Canopy Transpiration and Stomatal Responses to Prolonged Drought by a Dominant Desert Species in Central Asia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Environmental Variables
2.3. Canopy Transpiration and Stomatal Conductance
2.4. Data and Statistical Analysis
3. Results
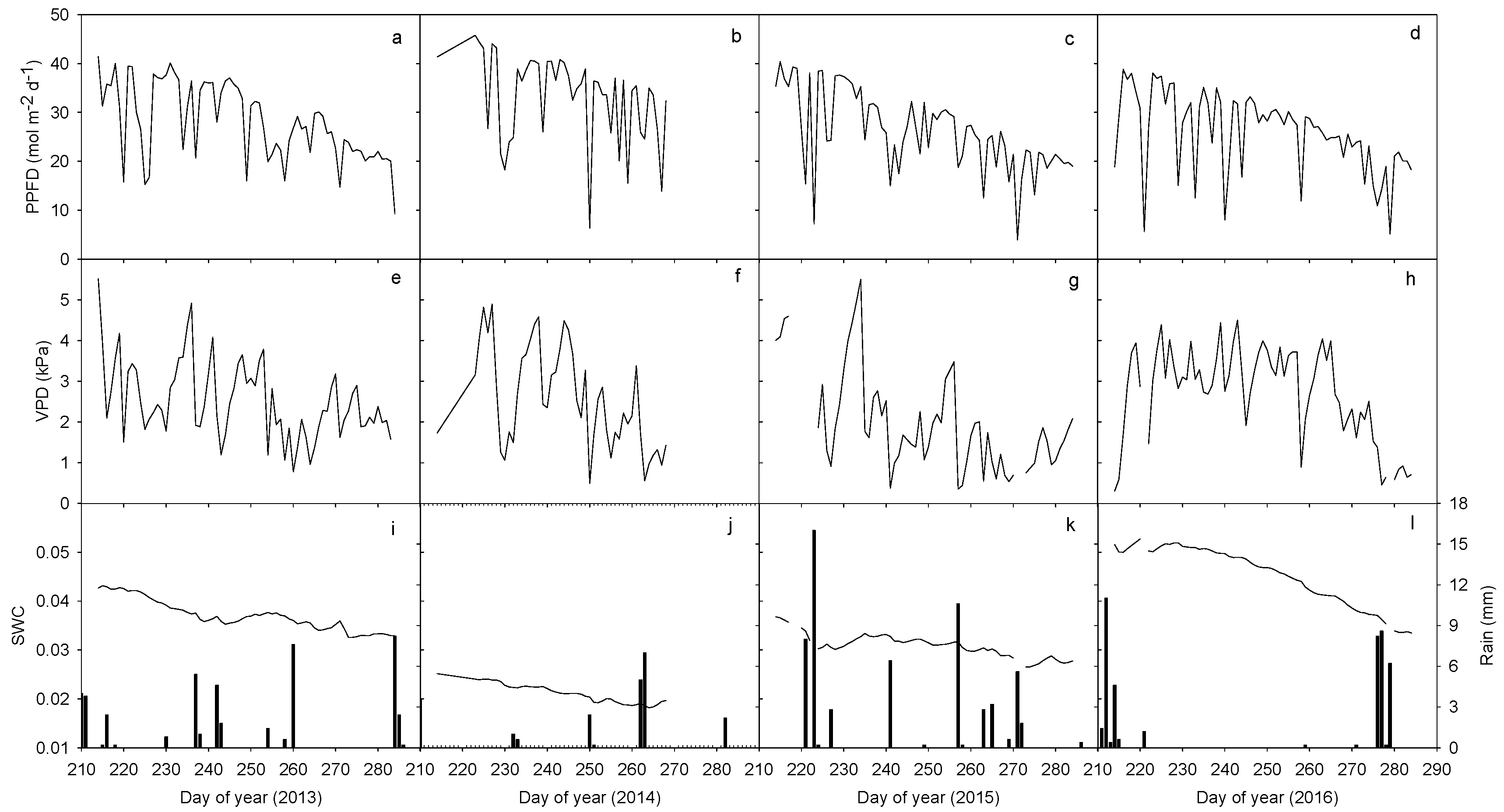
3.1. Soil Moisture and Microclimate
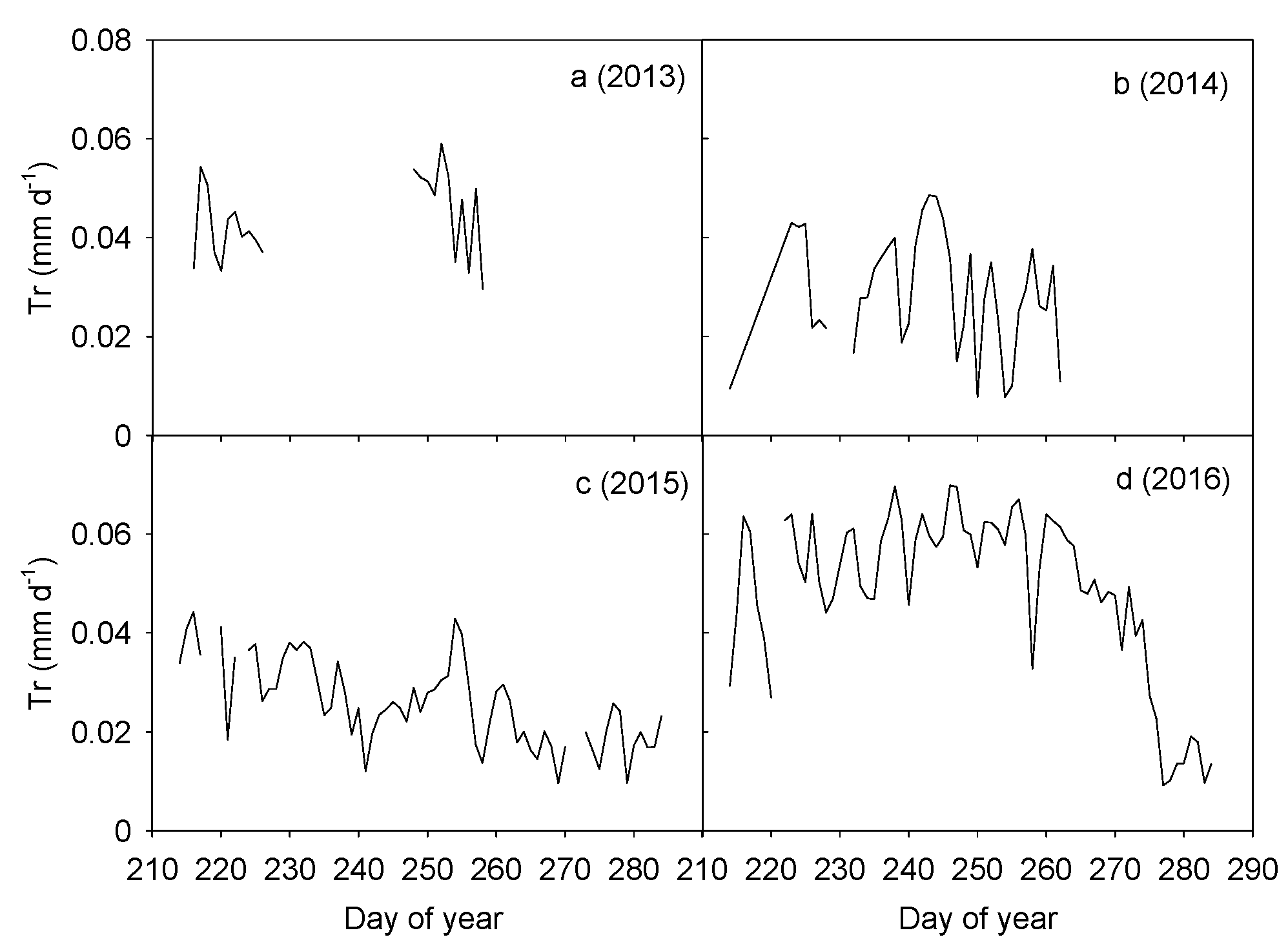
3.2. Canopy Transpiration
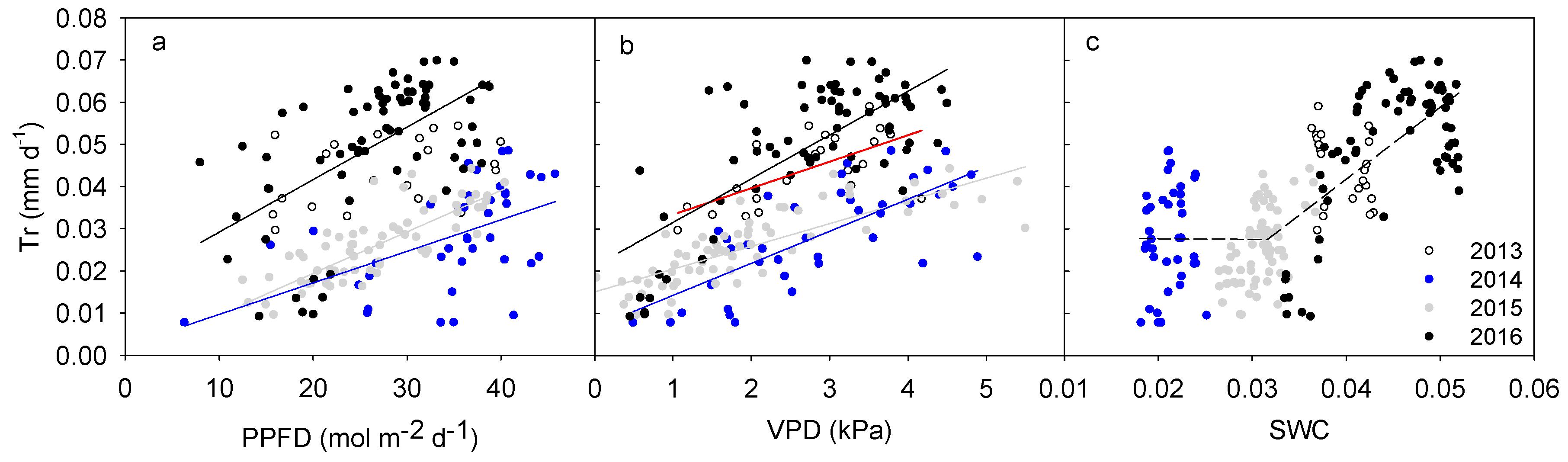
3.3. Response of Daily Canopy Transpiration to Environmental Variables
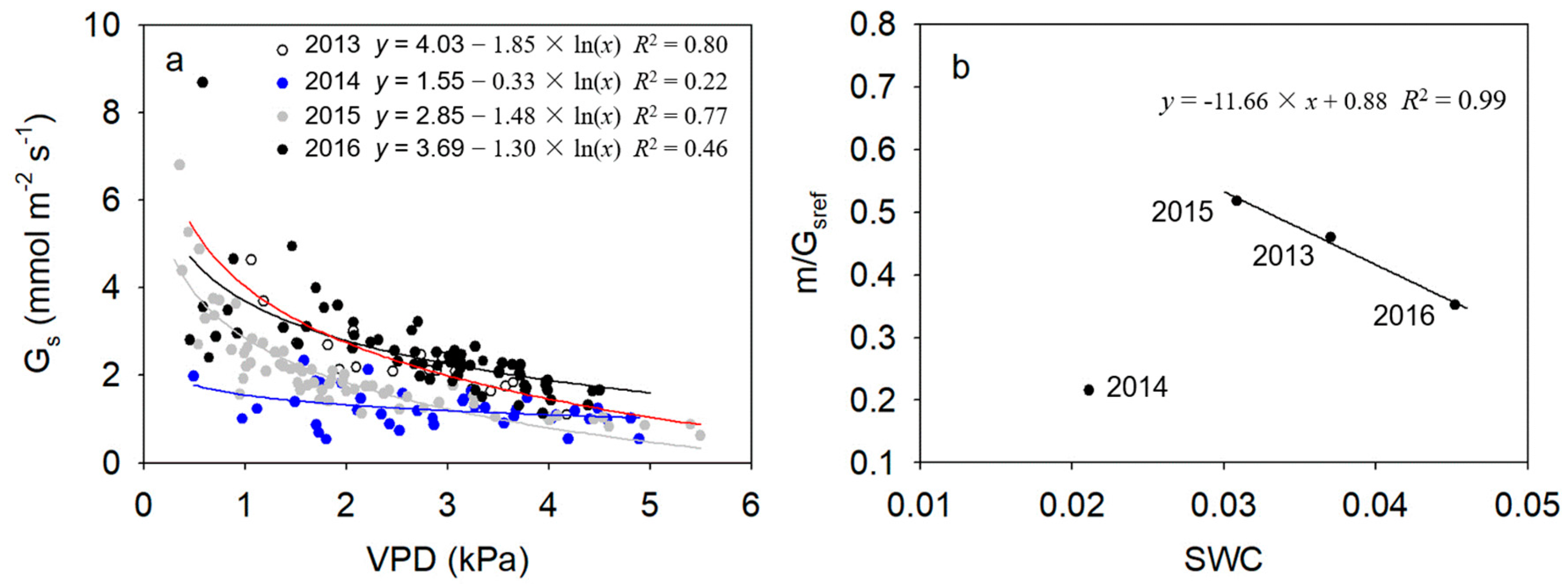
3.4. Response of Canopy Stomatal Conductance to VPD in Different Years
4. Discussion
4.1. Soil Drought Intensity Decides the Response of Canopy Transpiration to Climate
4.2. Soil Drought Intensity Decides Stomatal Regulation Ability for Water Loss
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Dyurgerov, M.B.; Meier, M.F. Twentieth century climate change: Evidence from small glaciers. Proc. Natl. Acad. Sci. USA 2000, 97, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shen, Y.; Kang, E.; Li, D.; Ding, Y.; Zhang, G.; Hu, R. Recent and future climate change in northwest China. Clim. Chang. 2007, 80, 379–393. [Google Scholar] [CrossRef]
- Sorg, A.; Bolch, T.; Stoffel, M.; Solomina, O.; Beniston, M. Climate change impacts on glaciers and runoff in Tien Shan (Central Asia). Nat. Clim. Chang. 2012, 2, 725–731. [Google Scholar] [CrossRef]
- Lal, M.; Harasawa, H. Future climate change scenarios for asia as inferred from selected coupled atmosphere-ocean global climate models. J. Meteorol. Soc. Jpn. Ser. II 2001, 79, 219–227. [Google Scholar] [CrossRef]
- Chou, C.; Chiang, J.C.H.; Lan, C.-W.; Chung, C.-H.; Liao, Y.-C.; Lee, C.-J. Increase in the range between wet and dry season precipitation. Nat. Geosci. 2013, 6, 263–267. [Google Scholar] [CrossRef]
