Atmospheric and Surface-Condition Effects on CO2 Exchange in the Liaohe Delta Wetland, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. EC Measurements
2.3. Abiotic Measurements
2.4. Meteorological Data
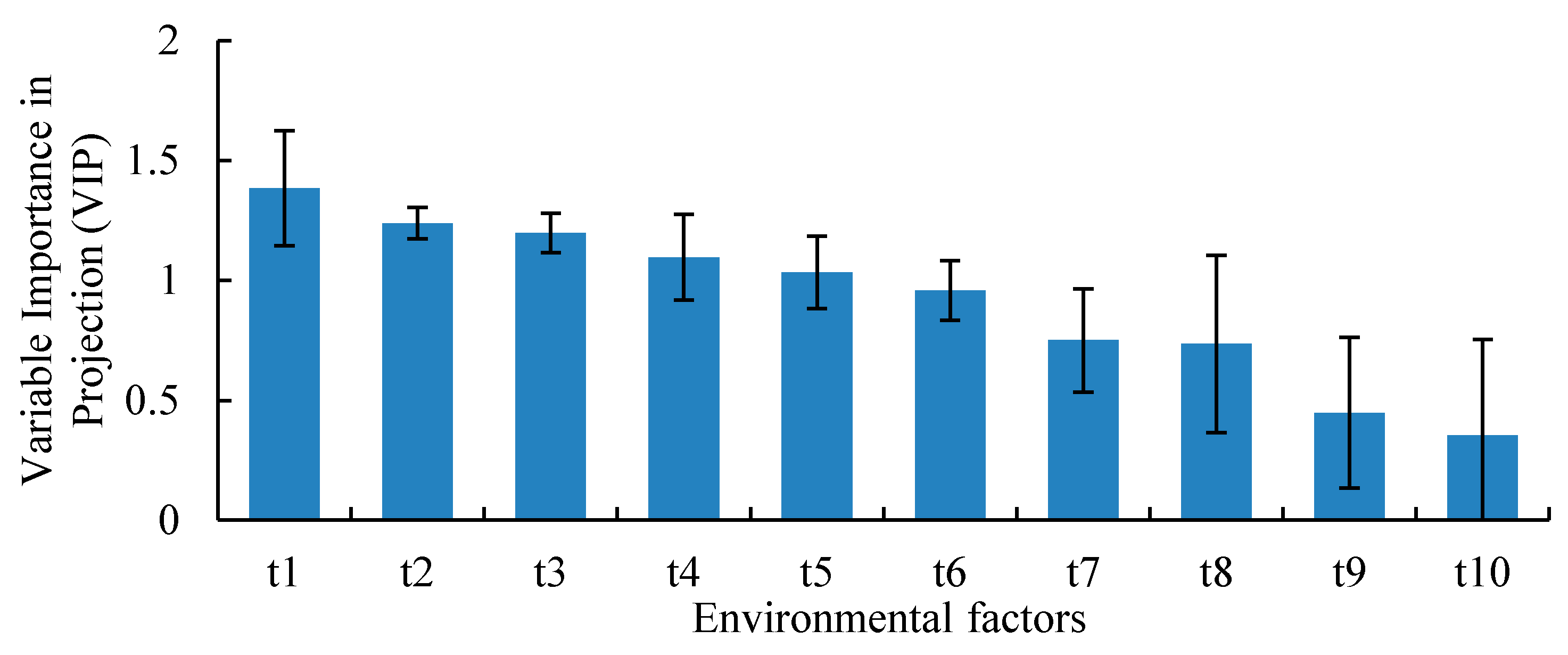
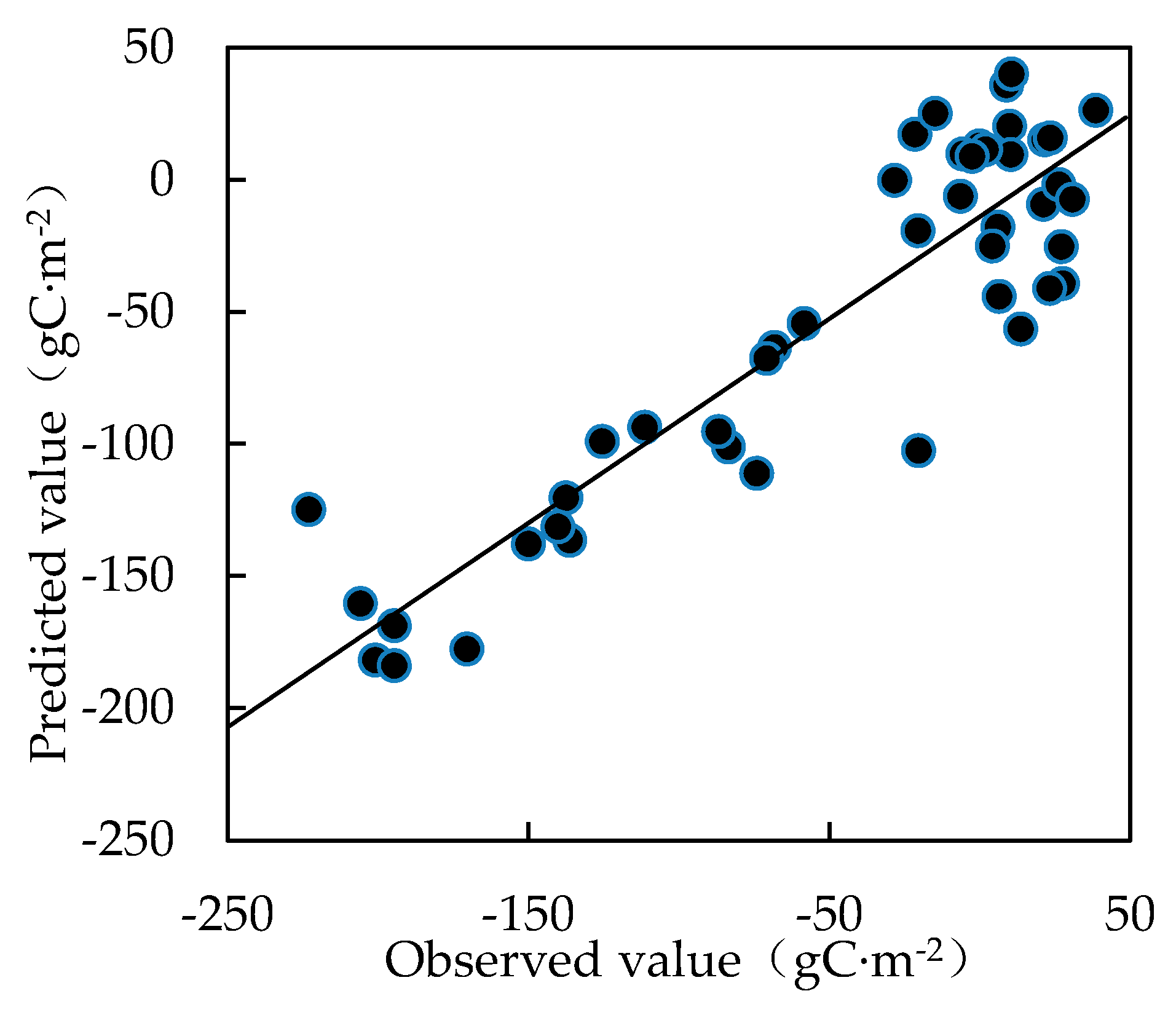
