (Lampsilis cardium) in laboratory sediment toxicity tests. Environ. Toxicol. Chem. 2003, 22, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ingersoll, C.G.; Hardesty, D.K.; Ivey, C.D.; Kunz, J.L.; May, T.W.; Dwyer, F.J.; Roberts, A.D.; Augspurger, T.; Kane, C.M.; et al. Acute toxicity of copper, ammonia, and chlorine to glochidia and juveniles of freshwater mussels (Unionidae). Environ. Toxicol. Chem. 2007, 26, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.; Downing, J.A. Relationship of declining mussel biodiversity to stream-reach and watershed characteristics in an agricultural landscape. J. N. Am. Benthol. Soc. 2004, 23, 114–125. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Aquatic Life Ambient Water Quality Criteria for Ammonia—Freshwater; EPA 822-R-13-001; U.S. Environmental Protection Agency, Office of Water, Office of Science and Technology: Washington, DC, USA, 2013; pp. 1–242.
- Studer, I.; Boeker, C.; Geist, J. Physicochemical and microbiological indicators of surface water body contamination with different sources of digestate from biogas plants. Ecol. Indic. 2017, 77, 314–322. [Google Scholar] [CrossRef]
- Constable, M.; Charlton, M.; Jensen, F.; McDonald, K.; Craig, G.; Taylor, K.W. An ecological risk assessment of ammonia in the aquatic environment. Hum. Ecol. Risk Assess. 2003, 9, 527–548. [Google Scholar] [CrossRef]
- Erickson, R.J.; Nichols, J.W.; Cook, P.M.; Ankley, G.T. Bioavailability of Chemical Contaminants in Aquatic Systems. In The Toxicology of Fishes; DiGiulio, R.T., Hinton, D.E., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 9–54. [Google Scholar]
- Hartmann, J.T.; Beggel, S.; Auerswald, K.; Stoeckle, B.C.; Geist, J. Establishing mussel behavior as a biomarker in ecotoxicology. Aquat. Toxicol. 2016, 170, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Beggel, S.; Auerswald, K.; Geist, J. Determination of the most suitable adhesive for tagging freshwater mussels and its use in an experimental study of filtration behavior and biological rhythm. J. Molluscan Stud. 2016, 82, 415–421. [Google Scholar] [CrossRef]
- Van Hassel, J.H.; Farris, J.L. A review of the use of Unionid mussels as biological indicators of ecosystem health. In Freshwater Bivalve Ecotoxicology; Farris, J.L., Van Hassel, J.H., Eds.; CRC Press and SETAC Press: Boca Raton, FL, USA; Pensacola, FL, USA, 2007; pp. 19–49. [Google Scholar]
- Cope, W.G.; Bringolf, R.B.; Buchwalter, D.B.; Newton, T.J.; Ingersoll, C.G.; Wang, N.; Augspurger, T.; Dwyer, F.J.; Barnhart, C.; Neves, R.J.; et al. Differential exposure, duration, and sensitivity of unionoidean bivalve life stages to environmental contaminants. J. N. Am. Benthol. Soc. 2008, 27, 451–462. [Google Scholar] [CrossRef]
- McIvor, A.L. Freshwater Mussels as Biofilters. Ph.D. Thesis, Department of Zoology, University of Cambridge, Cambridge, UK, 2004. [Google Scholar]
- Naimo, T.J.; Damschen, E.D.; Rada, R.G.; Monroe, E.M. Nonlethal evaluation of the physiological health of unionid mussels: Methods for biopsy and glycogen analysis. J. N. Am. Benthol. Soc. 1998, 17, 121–128. [Google Scholar] [CrossRef]
- Jokela, J.; Uotila, L.; Taskinen, J. Effect of the castrating trematode parasite Rhipidocotyle fennica on energy allocation of fresh-water clam Anodonta piscinalis. Funct. Ecol. 1993, 7, 332–338. [Google Scholar] [CrossRef]
