Effects of Dredging and Lanthanum-Modified Clay on Water Quality Variables in an Enclosure Study in a Hypertrophic Pond
Abstract
:1. Introduction
2. Materials and Methods
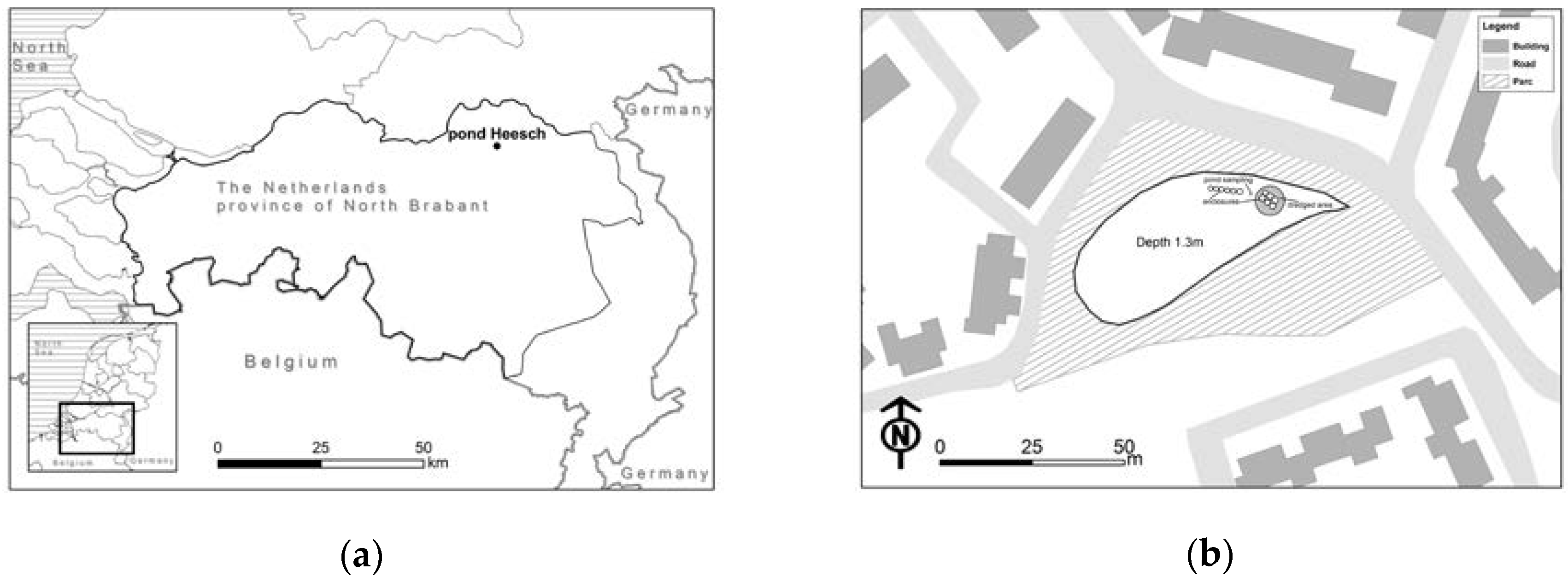
2.1. Site Description
2.2. Enclosure Experiment
2.3. Water Quality Variables
2.4. Data Analysis