- Farquhar, G.; Richards, R. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Jasechko, S.; Sharp, Z.D.; Gibson, J.J.; Birks, S.J.; Yi, Y.; Fawcett, P.J. Terrestrial water fluxes dominated by transpiration. Nature 2013, 496, 347–350. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Kolle, O.; Trumbore, S. Thirst beats hunger-declining hydration during drought prevents carbon starvation in norway spruce saplings. New Phytol. 2013, 200, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Edwards, G.E.; Ku, M.S. Control of photosynthesis and stomatal conductance in Ricinus communis L. (castor bean) by leaf to air vapor pressure deficit. Plant Physiol. 1992, 99, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Sperry, J.S.; Katul, G.; Pataki, D.; Ewers, B.; Phillips, N.; Schäfer, K. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Hutton, R.J.; Clarke, S.J.; Hutchinson, P.A.; Somers, A. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 2012, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Bourne, A.E.; Haigh, A.M.; Ellsworth, D.S. Stomatal sensitivity to vapour pressure deficit relates to climate of origin in Eucalyptus species. Tree Physiol. 2015, 35, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S. Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Trees 2004, 18, 399–407. [Google Scholar] [CrossRef]
- Lioubimtseva, E.; Henebry, G.M. Climate and environmental change in arid Central Asia: Impacts, vulnerability, and adaptations. J. Arid Environ. 2009, 73, 963–977. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Q. Seasonal and annual variation in transpiration of a dominant desert species, Haloxylon ammodendron, in Central Asia up-scaled from sap flow measurement. Ecohydrology 2015, 8, 948–960. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Q. Water-use response to climate factors at whole tree and branch scale for a dominant desert species in Central Asia: Haloxylon ammodendron. Ecohydrology 2014, 7, 56–63. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Xu, G.; Zou, T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ. 2007, 30, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Q.; Li, Y.; Xu, H. Seasonal variation in plant hydraulic traits of two co-occurring desert shrubs, Tamarix ramosissima and Haloxylon ammodendron, with different rooting patterns. Ecol. Res. 2011, 26, 1071–1080. [Google Scholar] [CrossRef]
- Liu, R.; Pan, L.-P.; Jenerette, G.D.; Wang, Q.-X.; Cieraad, E.; Li, Y. High efficiency in water use and carbon gain in a wet year for a desert halophyte community. Agric. For. Meteorol. 2012, 162–163, 127–135. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer-Verlag: New York, NY, USA, 1998; p. 286. [Google Scholar]
- Granier, A. Evaluation of transpiration in a douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Granier, A.; Huc, R.; Barigah, S.T. Transpiration of natural rain forest and its dependence on climatic factors. Agric. For. Meteorol. 1996, 78, 19–29. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Law, B.; Falge, E.; Gu, L.; Baldocchi, D.; Bakwin, P.; Berbigier, P.; Davis, K.; Dolman, A.; Falk, M.; Fuentes, J. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteorol. 2002, 113, 97–120. [Google Scholar] [CrossRef]
- Nicolás, E.; Barradas, V.; Ortuño, M.; Navarro, A.; Torrecillas, A.; Alarcón, J. Environmental and stomatal control of transpiration, canopy conductance and decoupling coefficient in young lemon trees under shading net. Environ. Exp. Bot. 2008, 63, 200–206. [Google Scholar] [CrossRef]
- Clausnitzer, F.; Köstner, B.; Schwärzel, K.; Bernhofer, C. Relationships between canopy transpiration, atmospheric conditions and soil water availability—Analyses of long-term sap-flow measurements in an old Norway spruce forest at the Ore Mountains/Germany. Agric. For. Meteorol. 2011, 151, 1023–1034. [Google Scholar] [CrossRef]