- Hinzmann, M.F.; Lopes-Lima, M.; Bobos, I.; Ferreira, J.; Domingues, B.; Machado, J. Morphological and chemical characterization of mineral concretions in the freshwater bivalve Anodonta cygnea (Unionidae). J. Morphol. 2014, 276, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C. Bivalves. In Invertebrate Blood Cells; Ratcliffe, N.A., Rowley, A.F., Eds.; Academic Press: London, UK, 1981; Volume 1, pp. 231–300. [Google Scholar]
- Hine, P.M. The inter-relationships of bivalve haemocytes. Fish Shellfish Immunol. 1999, 9, 367–385. [Google Scholar] [CrossRef]
- Antunes, F.; Hinzmann, M.F.; Lopes-Lima, M.; Machado, J.; Martins da Costa, P. Association between environmental microbiota and indigenous bacteria found in hemolymph, extrapallial fluid and mucus of Anodonta cygnea (Linnaeus, 1758). Microbiol. Ecol. 2010, 60, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Hinzmann, M.F.; Lopes-Lima, M.; Vaz-Pirez, P.; Ferreira, S.; Domingues, B.; Machado, J. Antibacterial effects of Anodonta cygnea fluids on Escherichia coli and enterococci multi-drug-resistant strains: Environmental implications. Toxicol. Environ. Chem. 2014, 96, 880–889. [Google Scholar] [CrossRef]
- Van Damme, D. Unio. pictorum. The IUCN Red List of Threatened Species, 2011, e.T155543A4795613; doi:10.2305/IUCN.UK.2011-2.RLTS.T155543A4795613.en. Available online: http://www.iucnredlist.org/details/155543/0 (accessed on 22 June 2017).
- Van Vliet, M.T.H.; Franssen, W.H.P.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- ASTM. ASTME2455-06. In Standard Guide for Conducting Laboratory Toxicity Tests with Freshwater Mussels; ASTM International: West Conshohocken, PA, USA, 2006; Available online: www.astm.org (accessed on 5 November 2011). [CrossRef]
- Coughlan, J. The estimation of filtration rates from the clearance of suspensions. Mar. Biol. 1969, 2, 256–258. [Google Scholar] [CrossRef]
- Gagnaire, B.; Thomas-Guyon, H.; Renault, T. In vitro effects of cadmium and mercury on Pacific oyster, Crassostrea gigas (Thunberg), haemocytes. Fish Shellfish Immunol. 2004, 16, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Soares-da-Silva, I.M.; Ribeiro, J.; Valongo, C.; Pinto, R.; Vilanova, M.; Machado, J. Cytometric, morphologic and enzymatic characterisation of haemocytes in Anodonta cygnea. Comp. Biochem. Phys. A 2002, 132, 541–553. [Google Scholar] [CrossRef]
- Hinzmann, M.F.; Lopes-Lima, M.; Gonçalves, J.; Machado, J. Antiaggregant and toxic properties of different solutions on hemocytes of three freshwater bivalves. Toxicol. Environ. Chem. 2013, 95, 790–805. [Google Scholar] [CrossRef]
- Ford, S.E.; Haskin, H.H. Comparison of in vitro salinity tolerance of the oyster Parasite, Haplosporidium. nelsoni (msx) and hemocytes from the host, Crassostrea virginica. Comp. Biochem. Phys. A 1988, 90, 183–187. [Google Scholar] [CrossRef]
- Tirard, C.T.; Grossfeld, R.M.; Levine, J.F.; Kennedy-Stoskopf, S. Effect of Osmotic Shock on Protein Synthesis of Oyster Hemocytes in Vitro. Comp. Biochem. Phys. A 1997, 116, 43–49. [Google Scholar] [CrossRef]
