Performance of Denitrifying Bioreactors at Reducing Agricultural Nitrogen Pollution in a Humid Subtropical Coastal Plain Climate
Abstract
:1. Introduction
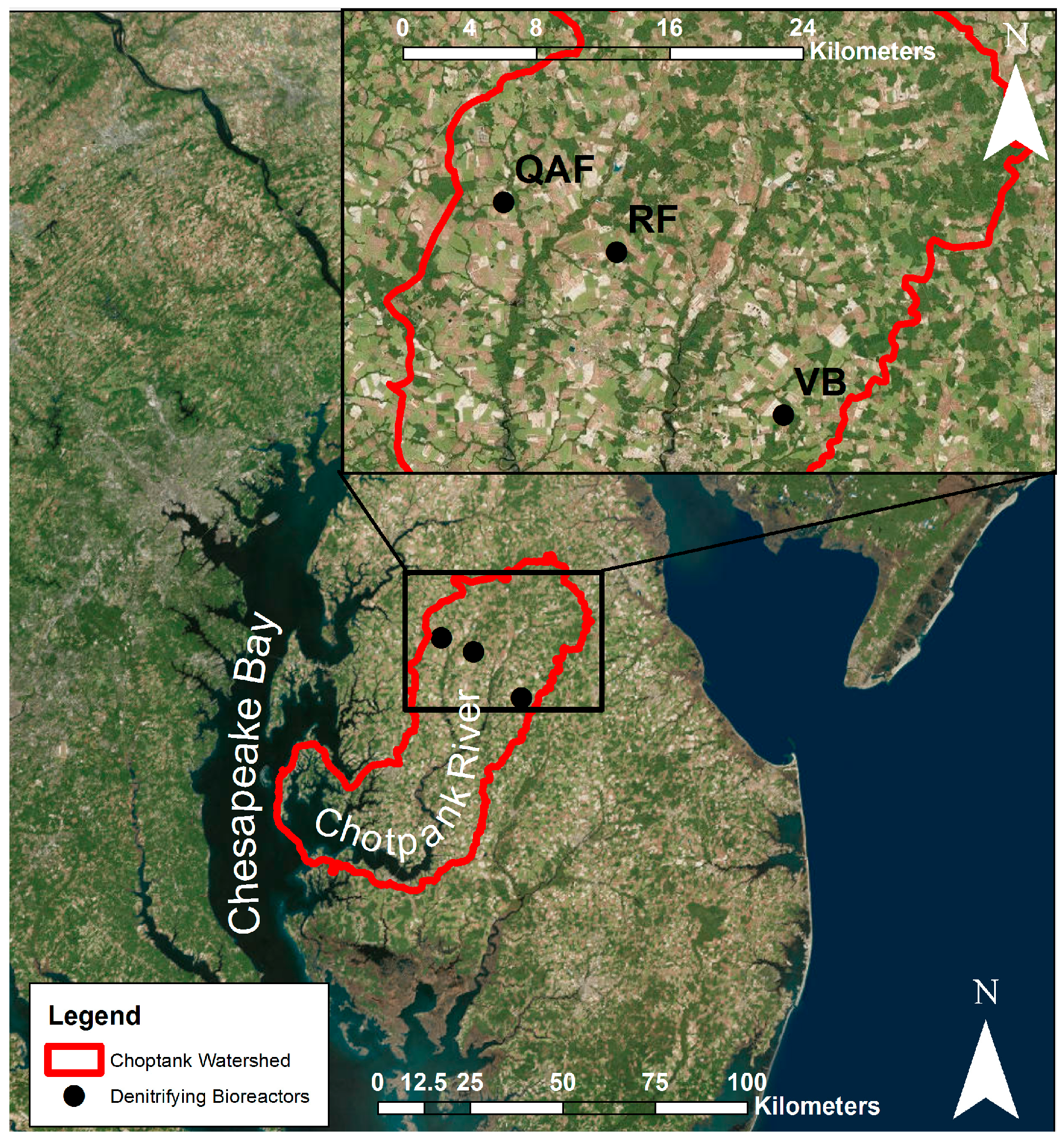
2. Materials and Methods
2.1. Ridgely Farm (RF)
2.2. Queen Anne Farm (QAF)
2.3. Voorhees Farm
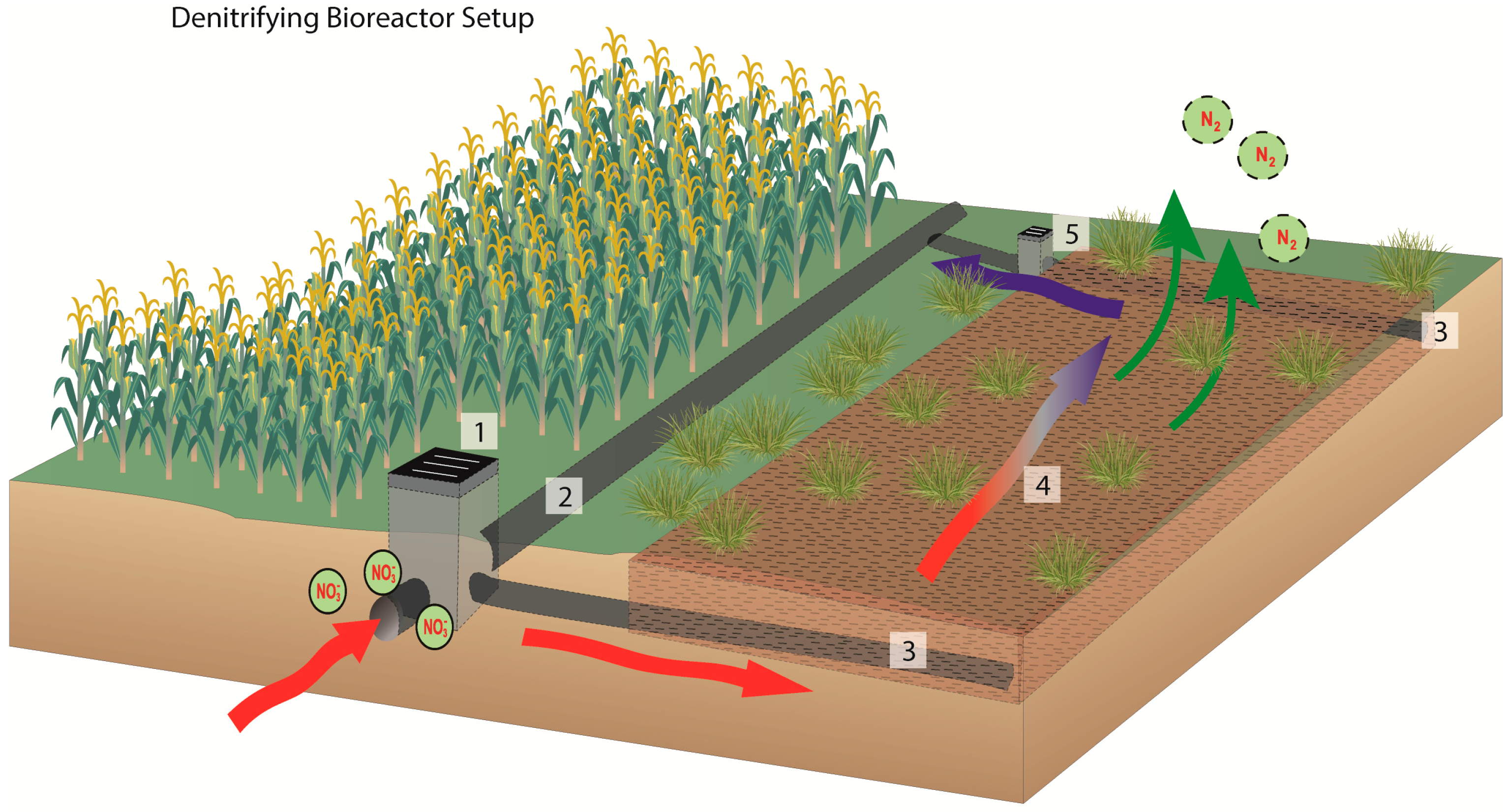
2.4. Bioreactor Sampling and Analysis
3. Results and Discussion
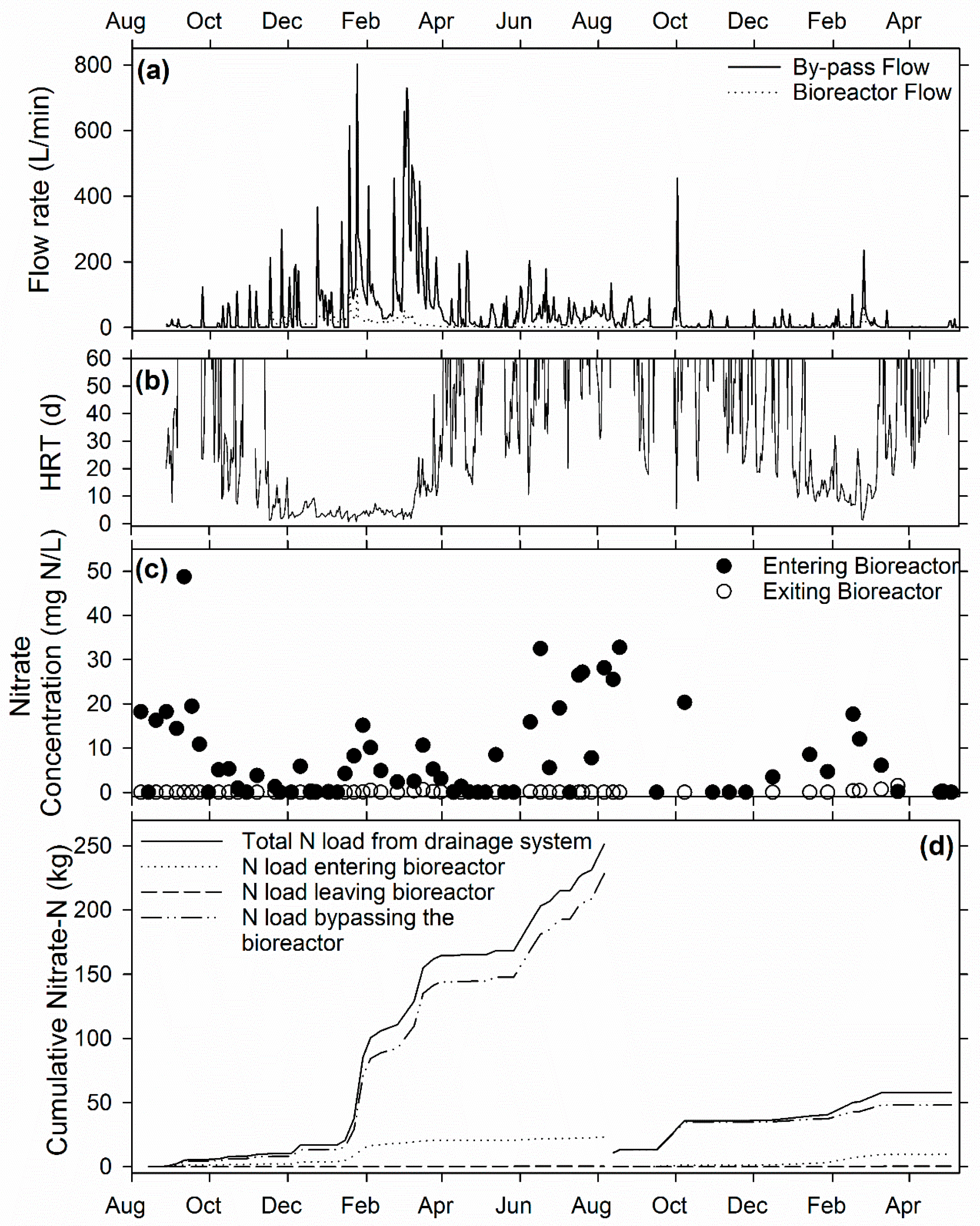