- Jung, E.Y.; Otieno, D.; Lee, B.; Lim, J.H.; Kang, S.K.; Schmidt, M.W.T.; Tenhunen, J. Up-scaling to stand transpiration of an Asian temperate mixed-deciduous forest from single tree sapflow measurements. Plant Ecol. 2010, 212, 383–395. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, S.; Wei, X.; Wang, X. Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid northwest China. Agric. Water Manag. 2014, 136, 23–33. [Google Scholar] [CrossRef]
- Daly, E.; Porporato, A. A review of soil moisture dynamics: From rainfall infiltration to ecosystem response. Environ. Eng. Sci. 2005, 22, 9–24. [Google Scholar] [CrossRef]
- Cammalleri, C.; Rallo, G.; Agnese, C.; Ciraolo, G.; Minacapilli, M.; Provenzano, G. Combined use of eddy covariance and sap flow techniques for partition of ET fluxes and water stress assessment in an irrigated olive orchard. Agric. Water Manag. 2013, 120, 89–97. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y. Water-use strategy of three Central Asian desert shrubs and their responses to rain pulse events. Plant Soil 2006, 285, 5–17. [Google Scholar] [CrossRef]
- Jiang, J. The physioecological characters of transpiration of two species of haloxylon. Arid Zone Res. 1992, 9, 14–17. [Google Scholar]
- O’Grady, A.P.; Mitchell, P.J.; Pinkard, E.A.; Tissue, D.T. Thirsty roots and hungry leaves: Unravelling the roles of carbon and water dynamics in tree mortality. New Phytol. 2013, 200, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.A.; Wadsworth, W.D.; Pangle, R.; Yepez, E.A.; McDowell, N.G.; Pockman, W.T. Reduced transpiration response to precipitation pulses precedes mortality in a pinon-juniper woodland subject to prolonged drought. New Phytol. 2013, 200, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Johnson, D.M. Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. cultivars? Tree Physiol. 