- Keppler, D.; Decker, K. Glycogen determination with amyloglucosidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; Volume 3, pp. 1127–1131. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Denic, M.; Stoeckl, K.; Gum, B.; Geist, J. Physicochemical assessment of Unio crassus habitat quality in a small upland stream and implications for conservation. Hydrobiologia 2014, 735, 111–122. [Google Scholar] [CrossRef]
- Augspurger, T.; Dwyer, F.J.; Ingersoll, C.G.; Kane, C.M. Advances and opportunities in assessing contaminant sensitivity of freshwater mussel (Unionidae) early life stages. Environ. Toxicol. Chem. 2007, 26, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Haag, W.R. North American Freshwater Mussels: Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Ward, S.; Augspurger, T.; Dwyer, F.J.; Kane, C.; Ingersoll, C.G. Risk assessment of water quality in three North Carolina, USA, streams supporting federally endangered freshwater mussels (Unionidae). Environ. Toxicol. Chem. 2007, 26, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ivey, C.D.; Ingersoll, C.G.; Brumbaugh, W.G.; Alvarez, D.; Hammer, E.; Bauer, C.R.; Augspurger, T.; Raimondo, S.; Barnhart, M.C. Acute sensitivity of a broad range of freshwater mussels to chemicals with different modes of toxic action. Environ. Toxicol. Chem. 2017, 36, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Ganser, A.M.; Newton, T.J.; Haro, R.J. The effects of elevated water temperature on native juvenile mussels: Implications for climate change. Freshw. Sci. 2013, 32, 1168–1177. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Borgmann, U. Chronic toxicity of ammonia to the amphipod Hyalella azteca; Importance of ammonium ion and water hardness. Environ. Pollut. 1994, 86, 329–335. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Chippendale, D.; Knight, A.W.; Colt, J.E. Interaction of ionized and un-ionized ammonia on short-term survival and growth of prawn larvae, Macrobrachium rosenbergii. Biol. Bull. 1978, 154, 15–31. [Google Scholar] [CrossRef]
- Hickey, C.W.; Martin, M.L. Chronic Toxicity of Ammonia to the Freshwater Bivalve Sphaerium novaezelandiae. Arch. Environ. Contam. Toxicol. 1999, 36, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Gatta, T.; Ravera, O. Relationship between anti-oxidant capacity and manganese accumulation in the soft tissues of two freshwater molluscs: Unio pictorum mancus (Lamellibranchia, Unionidae) and Viviparus ater (Gastropoda, Prosobranchia). J. Limnol. 2005, 64, 153–158. [Google Scholar] [CrossRef]
- Beggel, S.; Geist, J. Acute effects of salinity exposure on glochidia viability and host infection of the freshwater mussel Anodonta anatina (Linnaeus, 1758). Sci. Total Environ. 2014, 502, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.J.S.; Fang, J.G.; Pascoe, P.L.; Zhang, J.H.; Zhang, X.L.; Zhu, M.Y. Modelling short-term responsive adjustments in particle clearance rate among bivalve suspension-feeders: Separate unimodal effects of seston volume and composition in the scallop Chlamys farreri. J. Exp. Mar. Biol. Ecol. 2001, 262, 61–73. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Gan, J. Trace element accumulation in bivalve mussels Anodonta woodiana from Taihu Lake, China. Arch. Environ. Contam. Toxicol. 