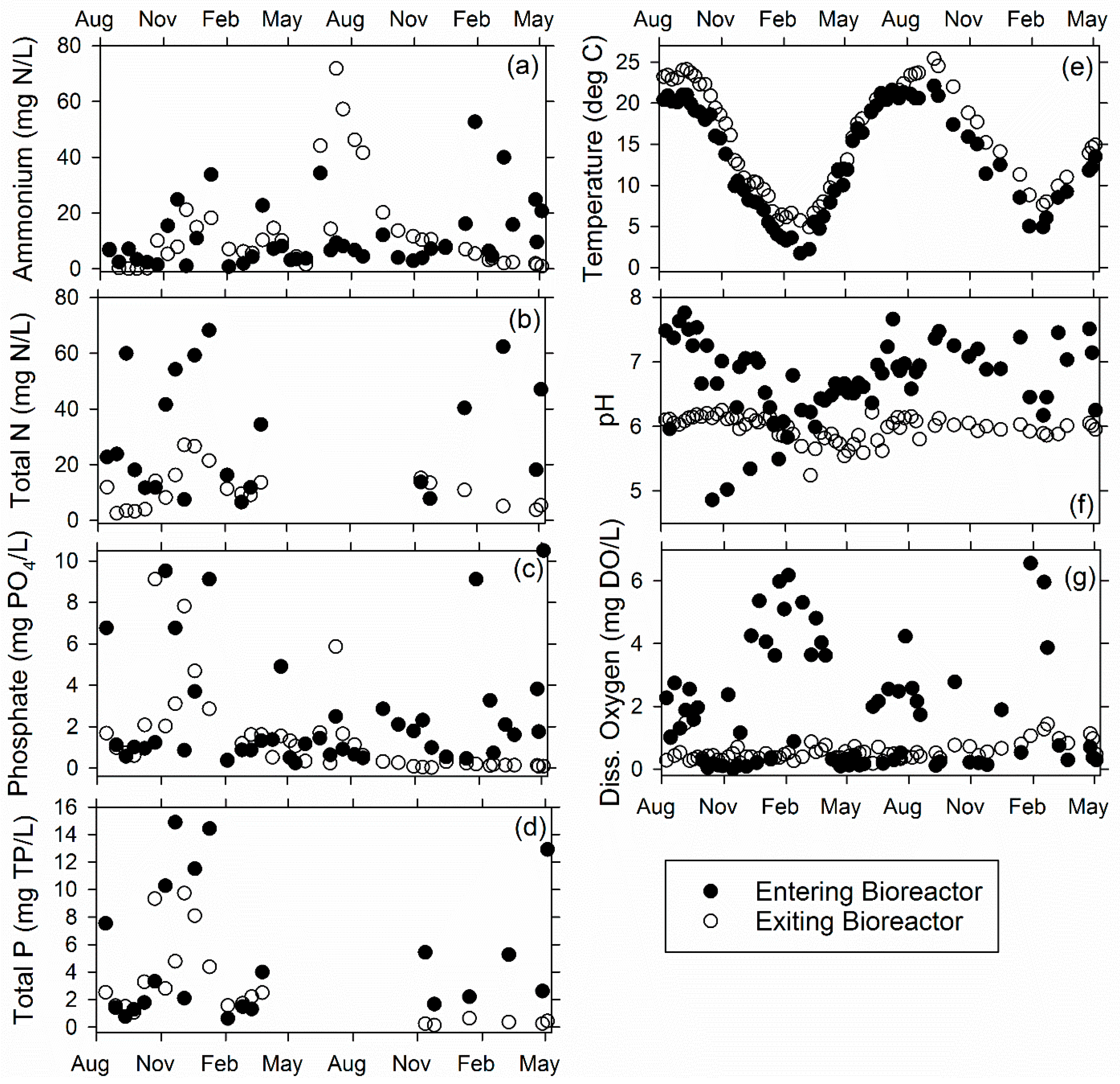
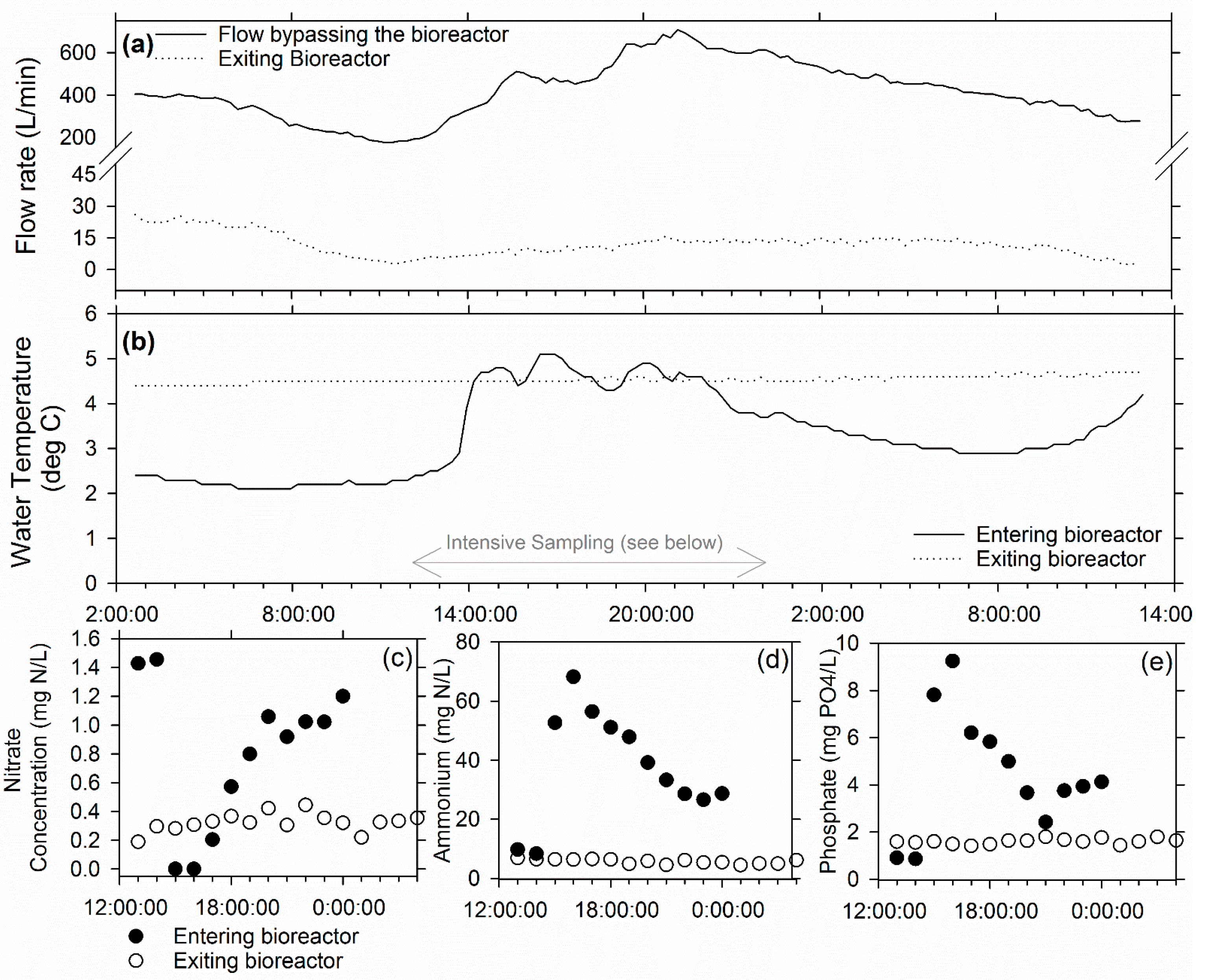
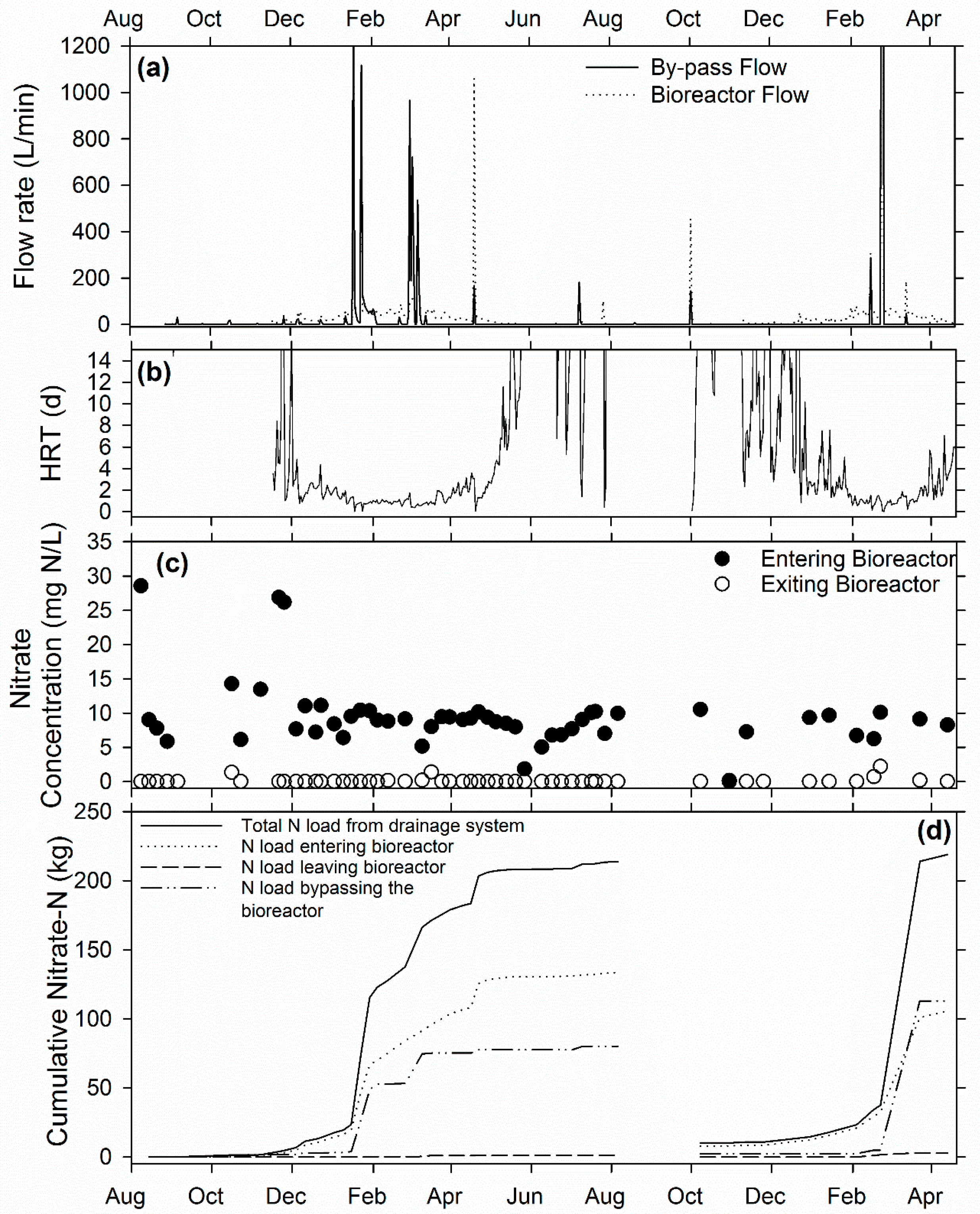
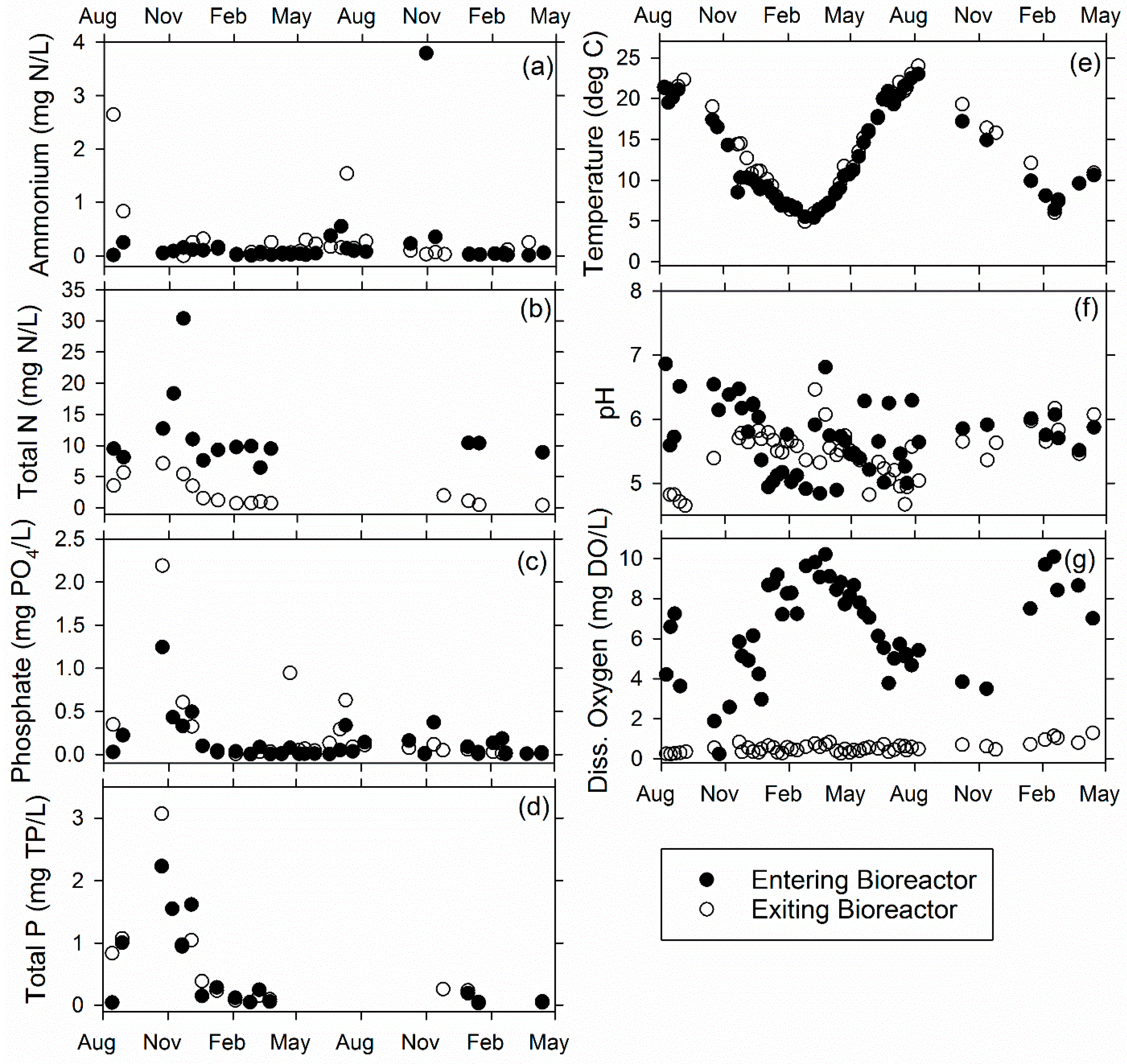
3.1. Ridgely Farm Bioreactor
3.2. Queen Anne Farm Bioreactor
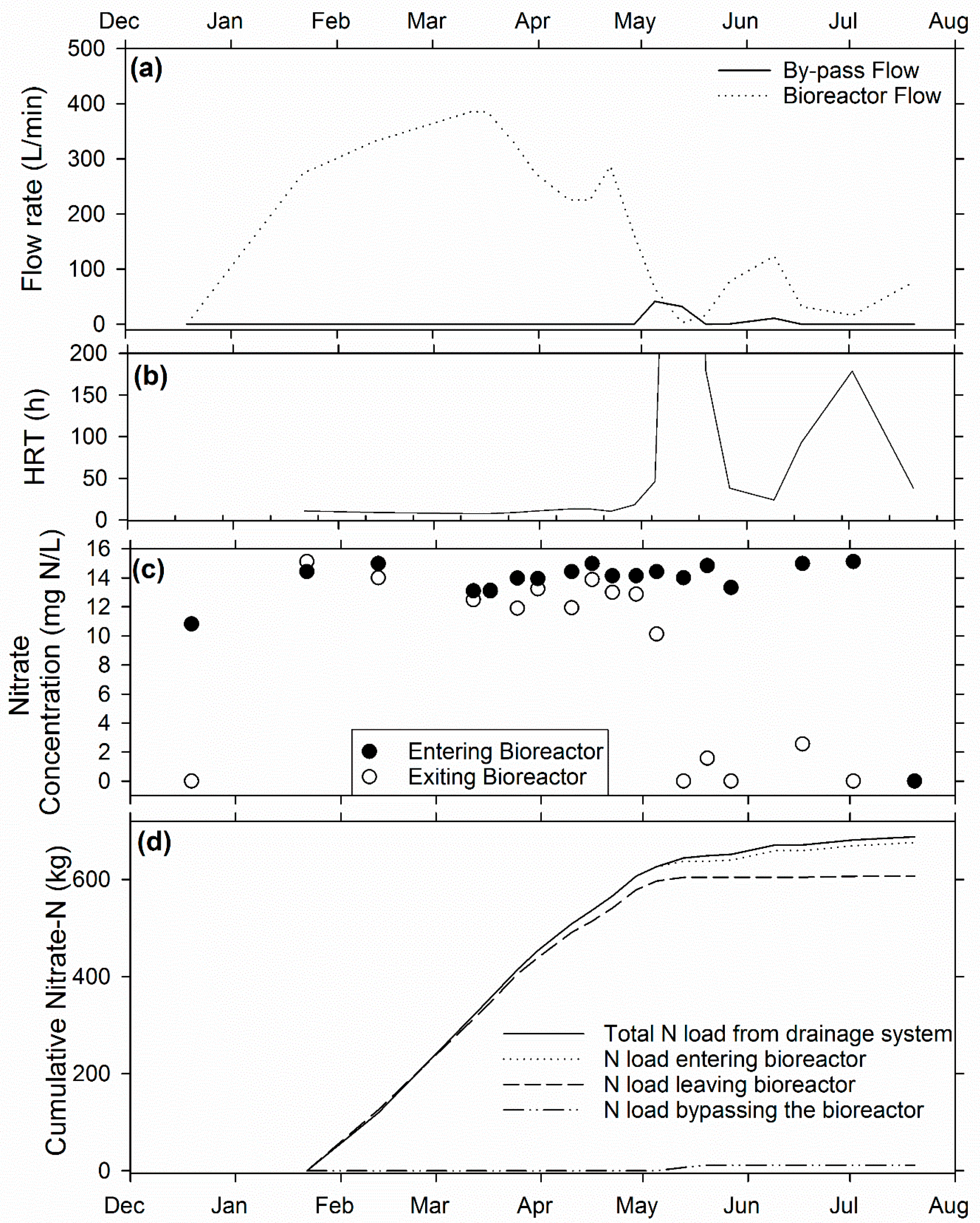
3.3. Voorhees Farm Bioreactor (VB)
3.4. Overall Bioreactor Performance and Comparison
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christianson, L.E.; Bhandari, A.; Helmers, M.J. A