2012, 32, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Oren, R.; Kang, S. Spatiotemporal variation of crown-scale stomatal conductance in an arid Vitis vinifera L. cv. Merlot Vineyard: Direct effects of hydraulic properties and indirect effects of canopy leaf area. Tree Physiol. 2012, 32, 262–279. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, Y.; Zou, T. Hydraulic resistance partitioning between shoot and root system and plant water status of Haloxyolon ammodendron growing at sites of contrasting soil texture. J. Arid Land 2010, 2, 98–106. [Google Scholar] [CrossRef]
- Schafer, K.V. Canopy stomatal conductance following drought, disturbance, and death in an upland oak/pine forest of the New Jersey Pine Barrens, USA. Front. Plant Sci. 2011, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y. Precipitation Partitioning and Its Ecological Significance for Desert Dominant Species Haloxylon ammodendron and Haloxylon persicum. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. [Google Scholar]
- Zou, T.; Li, Y.; Xu, H.; Xu, G.-Q. Responses to precipitation treatment for Haloxylon ammodendron growing on contrasting textured soils. Ecol. Res. 2010, 25, 185–194. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, D.; Wang, Q.; Otieno, D. Canopy Transpiration and Stomatal Responses to Prolonged Drought by a Dominant Desert Species in Central Asia. Water 2017, 9, 404. https://doi.org/10.3390/w9060404
Gu D, Wang Q, Otieno D. Canopy Transpiration and Stomatal Responses to Prolonged Drought by a Dominant Desert Species in Central Asia. Water. 2017; 9(6):404. https://doi.org/10.3390/w9060404
Chicago/Turabian StyleGu, Daxing, Quan Wang, and Dennis Otieno. 2017. "Canopy Transpiration and Stomatal Responses to Prolonged Drought by a Dominant Desert Species in Central Asia" Water 9, no. 6: 404. https://doi.org/10.3390/w9060404