2010, 59, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Riisgard, H.U.; Kittner, C.; Seerup, D.F. Regulation of opening state and filtration rate in filter-feeding bivalves (Cardium edule, Mytilus edulis, Mya arenaria) in response to low algal concentration. J. Exp. Mar. Biol. Ecol. 2003, 284, 105–127. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L.; Tan, J.W.; Chen, X.Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T. Apoptosis and its functional significance in molluscs. Apoptosis 2010, 15, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Madec, L. Haemocyte apoptosis as a general cellular immune response of the snail, Lymnaea stagnalis, to a toxicant. Cell Tissue Res. 2007, 328, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Lanz, H.; Tsutsumi, V.; Arechiga, A. Morphological and biochemical characterisation of Procambarus clarki blood cells. Dev. Comp. Immunol. 1993, 17, 389–397. [Google Scholar] [CrossRef]
- Bayne, B.L.; Moore, M.N.; Widdows, J.; Livingstone, D.R.; Salkeld, P.; Crisp, D.J.; Morris, R.J.; Gray, J.S.; Holden, A.V.; Newell, R.C.; et al. Measurement of the responses of individuals to environmental stress and pollution: Studies with bivalve molluscs. Philos. Trans. R. Soc. B 1979, 286, 562–581. [Google Scholar] [CrossRef]


| Nominal | Measured (17 °C) | Measured (25 °C) | ||
|---|---|---|---|---|
| NH4+ | NH4-N | NH3-N a | NH4-N | NH3-N a |
| (mg·L−1) | (mg·L−1) | (mg·L−1) | (mg·L−1) | (mg·L−1) |
| 0.0 | 0.68 (0.04) | 0.12 (0.01) | 1.08 (0.17) | 0.28 (0.05) |
| 0.3 | 0.94 (0.16) | 0.17 (0.03) | 1.23 (0.42) | 0.32 (0.10) |
| 0.9 | 1.26 (0.24) | 0.22 (0.04) | 1.48 (0.17) | 0.39 (0.03) |
| 3.0 | 3.63 (0.37) | 0.64 (0.08) | 3.68 (0.16) | 0.97 (0.06) |
| 9.0 | 8.63 (0.99) | 1.51 (0.12) | 10.56 (0.53) | 2.78 (0.07) |
| Wilks’ Λ | F | df | Error | p | Partial ε2 | |
|---|---|---|---|---|---|---|
| Constant term | 0.025 | 285.454 | 7 | 52.000 | <0.001 | 0.975 |
| Temperature | 0.359 | 13.286 | 7 | 52.000 | <0.001 | 0.641 |
| Total ammonia N | 0.744 | 0.576 | 28 | 188.911 | 0.957 | 0.071 |
| Interaction | 0.628 | 0.929 | 28 | 188.911 | 0.572 | 0.108 |
| Temp | NH4-N (mg·L−1) | t = 0 h | t = 96 h | ||
|---|---|---|---|---|---|
| Visually Active | Measurable Active | Visually Active | Measureable Active | ||
| 17 °C | 0.68 | 28% | 28% | 40% | 50% |
| 0.94 | 0% | 33% | 20% | 40% | |
| 1.26 | 50% | 33% | 20% | 40% | |
| 3.63 | 17% | 33% | 0% | 20% | |
| 8.63 | 33% | 33% | 20% | 20% | |
| 25 °C | 1.08 | 44% | 44% | 70% | 50% |
| 1.23 | 0% | 50% | 80% | 80% | |
| 1.48 | 67% | 83% | 40% | 40% | |
| 3.68 | 67% | 50% | 100% | 60% | |
| 10.56 | 83% | 33% | 0% | 60% | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beggel, S.; Hinzmann, M.; Machado, J.; Geist, J. Combined Impact of Acute Exposure to Ammonia and Temperature Stress on the Freshwater Mussel Unio pictorum. Water 2017, 9, 455. https://doi.org/10.3390/w9070455
Beggel S, Hinzmann M, Machado J, Geist J. Combined Impact of Acute Exposure to Ammonia and Temperature Stress on the Freshwater Mussel Unio pictorum. Water. 2017; 9(7):455. https://doi.org/10.3390/w9070455
Chicago/Turabian StyleBeggel, Sebastian, Mariana Hinzmann, Jorge Machado, and Juergen Geist. 2017. "Combined Impact of Acute Exposure to Ammonia and Temperature Stress on the Freshwater Mussel Unio pictorum" Water 9, no. 7: 455. https://doi.org/10.3390/w9070455