practice-oriented review of woodchip bioreactors for subsurface agricultural drainage. Appl. Eng. Agric. 2012, 28, 861–874. [Google Scholar] [CrossRef]
- Schipper, L.A.; Robertson, W.D.; Gold, A.J.; Jaynes, D.B.; Cameron, S.C. Denitrifying bioreactors: An approach for reducing nitrate loads to receiving waters. Ecol. Eng. 2010, 36, 1532–1543. [Google Scholar] [CrossRef]
- Bell, W.H.; Favero, P. Moving Water a Report to the Chesapeake Bay Cabinet. Public Drainage Task Force, 2000. Available online: http://www.dnr.state.md.us/irc/docs/00003588.pdf (accessed on 26 January 2015).
- Cahill, B. Tax Ditches and Their Role in the Development Process. Drainage Program, Sussex County March 2006. Available online: http://www.dnrec.state.de.us/dnrec2000/Divisions/Soil/stormwater/CCRCourse/05-Tax%20Ditches%20and%20construction_BPC.pdf (accessed on 7 December 2016).
- Keppler, J. Agricultural Ditch Management on Maryland’s Eastern Shore. Roadside Ditch Management Workshop; Chesapeake Bay Program Scientific and Technical Advisory Committee Workshop: Easton, MD, USA, 2014; Available online: http://www.chesapeake.org/stac/presentations/235_Keppler%20Ditch%20Workshop.pdf (accessed on 6 July 2015).
- Cushing, E.M.; Kantrowitz, I.H.; Taylor, K.R. Water resources of the Delmarva Peninsula. U.S Geological Survey Professional Paper 822; 1973; pp. 1–58. Available online: https://pubs.usgs.gov/pp/0822/report.pdf (accessed on 25 November 2016). [Google Scholar]
- Denver, J.M.; Ator, S.W.; Debrewer, L.M.; Ferrari, M.J.; Barbaro, J.R.; Hancock, T.C.; Brayton, M.J.; Nardi, M.R. Water Quality in the Delmarva Peninsula, Delaware, Maryland, and Virginia, 1999–2001; U.S. Geological Survey Circular 1228; U.S. Geological Survey: Reston, VA, USA, 2004; pp. 1–26.