2.5. Chemical Equilibrium Modeling
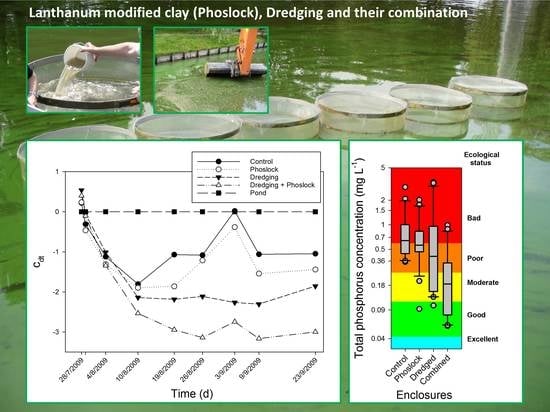
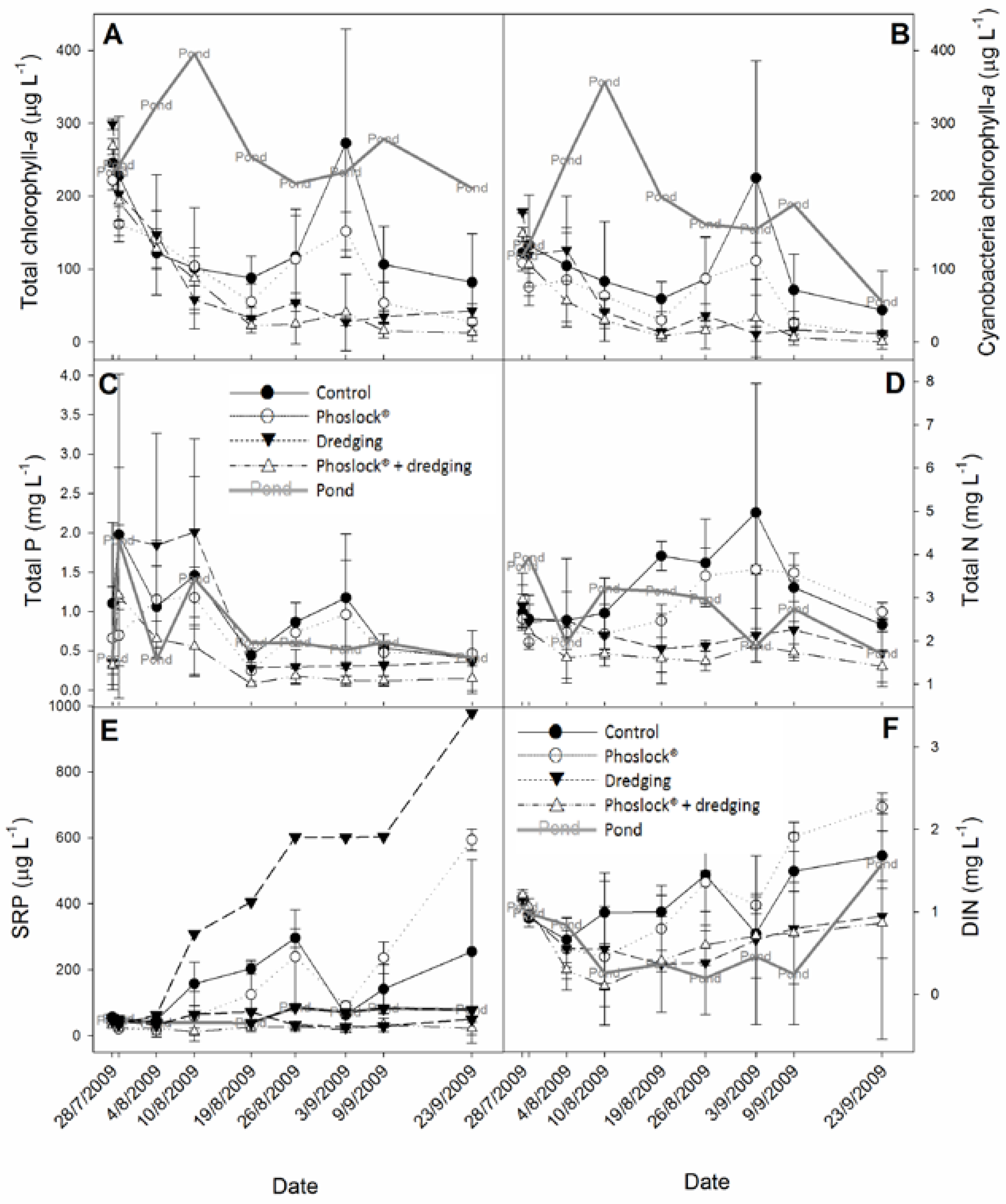
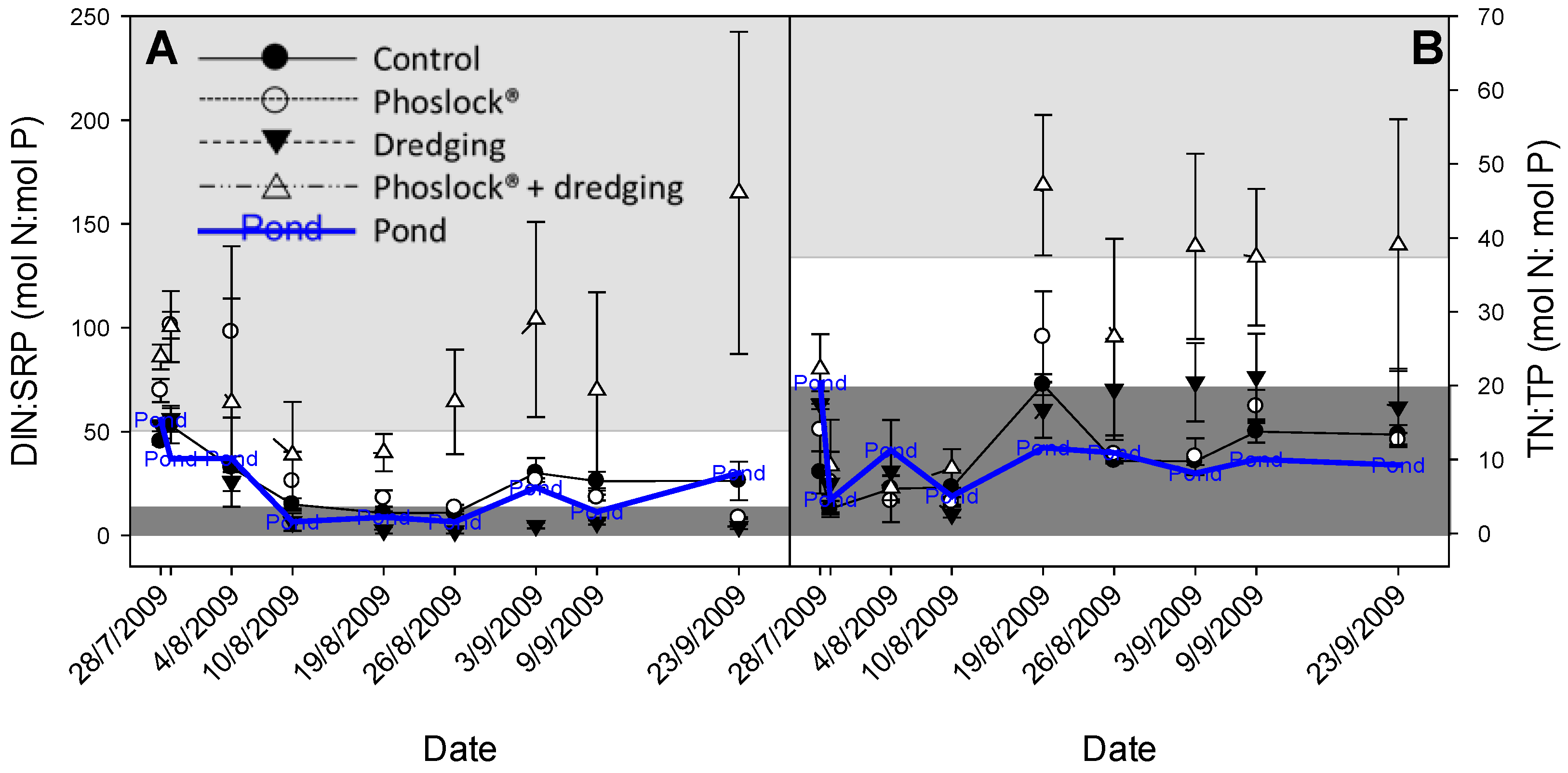
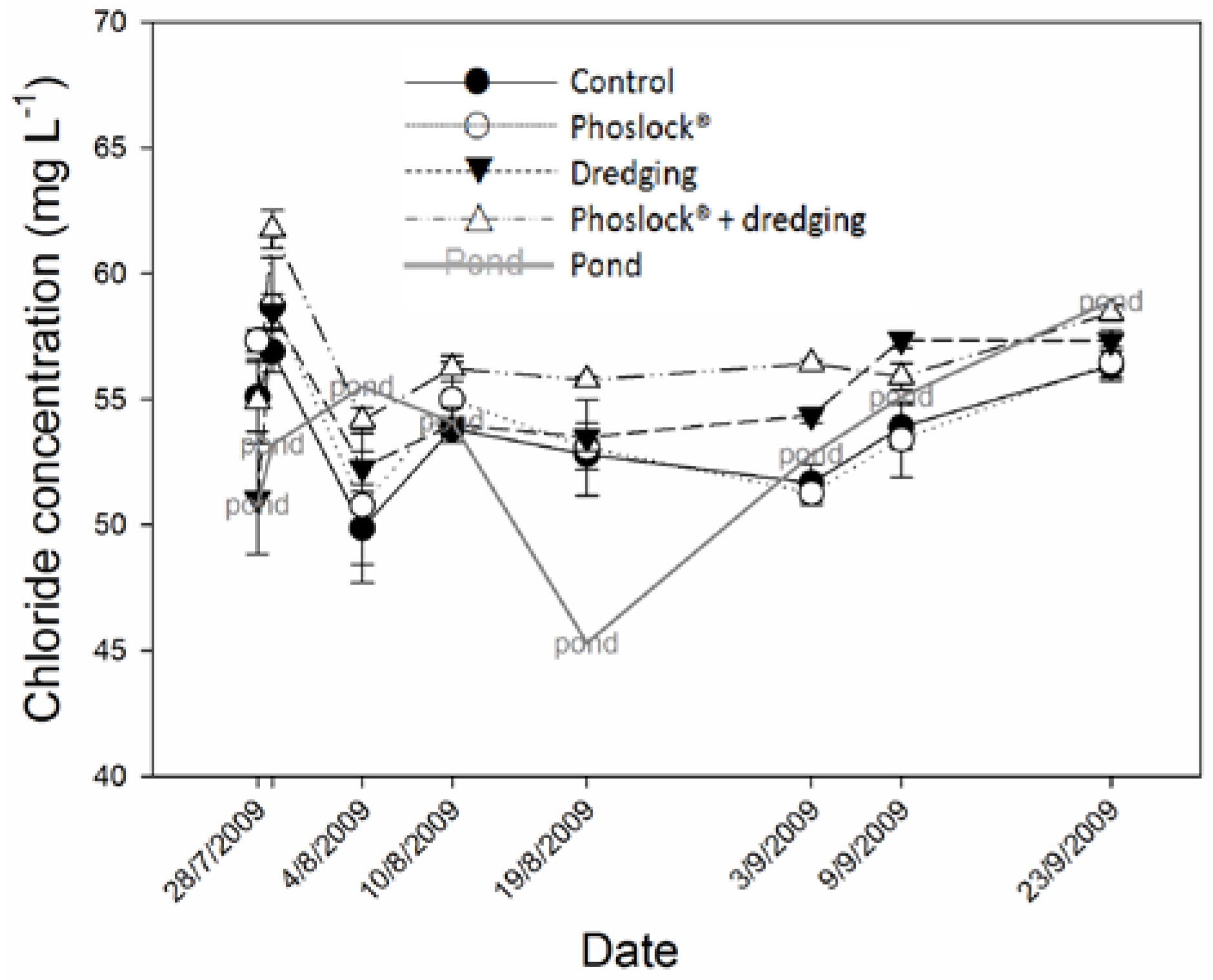
3. Results
3.1. Chlorophyll-a, Phosphorus, and Nitrogen Concentrations
3.2. Transparency, pH, Conductivity, Oxygen, and Temperature