2.5. Partial Least Squares Regression Analysis
3. Results
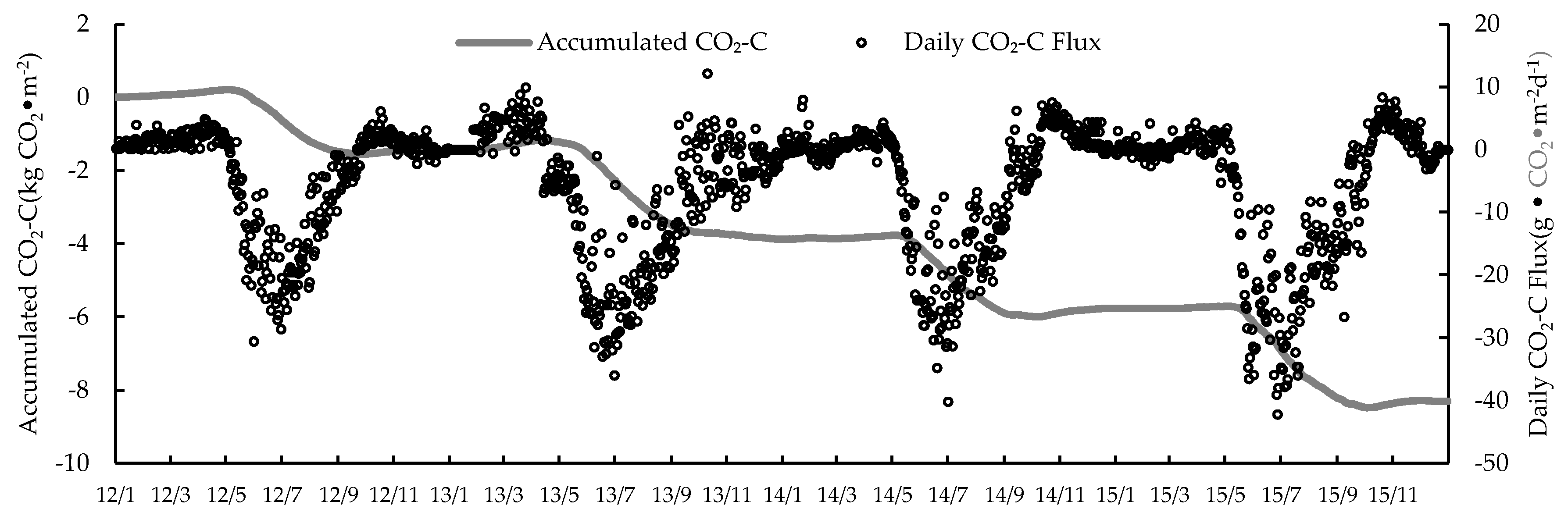
3.1. Seasonal and Inter-Annual Variation of the CO2 Exchange
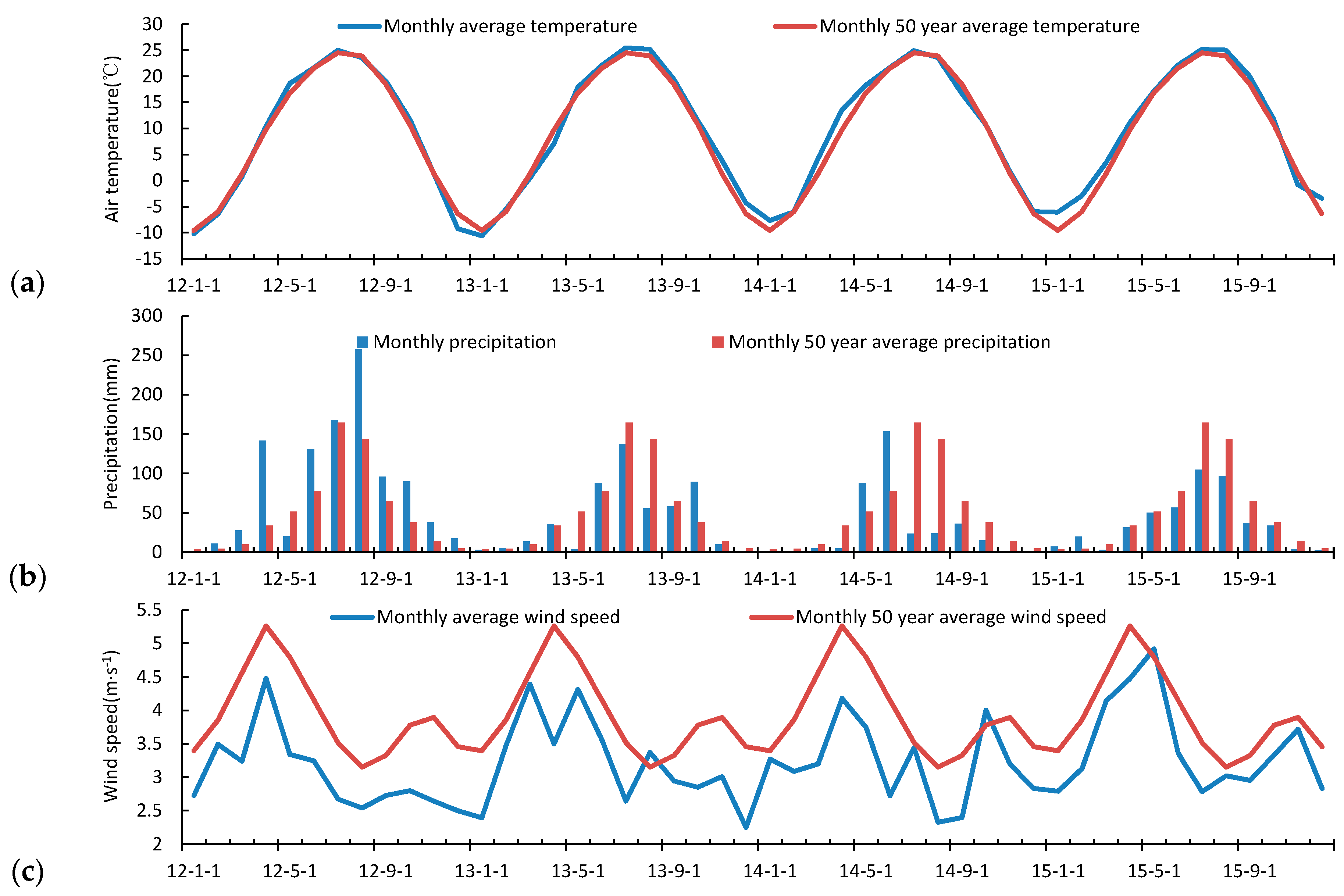
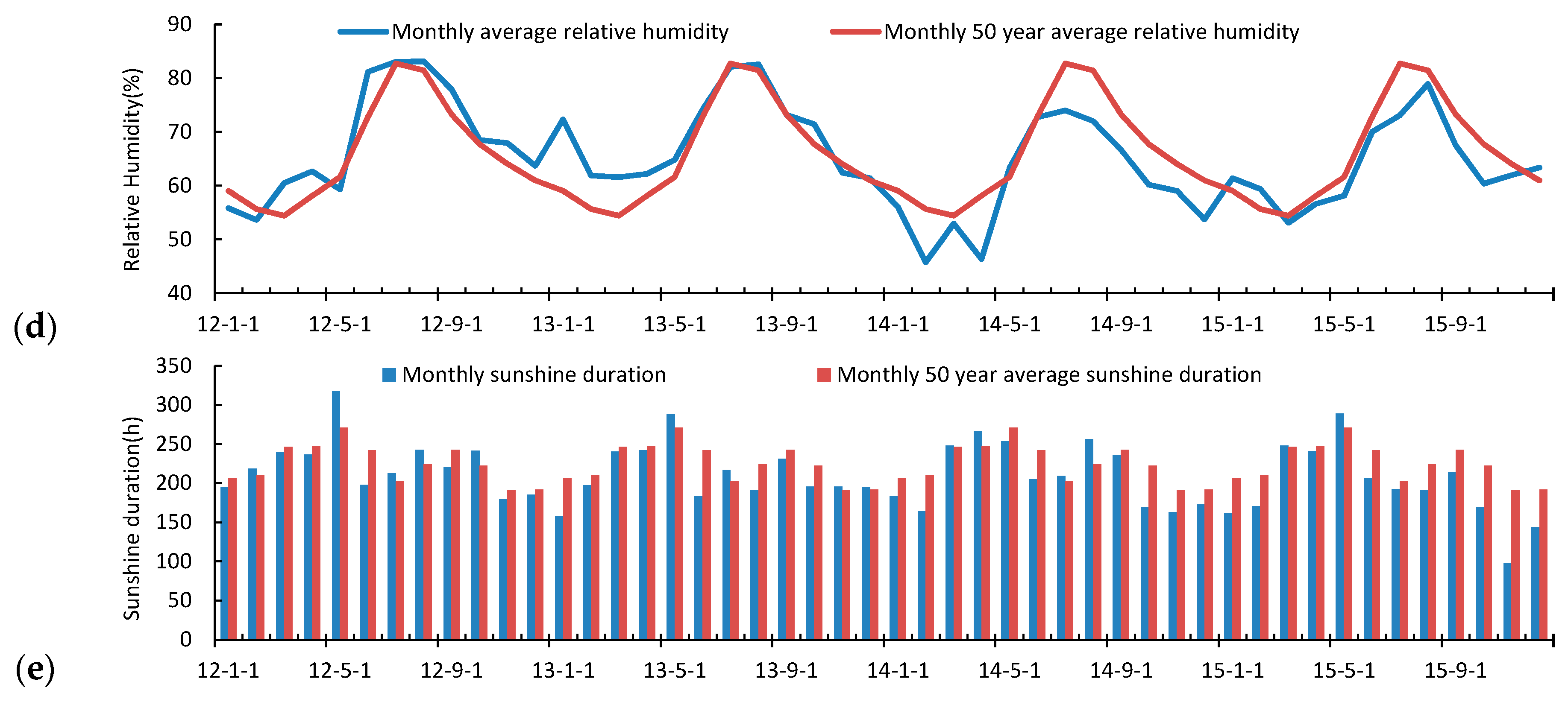
3.2. Seasonal and Inter-Annual Variation of the Atmospheric Measurements