- Follett, J.R.; Follett, R.F. Utilization and metabolism of nitrogen by humans. In Nitrogen in the Environment: Sources, Problems and Management; Follett, R., Hatfield, J.L., Eds.; Elsevier Science: New York, NY, USA, 2001; pp. 65–92. [Google Scholar]
- Maryland Department of the Environment (MDE). Maryland’s Phase II Watershed Implementation Plan for the Chesapeake Bay TMDL. Document Version; 26 October 2012. Available online: http://www.mde.state.md.us/programs/Water/TMDL/TMDLImplementation/Documents/FINAL_PhaseII_Report_Docs/Final_Documents_PhaseII/Final_Phase_II_WIP_MAIN_REPORT_102612.pdf (accessed on 11 November 2016). [Google Scholar]
- Flewelling, S.A.; Herman, J.S.; Hornberger, G.M.; Mills, A.L. Travel time controls the magnitude of nitrate discharge in groundwater bypassing the riparian zone to a stream on Virginia’s coastal plain. Hydrol. Process. 2011, 26, 1242–1253. [Google Scholar] [CrossRef]
- Christianson, L.E.; Schipper, L.A. Moving denitrifying bioreactors beyond proof of concept: Introduction to the special section. J. Environ. Qual. 2016, 45, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Bhattarai, R.; Goodwin, G.; Cooke, R.; Chun, J.A. Evaluation of Conservation Drainage Systems in Illinois: Bioreactors; ASABE Paper No. 10098942010; ASABE: St. Joseph, MI, USA, 2010. [Google Scholar]
- Woli, K.P.; David, M.B.; Cooke, R.A.; McIssac, G.F.; Mitchell, C.A. Nitrogen balance in and export from agricultural fields associated with controlled drainage systems and denitrifying bioreactors. Ecol. Eng. 2010, 36, 1558–1566. [Google Scholar] [CrossRef]
- Iowa Department of Agriculture and Land Stewardship (IDALS). Iowa Nutrient Reduction Strategy: A Science and Technology-Based Framework to Assess and Reduce Nutrients to Iowa Waters and the Gulf of Mexico; Iowa Department of Agriculture and Land Stewardship, Iowa Department of Natural Resources, and Iowa State University: Des Moines, IA, USA, 2014.
- IDOA. Illinois Nutrient Loss Reduction Strategy. Illinois Department of Agriculture and Illinois Environmental Protection Agency: Springfield, IL, USA, 2015. Available online: http://www.epa.illinois.gov/Assets/iepa/water-quality/watershed-management/nlrs/nlrs-final-revised-083115.pdf (accessed on 18 January 2017). [Google Scholar]
- Christianson, L.; Bhandari, A.; Helmers, M.; Kult, K.; Sutphin, T.; Wolf, R. Performance evaluation of four field-scale agricultural drainage denitrification bioreactors in Iowa. Trans. ASABE 2012, 55, 2163–2174. [Google Scholar] [CrossRef]
- Addy, K.; Gold, A.J.; Christianson, L.E.; David, M.B.; Schipper, L.A.; Ratigan, N.A. Denitrifying Bioreactors for Nitrate Removal: A Meta-Analysis. J. Environ. Qual. 2016, 45, 873–881. [Google Scholar] [CrossRef] [PubMed]
- David, M.B.; Gentry, L.E.; Cooke, R.A.; Herbstritt, S.M. Temperature and Substrate Control Woodchip Bioreactor Performance in Reducing Tile Nitrate Loads in East-Central Illinois. J. Environ. Qual. 2016, 45, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Community Collaborative Rain, Hail, and Snow Network (CoCoRaHS). Available online: http://www.cocorahs.org/state.aspx?state=md (accessed on 12 November 2016).
- Easton, Z.M.; (Virginia Tech University). Personal communication, 2013.
- Christianson, L.; Christianson, R.; Helmers, M.; Pederson, C.; Bhandari, A. Modeling and Calibration of Drainage Denitrification Bioreactor Design Criteria. J. Irrig. Drain. Eng. 2013, 139, 699–709. [Google Scholar] [CrossRef]
- Iowa United States Department of Agriculture Natural Resource Conservation Service (NRCS). IADentrifyingBioreactor.xlsx v1.0. Amiable online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/ia/technical/engineering/?cid=nrcs142p2_008213 (accessed on 15 November 2015).