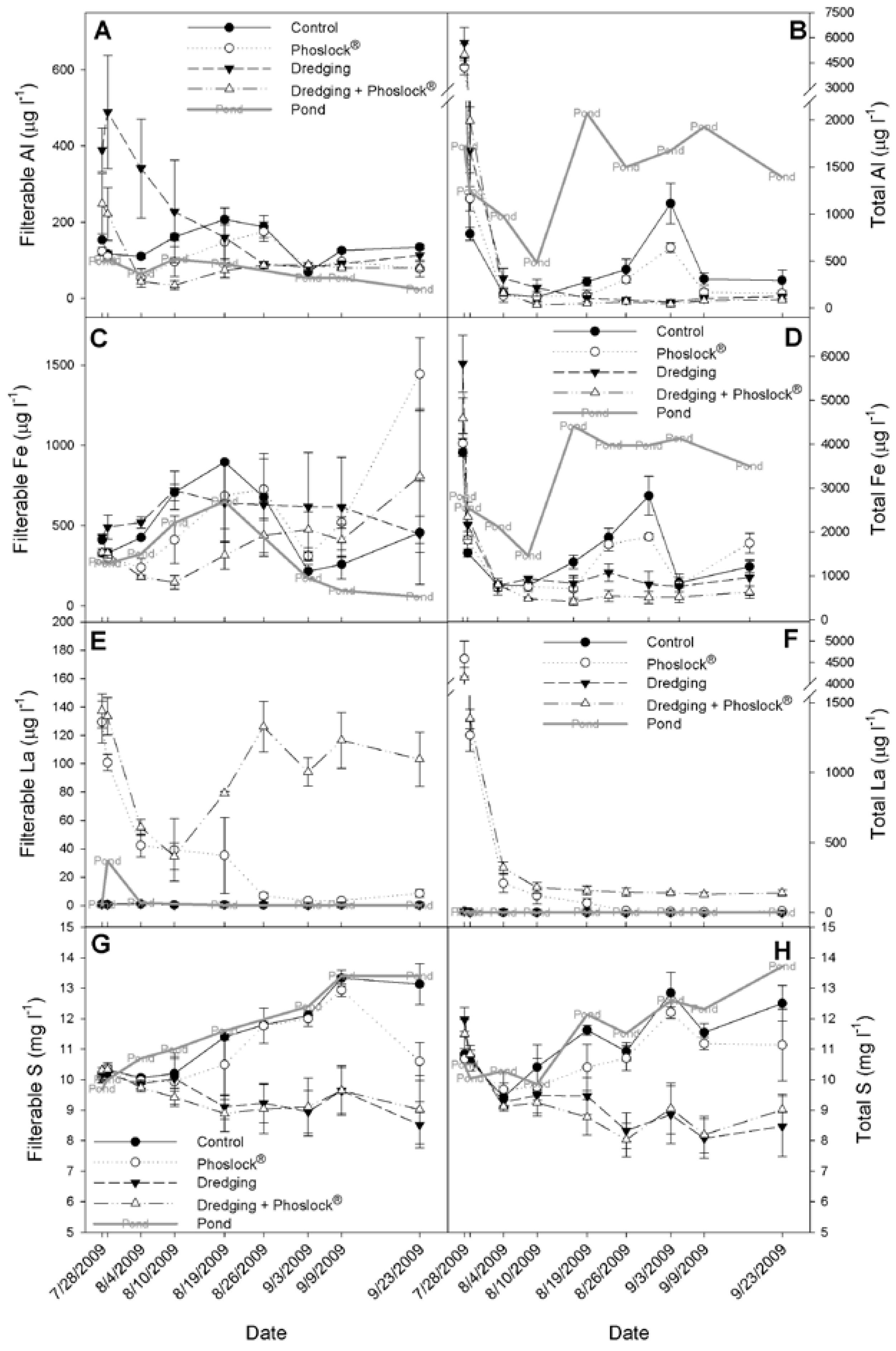
3.3. Metals, Sulfur, Chloride
3.4. Zooplankton
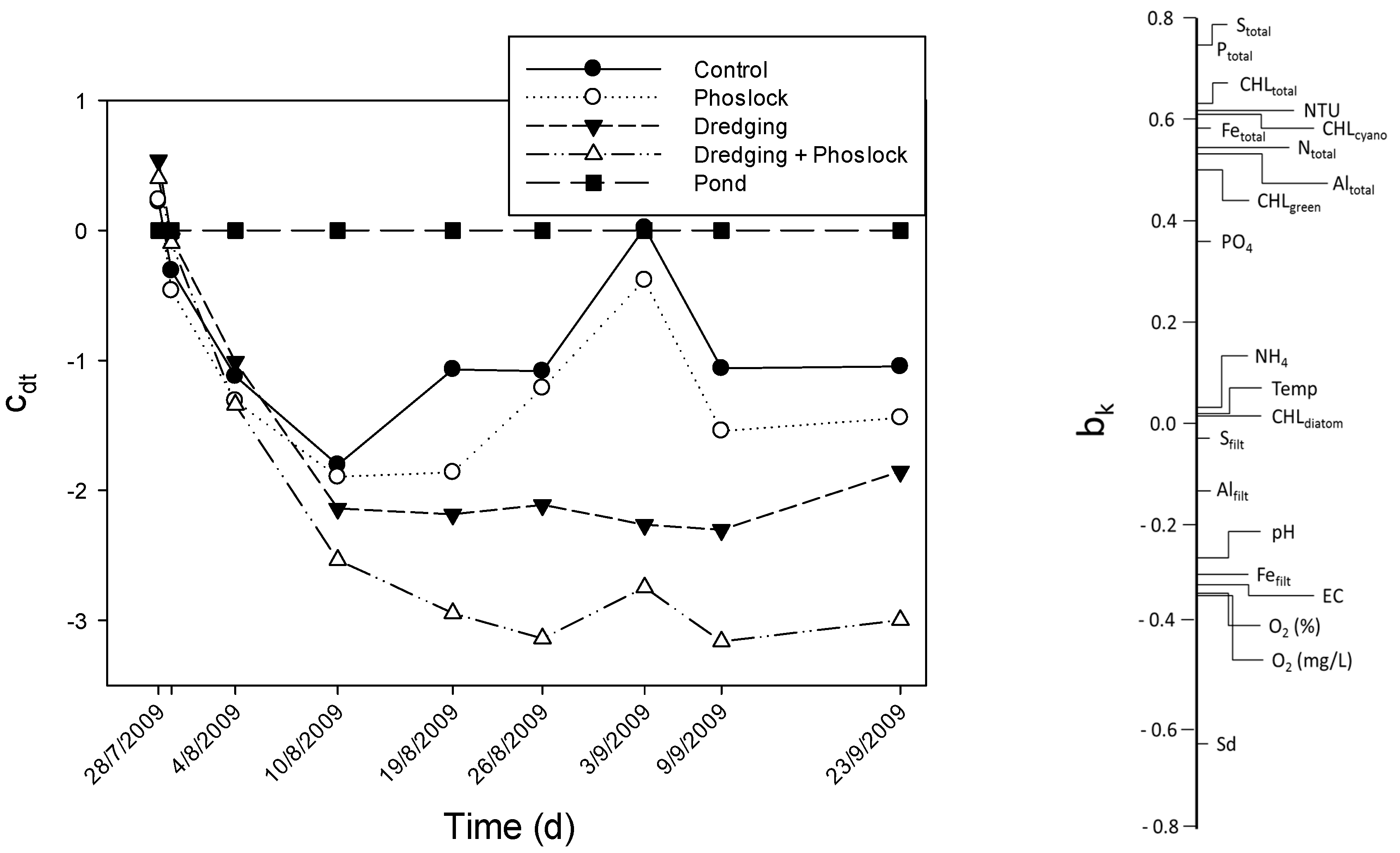
3.5. Multivariate Analysis
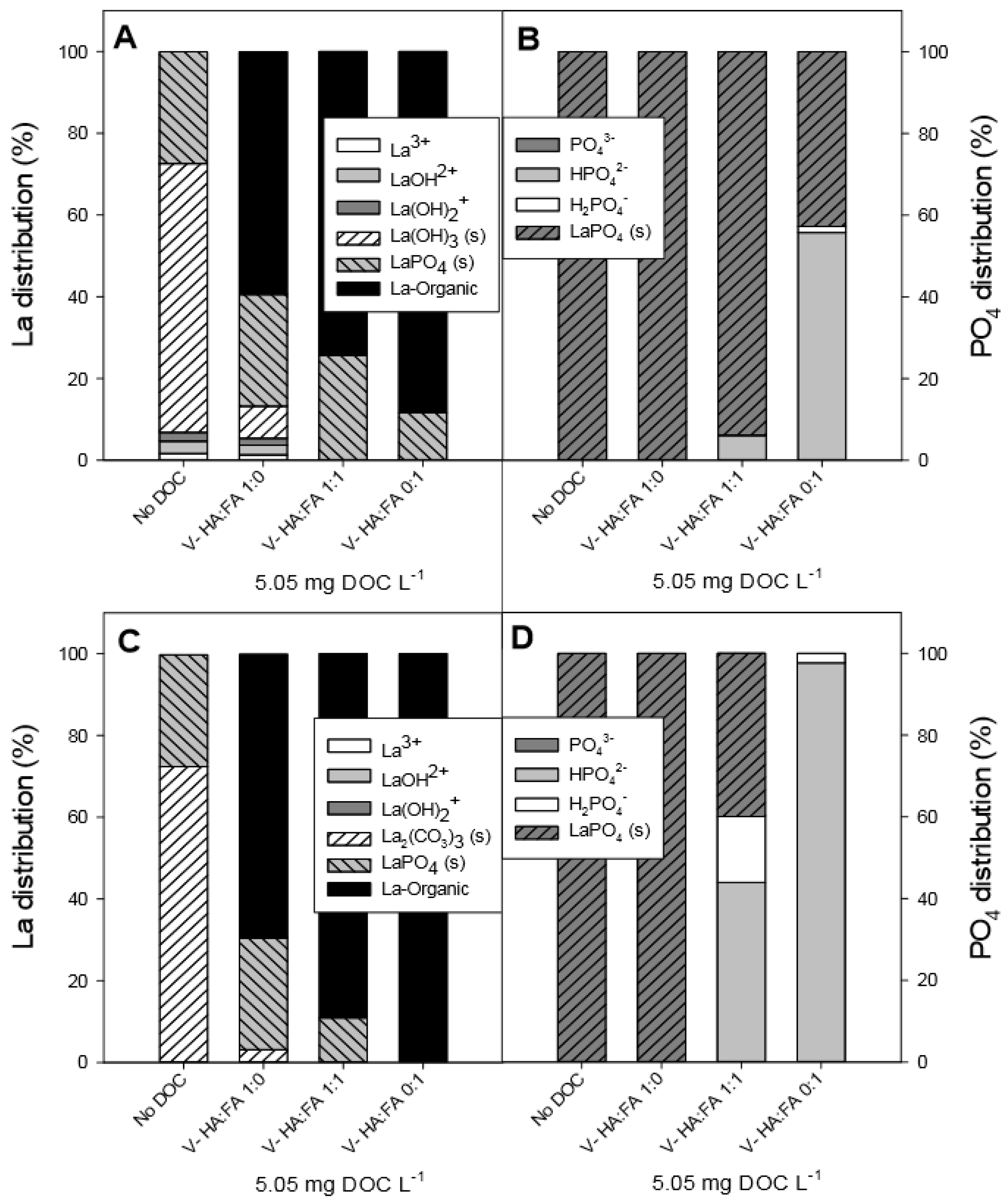
3.6. Chemical Equilibrium Modeling
4. Discussion
5. Conclusions
- The combination of dredging and LMB addition improved the water quality in the enclosures the most.
- The combination of dredging and LMB treatment had a serious drawback: a persistently high filterable La concentration of 100 μg L−1, which exceeds by 10-fold the maximum permissible concentration of lanthanum in Dutch surface water.