3.3. Seasonal and Inter-Annual Variation of Phragmites Communis
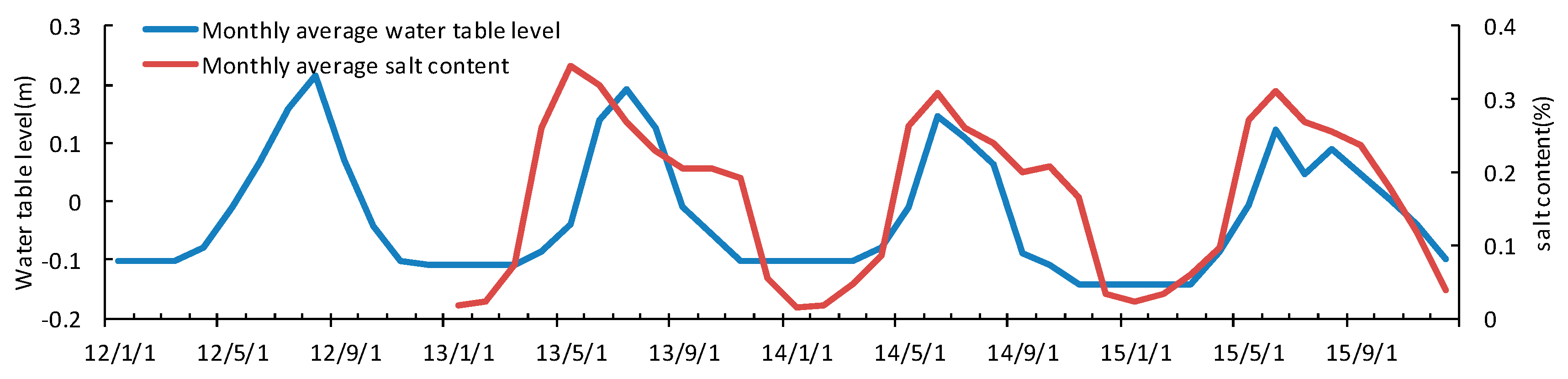
3.4. Seasonal and Inter-Annual Variation of Soil Water and Salinity
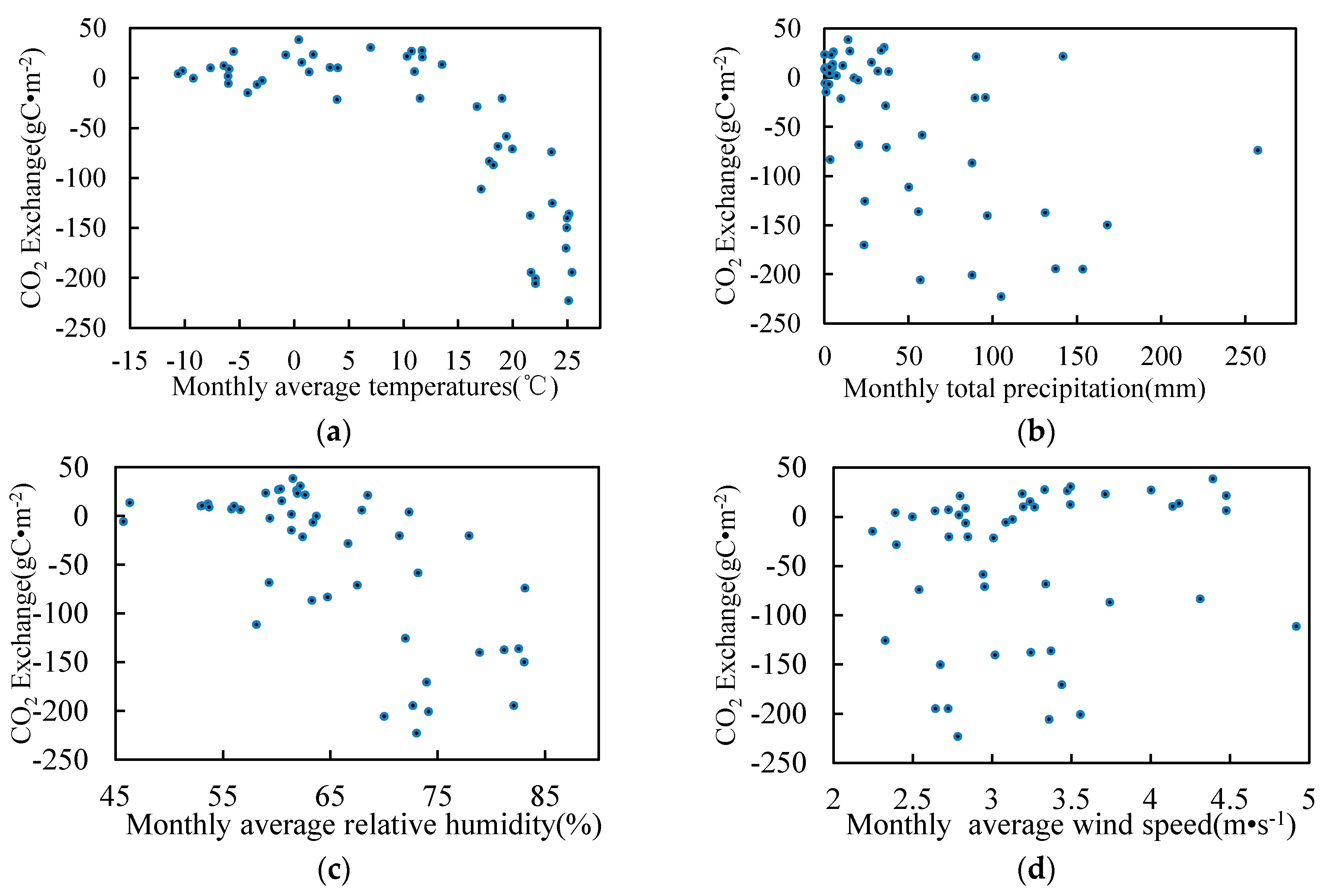
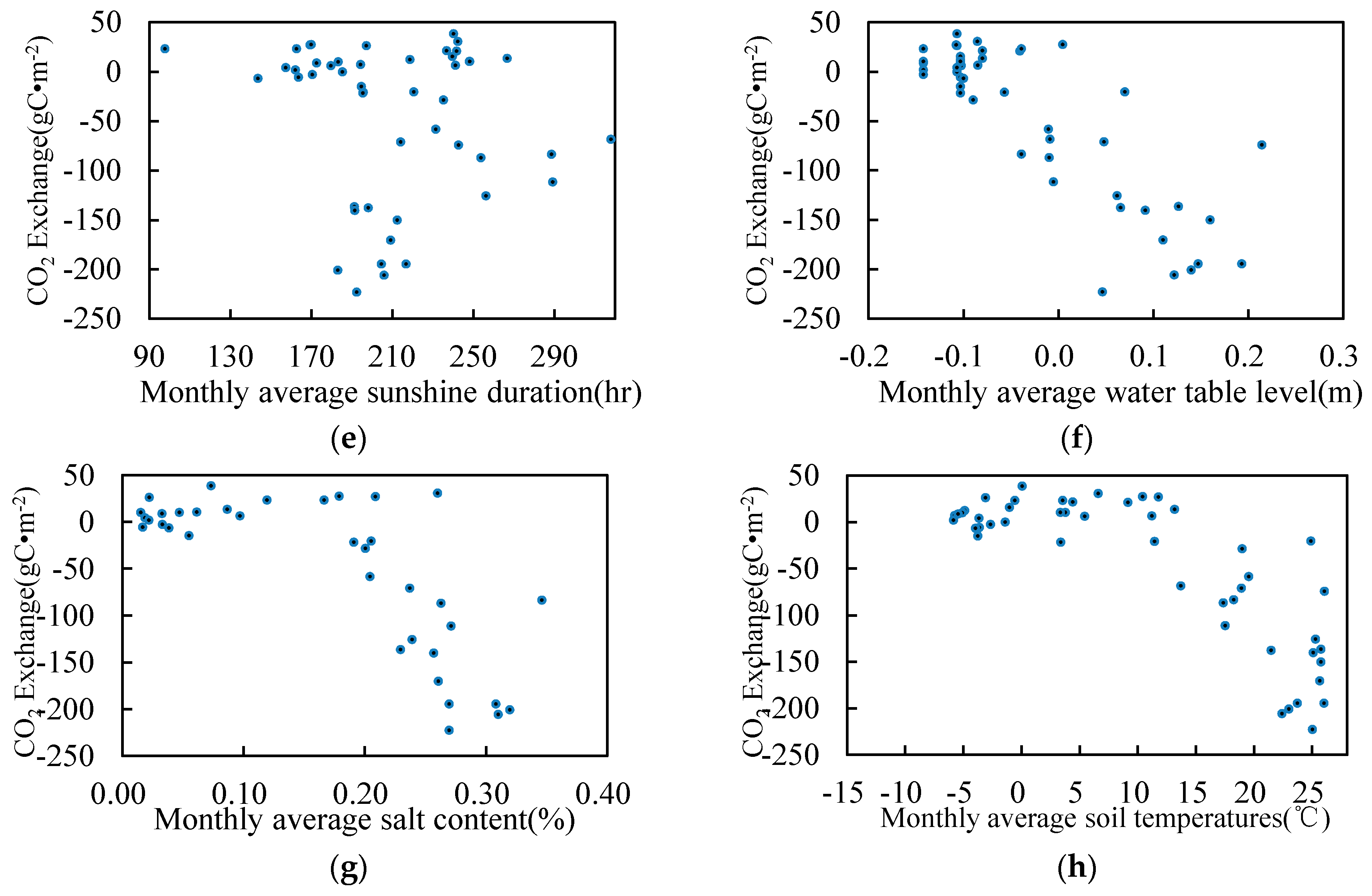