- Wang, X.; Frankenberger, J.R.; Kladivko, E.J. Estimating nitrate-N losses from subsurface drains using variable water sampling frequencies. Trans. ASABE 2003, 464, 1033–1040. [Google Scholar] [CrossRef]
- Partheban, C. Demonstrating the Effectiveness of Nitrate-Nitrogen Removal of Denitrifying Bioreactors in South Dakota for Improved Drainage Water Management. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 2014. [Google Scholar]
- Chun, J.A.; Cooke, R.A. Calibrating AgriDrain Water Level Control Structures Using Generalized Weir and Orifice Equations. Appl. Eng. Agric. 2008, 24, 595–602. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Ambient Water Quality Criteria Recommendations Information Supporting the Development of State and Tribal Nutrient Criteria Rivers and Streams in Nutrient Ecoregion XIV. EPA 822-B-00-022; 2000; pp. 1–84. Available online: https://www.epa.gov/sites/production/files/documents/rivers14.pdf (accessed on 7 December 2016). [Google Scholar]
- Sharrer, K.; Christianson, L.E.; Lepine, C.; Summerfelt, S.T. Modeling and mitigation of denitrification ‘woodchip’ bioreactor phosphorus releases during treatment of aquaculture wastewater. Ecol. Eng. 2016, 93, 135–143. [Google Scholar] [CrossRef]
| Bioreactor | Location | Installation Date | Farm Details | Soil Types (% of Catchment) † | Drainage Treatment Area (ha) | Length × Width × Depth (m) |
|---|---|---|---|---|---|---|
| Ridgely Farm (RF) | Caroline Co., MD | November 2013 | 700 head dairy; triticale (×Triticosecale) and feed corn (Zea mays) | Sassafras sandy loam (47.2), Fallsington loam (27.5), Hambrook loam (16.9), Woodstown sandy loam (4.4), Lenni Loam (4.0) | 34.7 ha | 30.5 × 6.1 × 0.91 |
| Queen Anne Farm (QAF) | Queen Anne’s Co., MD | December 2013 | Organic rotation of corn (Zea mays), winter wheat (Triticum aestivum) or barley (Hordeum vulgare), and soybeans (Glycine max) | Ingleside sandy loam (29.5), Unicorn-Sassafras loam (24.6), Carmichael loam (9.8), Hammonton sandy loam (9.8), Whitemarsh silt loam (8.2), Fallsington loam (6.6), Pineyneck silt loam (6.5), Mattapex-Betterton silt loam (4.9) | 25.2 ha | 26 × 4.6 × 0.76 |
| Voorhees Farm | Caroline Co., MD | November 2014 | Rotation of corn (Zea mays), winter wheat (Triticum aestivum) and soybeans (Glycine max) | Sassafras sandy loam (32.1), Hambrook sandy loam (23.1), Fallsington sandy loam (19.8), Woodstown sandy loam (15.9), Ingleside sandy loam (3.6), Ingleside loamy sand (3.6), Woodstown loam (2.0) | 40.1 ha | 30.5 × 9.1 × 0.91 |
| Flow | Bioreactor | Total (Including Bypass Flow) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Volume from Field | Percent Treated in Bioreactor | Flow-Weighted Concentration: IN | Flow-Weighted Concentration: OUT | Nitrate Load: IN | Nitrate Load: OUT | Nitrate Removal Efficiency | Nitrate Removal Rate † | Nitrate Load: IN | Nitrate Load: OUT | Nitrate Removal Efficiency | |
| m3 | % | mg NO3-N/L | kg N | % | g N Removed per m3 Bioreactor per day | kg N | % | ||||
| Ridgely Farm | |||||||||||
| 8 August 2014–6 August 2015 | 37,000 | 13% | 4.65 | 0.06 | 23 | 0.3 | 99% | 0.40 | 251 | 229 | 9.0% |
| 6 August 2015–4 May 2016 ‡ | 5860 | 21% | 7.64 | 0.30 | 9.6 | 0.4 | 96% | 0.21 | 58 | 48 | 16% |
| Queen Anne Farm | |||||||||||
| 8 August 2014–6 August 2015 | 24,400 | 59% | 9.22 | 0.08 | 134 | 1.1 | 99% | 5.36 | 214 | 81 | 62% |
| 6 August 2015–13 April 2015 ‡ | 24,800 | 50% | 8.60 | 0.23 | 106 | 2.9 | 97% | 5.12 | 219.0 | 115.92 | 47% |
| Voorhees Farm | |||||||||||
| 19 December 2104–20 July 2015 ‡ | 49,700 | 98% | 13.46 | 11.57 | 677 | 607 | 10% | 1.53 | 688 | 618 | 10% |
| Nitrate-N | Ammonium | Total Nitrogen | Orthophosphate | Total Phosphorus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg N/L | mg P/L | |||||||||
| Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | |
| Ridgely Farm | 8.17 ± 10.5 (66) † | 0.07 ± 0.2 (66) † | 11.25 ± 11.8 (41) | 12.78 ± 16.3 (41) | 30.3 ± 20.9 (21) † | 11.2 ± 7.2 (21) † | 2.52 ± 2.8 (41) † | 1.43 ± 2.1 (41) † | 5.08 ± 4.8 (21) | 2.81 ± 2.9 (21) |
| Queen Anne Farm | 9.49 ± 5.0 (52) † | 0.12 ± 0.4 (53) † | 0.22 ± 0.7 (32) | 0.26 ± 0.5 (32) | 11.5 ± 5.9 (15) † | 2.33 ± 2.2 (15) † | 0.15 ± 0.2 (32) | 0.21 ± 0.4 (32) | 0.57 ± 0.7 (15) | 0.57 ± 0.8 (15) |
| Temperature | Dissolved Oxygen | Specific Conductance | pH | Oxidation Reduction Potential | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| °C | mg DO/L | μs/cm | mV | |||||||
| Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | |
| Ridgely Farm | 13.3 ± 6.4 † | 15.6 ± 6.3 † | 1.8 ± 1.9 | 0.5 ± 0.3 | 655 ± 386 | 697 ± 239 | 6.7 ± 0.6 † | 6.0 ± 0.2 † | −8.3 ± 95 | −15 ± 36 |
| Queen Anne Farm | 12.7 ± 5.6 | 13.5 ± 5.8 | 6.6 ± 2.4 † | 0.5 ± 0.2 † | 223 ± 121 | 219 ± 87 | 5.6 ± 1.0 † | 5.4 ± 0.5 † | 126 ± 76 † | −11 ± 60 † |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosen, T.; Christianson, L. Performance of Denitrifying Bioreactors at Reducing Agricultural Nitrogen Pollution in a Humid Subtropical Coastal Plain Climate. Water 2017, 9, 112. https://doi.org/10.3390/w9020112
Rosen T, Christianson L. Performance of Denitrifying Bioreactors at Reducing Agricultural Nitrogen Pollution in a Humid Subtropical Coastal Plain Climate. Water. 2017; 9(2):112. https://doi.org/10.3390/w9020112
Chicago/Turabian StyleRosen, Timothy, and Laura Christianson. 2017. "Performance of Denitrifying Bioreactors at Reducing Agricultural Nitrogen Pollution in a Humid Subtropical Coastal Plain Climate" Water 9, no. 2: 112. https://doi.org/10.3390/w9020112