- Dredging was more effective in mitigating eutrophication than LMB application, but one dredged enclosure showed no improved water quality, suggesting methodological issues with the employed wet dredging.
- LMB application did not bring SRP to limiting concentrations and did not hamper sediment SRP efflux under anoxia.
- The effectiveness of LMB is most probably negatively influenced by humic substances.
- For rehabilitation of the pond, drawdown followed by bulldozer and scraper removal of sediment, drastic fish stock reduction, and dialogue with neighbors is recommended.
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
Appendix C
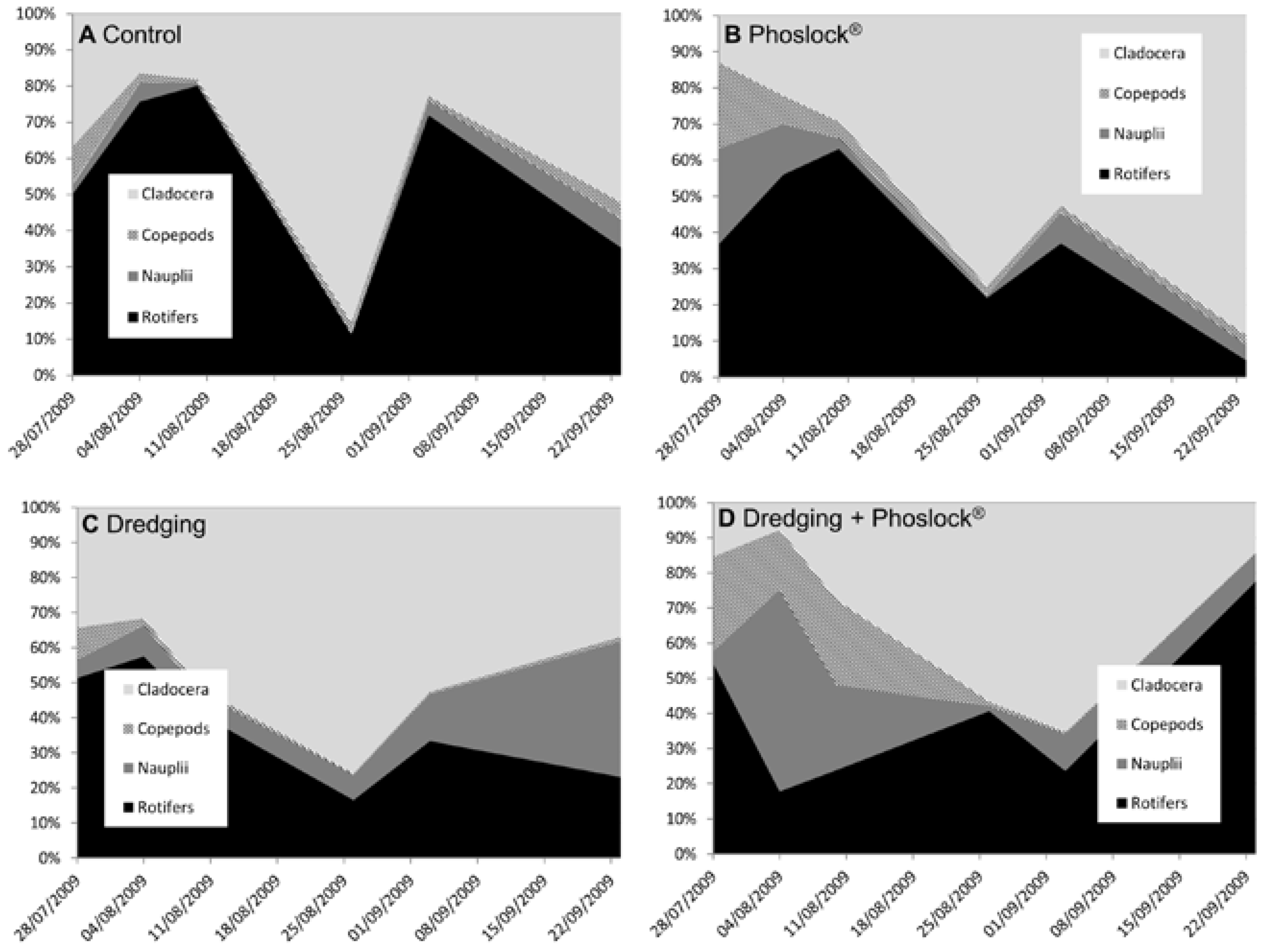
Appendix D
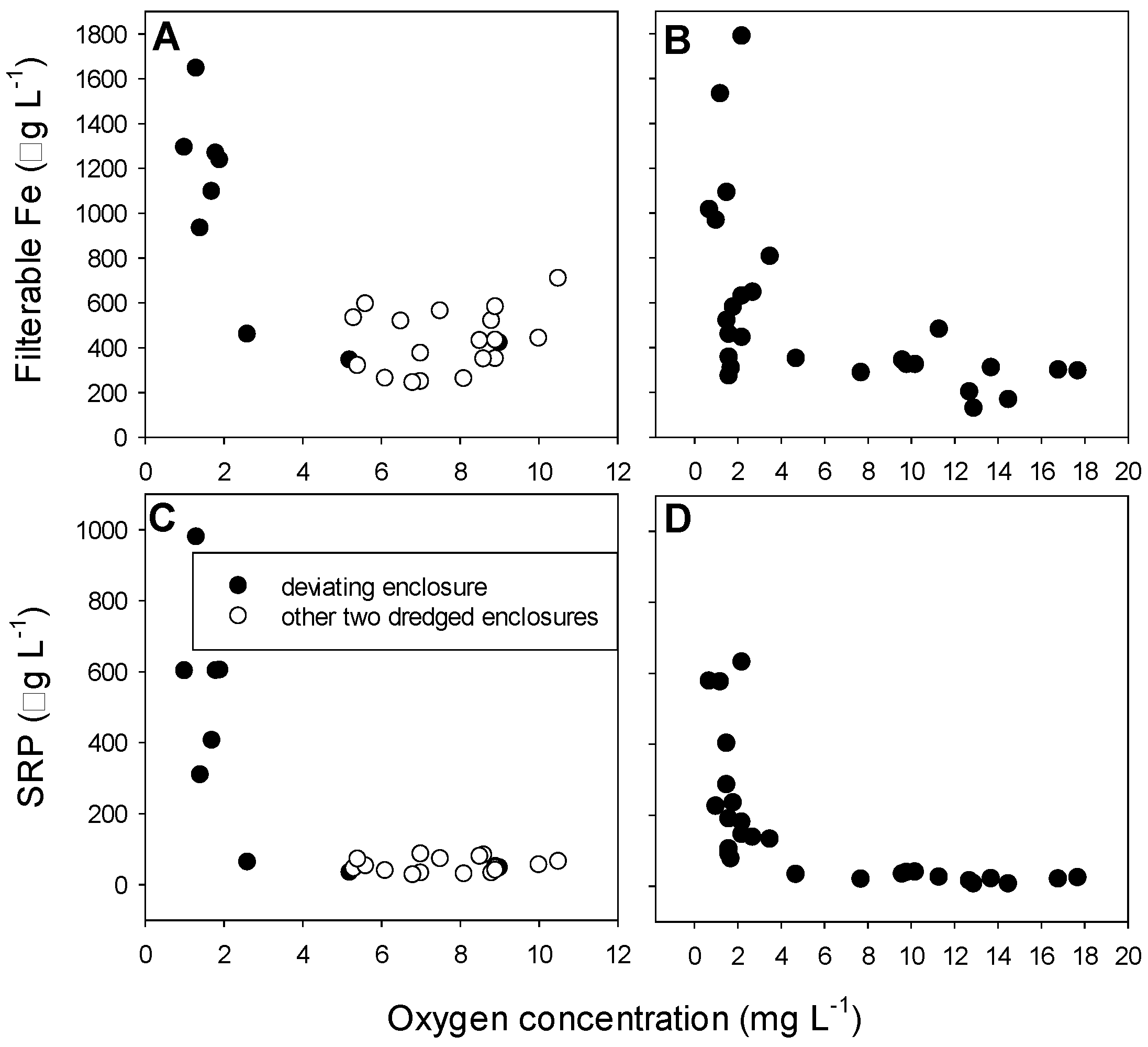
References
- Brönmark, C.; Hansson, L.-A. Environmental issues in lakes and ponds: Current state and perspectives. Environ. Conserv. 2002, 29, 290–306. [Google Scholar] [CrossRef]
- Gledhill, D.G.; James, P.; Davies, D.H. Pond density as a determinant of aquatic species richness in an urban landscape. Landsc. Ecol. 2008, 23, 1219–1230. [Google Scholar] [CrossRef]
- Waajen, G.W.A.M.; Faassen, E.J.; Lürling, M. Eutrophic urban ponds suffer from cyanobacterial blooms: Dutch examples. Environ. Sci. Pollut. Res. 2014, 21, 9983–9994. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.; McCaskie, J. Shallow urban lakes: A challenge for lake management. Hydrobiologia 1999, 395–396, 365–377. [Google Scholar] [CrossRef]
- Steffensen, D.A. Economic cost of cyanobacterial blooms. Adv. Exp. Med. Biol. 2008, 619, 855–865. [Google Scholar] [PubMed]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, A.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Cooke, G.D.; Welch, E.B.; Peterson, S.A.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Moss, B.; Balls, H.; Irvine, K.; Stansfield, J. Restoration of two lowland lakes by isolation from nutrient-rich water sources with and without removal of sediment. J. Appl. Ecol. 1986, 23, 391–414. [Google Scholar] [CrossRef]
- Moss, B.; Stansfield, J.; Irivine, K.; Perrow, M.; Phillips, G. Progressive restoration of a shallow lake: A 12-year experiment in isolation, sediment removal and biomanipulation. J. Appl. Ecol. 1996, 33, 71–86. [Google Scholar] [CrossRef]
- Muhammetoğlu, A.; Muhammetoğlu, H.; Soyupak, S. Evaluation of efficiencies of diffuse allochtonous and autochtonous nutrient input in restoration of a highly eutrophic lake. Water Sci. Technol. 2002, 45, 195–203. [Google Scholar] [PubMed]
- Douglas, G.B. Remediation Material and Remediation Process for Sediments. U.S. Patent 6350383, 26 February 2002. [Google Scholar]
- Robb, M.; Greenop, B.; Goss, Z.; Douglas, G.; Adeney, J. Application of PhoslockTM, an innovative phosphorus binding clay, to two Western Australian waterways: Preliminary findings. Hydrobiologia 2003, 494, 237–243. [Google Scholar] [CrossRef]
- Ross, G.; Haghseresht, F.; Cloete, T.M. The effect of pH and anoxia on the performance of Phoslock®, a phosphorus binding clay. Harmful Algae 2008, 7, 545–550. [Google Scholar] [CrossRef]
- Van Oosterhout, F.; Lürling, M. The effect of phosphorus binding clay (Phoslock®) in mitigating cyanobacterial nuisance: A laboratory study on the effects on water quality variables and plankton. Hydrobiologia 2013, 710, 265–277. [Google Scholar] [CrossRef]
- Copetti, D.; Finsterle, K.; Marziali, L.; Stefani, F.; Tartari, G.; Douglas, G.; Reitzel, K.; Spears, B.M.; Winfield, I.J.; Crosa, G.; et al. Eutrophication management in surface waters using a lanthanum modified bentonite: A review. Water Res. 2016, 97, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Spears, B.M.; Lurling, M.; Yasseri, S.; Castro-Castellon, A.T.; Gibbs, M.; Meis, S.; McDonald, C.; McIntosh, J.; Sleep, D.; Van Oosterhout, F. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: An analysis of water column lanthanum data from 16 case study lakes. Water Res. 2013, 47, 5930–5942. [Google Scholar] [CrossRef] [PubMed]
- Spears, B.M.; Mackay, E.B.; Yasseri, S.; Gunn, I.D.M.; Waters, K.E.; Andrews, C.; Cole, S.; de Ville, M.; Kelly, A.; Meis, S.; et al. Lake responses following lanthanum-modified bentonite (Phoslock®) application: A meta-analysis of water quality and aquatic macrophyte responses across 18 lakes. Water Res. 2016, 97, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Meis, S.; Prochazkova, L.; Carvalho, L.; Mackay, E.B.; Woods, H.J.; Pottie, J.; Milne, I.; Taylor, C.; Maberly, S.C.; et al. Phytoplankton community responses in a shallow lake following lanthanum-bentonite application. Water Res. 2016, 97, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; van Oosterhout, F. Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res. 2013, 47, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Meis, S.; Spears, B.M.; Maberly, S.C.; O’Malley, M.B.; Perkins, R.G. Sediment amendment with Phoslock in Clatto Reservoir (Dundee, UK): Investigating changes in sediment elemental composition and phosphorus fractionation. J. Environ. Manag. 2012, 93, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Meis, S.; Spears, B.M.; Maberly, S.C.; O’Malley, M.B.; Perkins, R.G. Assessing the mode of action of Phoslock® in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK). Water Res. 2013, 47, 4460–4473. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Faassen, E.J. Controlling toxic cyanobacteria: Effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 2012, 46, 1447–1459. [Google Scholar]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M.; Waajen, G.; Van Oosterhout, F. Humic substances interfere with phosphate removal by lanthanum modified clay in controlling eutrophication. Water Res. 2014, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Dithmer, L.; Nielsen, U.G.; Lundberg, D.; Reitzel, K. Influence of dissolved organic carbon on the efficiency of P sequestration by a lanthanum modified clay. Water Res. 2016, 97, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Reitzel, K.; Andersen, F.O.; Egemose, S.; Jensen, H.S. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water. Water Res. 2013, 47, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Psenner, R.; Pucsko, R.; Sager, M. Fractionation of organic and inorganic phosphorus compounds in lake sediments. Arch. Hydrobiol. Suppl. 1984, 70, 111–155. [Google Scholar]
- Hupfer, M.; Gächter, R.; Giovanoli, R. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat. Sci. 1995, 57, 305–324. [Google Scholar] [CrossRef]
- Netherlands Normalization Institute (NEN). Water: Photometric Determination of the Content of Dissolved Orthophosphate and the Total Content of Phosphorous Compounds by Continuous Flow Analysis; NEN 6663; Netherlands Normalization Institute: Delft, The Netherlands, 1986. [Google Scholar]
- Schauser, I.; Chorus, I.; Lewandowski, J. Effects of nitrate on phosphorus release: Comparison of two Berlin lakes. Acta Hydroch. Hydrob. 2006, 34, 325–332. [Google Scholar] [CrossRef]
- Netherlands Normalization Institute (NEN). Water: Photometric Determination of the Content of Ammonium Nitrogen and the Sum of the Contents of Ammoniacal and Organically Bound Nitrogen according to Kjeldahl by Continuous Flow Analysis; NEN 6646; Netherlands Normalization Institute: Delft, The Netherlands, 1990. [Google Scholar]
- Netherlands Normalization Institute (NEN). Bepaling van het Stikstofgehalte in de Vorm van Nitriet en in de Vorm van Nitraat en de som van Beide Met Doorstroomanalyse (CFA en FIA) en Spectrometrische Detectie; NEN-EN-ISO 13395; Netherlands Normalization Institute: Delft, The Netherlands, 1997. [Google Scholar]
- Van den Brink, P.J.; van Donk, E.; Gylstra, R.; Crum, S.J.H.; Brock, T.C.M. Effects of chronic low concentrations of the pesticides chlorpyrifos and atrazine in indoor fresh-water microcosms. Chemosphere 1995, 31, 3181–3200. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Ter Braak, C.J.F. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ. Toxicol. Chem. 1999, 18, 138–148. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Verweij, W. CHEAQS, a Program for Calculating CHemical Equilibria in AQuatic Systems, PRO version (2010.3); 2010. Available online: http://www.cheaqs.eu/history.html. (accessed on 27 December 2010).
- Tipping, E. WHAM—A chemical equilibrium model and computer code for waters, sediments and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput. Geosci. 1994, 20, 973–1023. [Google Scholar] [CrossRef]
- Tipping, E.; Hurley, M.A. A unifying model of cation binding by humic substances. Geochim. Cosmochim. Acta 1992, 56, 3627–3641. [Google Scholar] [CrossRef]
- Tipping, E. Humic ion-binding model VI: An improved description of the interactions of protons and metal ions with humic substances. Aquat. Geochem. 1998, 4, 3–48. [Google Scholar] [CrossRef]
- Tipping, E.; Lofts, S.; Sonke, J.E. Humic Ion-Binding Model VII: A revised parameterisation of cation-binding by humic substances. Environ. Chem. 2011, 8, 225–235. [Google Scholar] [CrossRef]
- Tang, J.; Johannesson, K.H. Speciation of rare earth elements in natural terrestrial waters: Assessing the role of dissolved organic matter from the modeling approach. Geochim. Cosmochim. Acta 2003, 67, 2321–2339. [Google Scholar] [CrossRef]
- Pourret, O.; Davranche, M.; Gruau, G.; Dia, A. Competition between humic acid and carbonates for rare earth elements complexation. J. Colloid Interface Sci. 2007, 305, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.C.; Funk, W.H.; Lafer, J. Long-term effects of dredging on phosphorus availability from Liberty Lake sediments. Lake Reserv. Manag. 1988, 4, 293–301. [Google Scholar] [CrossRef]
- Burton, G.A., Jr. Sediment quality criteria in use around the world. Limnology 2002, 3, 65–75. [Google Scholar] [CrossRef]
- Brouwer, E.; Roelofs, J.G. M. Degraded softwater lakes: Possibilities for restoration. Restor. Ecol. 2001, 9, 155–166. [Google Scholar] [CrossRef]
- Peterson, S.A. Lake restoration by sediment removal. Water Resour. Bull. 1982, 18, 423–435. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, C.P.; Macdonald, D.D. Effects of suspended sediments on aquatic ecosystems. N. Am. J. Fish. Manag. 1991, 11, 72–82. [Google Scholar] [CrossRef]
- Poor, N.D. Effect of lake management efforts on the trophic state of a subtropical shallow lake in Lakeland, Florida, USA. Water Air Soil Pollut. 2010, 207, 333–347. [Google Scholar] [CrossRef]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the plankton ecology group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Wickham, S.A.; Gilbert, J.J. Relative vulnerabilities of natural rotifer and ciliate communities to cladocerans: Laboratory and field experiments. Freshw. Biol. 1991, 26, 77–86. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Xu, D.; Lin, J.; Cheng, S.; Wu, Z. Effects of sediment dredging on water quality and zooplankton community structure in a shallow of eutrophic lake. J. Environ. Sci. 2010, 22, 218–224. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Effects of lanthanum and lanthanum-modified clay on growth, survival and reproduction of Daphnia magna. Water Res. 2010, 44, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Sneller, F.E.C.; Kalf, D.F.; Weltje, L.; van Wezel, A.P. Maximum Permissible Concentrations and Negligible Concentrations for Rare Earth Elements (REEs); Report 601501011; RIVM: Bilthoven, The Nederlands, 5 July 2000. [Google Scholar]
- Haupt, F.; Stockenreiter, M.; Reichwaldt, E.S.; Baumgartner, M.; Lampert, W.; Boersma, M.; Stibor, H. Upward phosphorus transport by Daphnia diel vertical migration. Limnol. Oceanogr. 2010, 55, 529–534. [Google Scholar] [CrossRef]
- Santos-Magalhaes, I.; Lürling, M.; Roijackers, R.M.M. Vertical distribution of Daphnia in Lake Berendonck (The Netherlands) during progressive hypolimnion oxygen depletion. Verh. Int. Ver. Theor. Angew. Limnol. 2005, 29, 273–278. [Google Scholar]
- Kirk, K.L.; Gilbert, J.J. Suspended clay and the population dynamics of planktonic rotifers and cladocerans. Ecology 1990, 71, 1741–1755. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, C.; Liu, J.; Weng, Y.; Li, H.; Zhang, D. Assessing the impacts of phosphorus inactive clay on phosphorus release control and phytoplankton community structure in eutrophic lakes. Environ. Pollut. 2016, 219, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterhout, J.F.X.; Lürling, M. ffects of the novel ‘Flock & Lock’ lake restoration technique on Daphnia in Lake Rauwbraken (The Netherlands). J. Plankton Res. 2011, 33, 255–263. [Google Scholar]
- Epe, T.S.; Finsterle, K.; Yasseri, S. Nine Years of Phosphorus Management with Lanthanum Modified Bentonite (Phoslock) in a Eutrophic, Shallow Swimming Lake in Germany. Lake Reserv. Manag. 2017. [Google Scholar] [CrossRef]
- Stauber, J.L.; Binet, M.T. Canning River Phoslock Field Trial—Ecotoxicity Testing Final Report; Report No.: ET317R; Centre for Advanced Analytical Chemistry Energy Technology, CSIRO: Bangor, Australia, 2000; p. 52. [Google Scholar]
- Hu, X.; Ding, Z.; Wang, X.; Chen, Y.; Dai, L. Effects of lanthanum and cerium on the vegetable growth of wheat (Triticum aestivum L.) seedlings. Bull. Environ. Contam. Toxicol. 2002, 69, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Waajen, G.; van Oosterhout, F.; Douglas, G.; Lürling, M. Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant—Lanthanum modified bentonite treatment. Water Res. 2016, 97, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Waajen, G.; van Oosterhout, F.; Douglas, G.; Lürling, M. Geo-engineering experiments in two urban ponds to control eutrophication. Water Res. 2016, 97, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, M.; Jeppesen, E.; Lauridsen, T.L.; Skov, C.; Van Nes, E.H.; Roijackers, R.; Lammens, E.; Portielje, R. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2007, 44, 1095–1105. [Google Scholar] [CrossRef]
- Gibbs, M.M.; Hickey, C.W.; Özkundakci, D. Sustainability assessment and comparison of efficacy of four P-inactivation agents for managing internal phosphorus loads in lakes: Sediment incubations. Hydrobiologia 2011, 658, 253–275. [Google Scholar] [CrossRef]
- Dithmer, L.; Lipton, A.S.; Reitzel, K.; Warner, T.E.; Lundberg, D.; Nielsen, U.G. Characterization of phosphate sequestration by a lanthanum modified bentonite clay: A solid-state NMR, EXAFS and PXRD study. Environ. Sci. Technol. 2015, 49, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Sonke, J.E.; Salters, V.J.M. Lanthanide–humic substances complexation. I. Experimental evidence for a lanthanide contraction effect. Geochim. Cosmochim. Acta 2006, 70, 1495–1506. [Google Scholar] [CrossRef]
- Tang, J.; Johannesson, K.H. Ligand extraction of rare earth elements from aquifer sediments: Implications for rare earth element complexation with organic matter in natural waters. Geochim. Cosmochim. Acta 2010, 74, 6690–6705. [Google Scholar] [CrossRef]
- Weng, L.; van Riemsdijk, W.H.; Hiemstra, T. Humic nanoparticles at the oxide-water interface: Interactions with phosphate ion adsorption. Environ. Sci. Technol. 2008, 42, 8747–8752. [Google Scholar] [CrossRef] [PubMed]
- Dance, K.W. The role of the neighbourhood in rehabilitation of an urban aquatic ecosystem. Water Qual. Res. J. Can. 1997, 32, 245–256. [Google Scholar]
- Kosten, S.; Huszar, V.L.M.; Mazzeo, N.; Scheffer, M.; Sternberg, L.D.S.L.; Jeppesen, E. Lake and watershed characteristics rather than climate influence nutrient limitation in shallow lakes. Ecol. Appl. 2009, 19, 1791–1804. [Google Scholar] [CrossRef] [PubMed]


| Secchi-Depth | Turbidity | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 8.0 | 81.017 | <0.001 | 8 | 57.819 | <0.001 |
| Time × treatments | 24.0 | 9.211 | <0.001 | 24 | 5.351 | <0.001 |
| Error | 64.0 | 64 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 26.6 | <0.001 | 3 | 5.165 | 0.028 |
| Error | 8 | 8 | ||||
| pH | Conductivity | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 2.66 | 12.942 | <0.001 | 2.72 | 128.126 | <0.001 |
| Time × treatments | 7.98 | 1.576 | 0.191 | 8.19 | 6.576 | <0.001 |
| Error | 21.3 | 53.8 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 4.284 | 0.044 | 3 | 5.395 | 0.025 |
| Error | 8 | 8 | ||||
| Oxygen Concentration | Temperature | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 2.49 | 22.606 | <0.001 | 6.46 | 1794.29 | <0.001 |
| Time × treatments | 7.46 | 4.533 | 0.003 | 19.4 | 3.130 | 0.001 |
| Error | 19.89 | 64 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 2.465 | 0.137 | 3 | 3.706 | 0.061 |
| Error | 8 | 8 | ||||
| Filterable Aluminium (Al) | Total Aluminium | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 2.35 | 8.956 | 0.001 | 8 | 99.516 | <0.001 |
| Time × treatments | 7.05 | 4.674 | 0.003 | 24 | 6.958 | <0.001 |
| Error | 18.8 | 64 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 3.291 | 0.079 | 3 | 5.914 | 0.020 |
| Error | 8 | 8 | ||||
| Filterable Iron (Fe) | Total Iron | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 2.71 | 3.941 | 0.025 | 6.48 | 62.694 | <0.001 |
| Time × treatments | 8.12 | 3.578 | 0.008 | 19.4 | 5.511 | <0.001 |
| Error | 21.7 | 51.8 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 1.616 | 0.261 | 3 | 5.367 | 0.026 |
| Error | 8 | 8 | ||||
| Filterable Lanthanum (La) | Total Lanthanum | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 2.08 | 17.203 | <0.001 | 2.53 | 86.011 | <0.001 |
| Time × treatments | 6.23 | 6.129 | 0.001 | 7.60 | 6.703 | <0.001 |
| Error | 16.6 | 20.3 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 407.8 | <0.001 | 3 | 337.9 | <0.001 |
| Error | 8 | 8 | ||||
| Filterable sulfur (S) | Total sulfur | |||||
| Tests of within-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 7.06 | 8.492 | <0.001 | 2.70 | 20.148 | <0.001 |
| Time × treatments | 21.2 | 10.044 | <0.001 | 8.11 | 12.463 | <0.001 |
| Error | 56.5 | 21.6 | ||||
| Tests of between-subjects effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 3 | 22.358 | <0.001 | 3 | 22.239 | <0.001 |
| Error | 8 | 8 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waajen, M.L.G.; Engels, B.; Oosterhout, F.V. Effects of Dredging and Lanthanum-Modified Clay on Water Quality Variables in an Enclosure Study in a Hypertrophic Pond. Water 2017, 9, 380. https://doi.org/10.3390/w9060380
Waajen MLG, Engels B, Oosterhout FV. Effects of Dredging and Lanthanum-Modified Clay on Water Quality Variables in an Enclosure Study in a Hypertrophic Pond. Water. 2017; 9(6):380. https://doi.org/10.3390/w9060380
Chicago/Turabian StyleWaajen, Miquel Lürling Guido, Bart Engels, and Frank Van Oosterhout. 2017. "Effects of Dredging and Lanthanum-Modified Clay on Water Quality Variables in an Enclosure Study in a Hypertrophic Pond" Water 9, no. 6: 380. https://doi.org/10.3390/w9060380