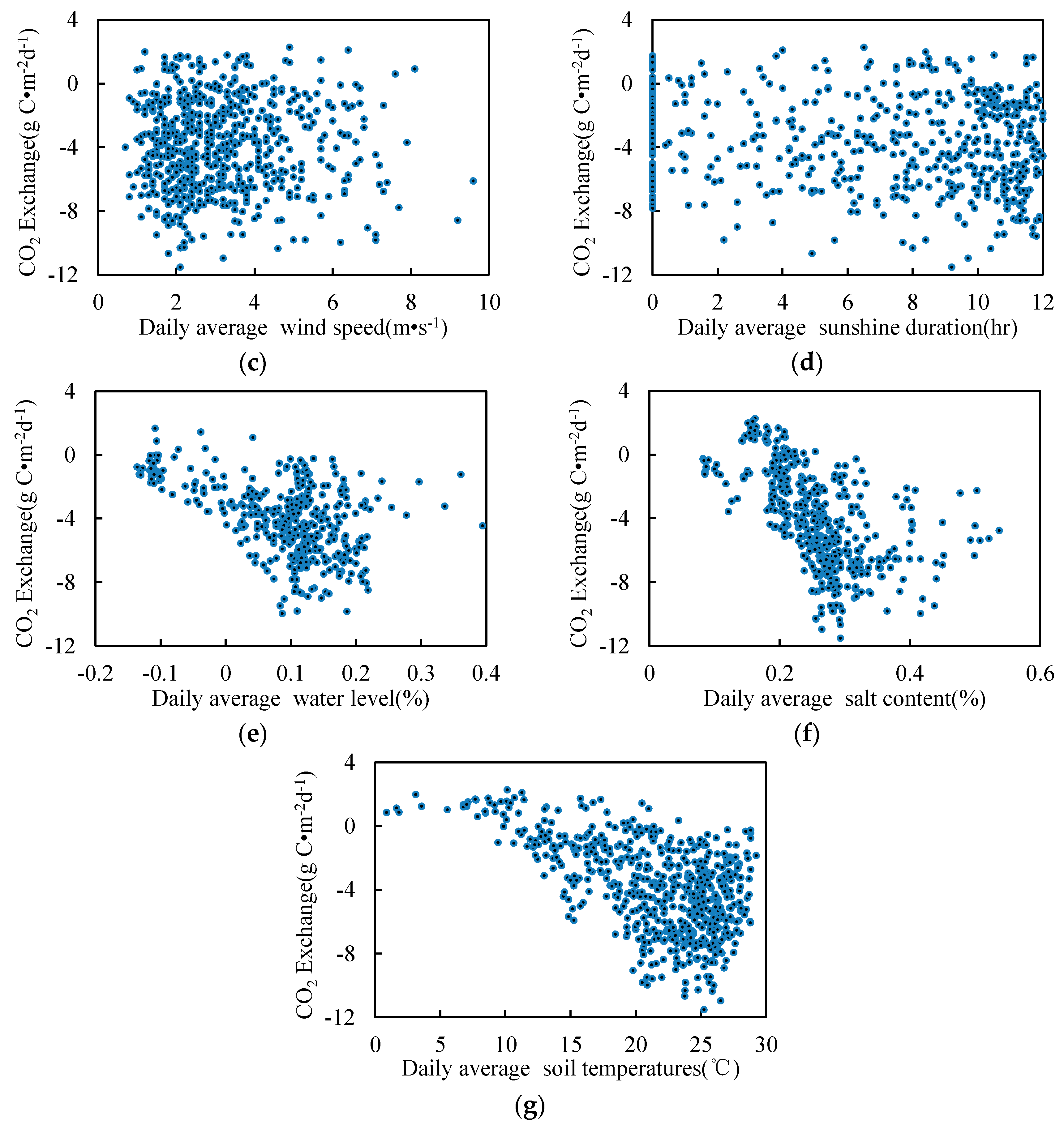
3.5. Relationship between Environmental Factors and CO2
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J. Wetlands, carbon, and climate change. Landsc. Ecol. 2012, 28, 1–15. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.H.; Manning, M. Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Yu, Z. Holocene carbon flux histories of the world’s peatlands: Global carbon-cycle implications. Holocene 2011, 21, 761–774. [Google Scholar] [CrossRef]
- Charman, D.J.; Beilman, D.W.; Blaauw, M.; Booth, R.K.; Brewer, S.; Chambers, F.M. Climate-related changes in peatland carbon accumulation during the last millennium. Biogeosci. Discuss. 2012, 9, 929–944. [Google Scholar] [CrossRef] [Green Version]
- Bu, Z.; Hans, J.; Li, H.; Zhao, G.; Zheng, X.; Ma, J.; Zeng, J. The response of peatlands to climate warming: A review. Acta Ecol. Sin. 2011, 31, 157–162. [Google Scholar] [CrossRef]
- Nilsson, M.; Sagerfors, J.; Buffam, I.; Laudon, H.; Eriksson, T.; Grelle, A.; Klemedtsson, L.; Weslien, P.; Lindroth, A. Contemporary carbon accumulation in a boreal oligotrophic minerogenic mire a significant sink after accounting for all C-fluxes. Glob. Chang. Biol. 2008, 14, 2317–2332. [Google Scholar] [CrossRef]
- Koehler, A.-K.; Sottocornola, M.; Kiely, G. How strong is the current carbon sequestration of an Atlantic blanket bog? Glob. Chang. Biol. 2015, 17, 309–319. [Google Scholar] [CrossRef]
- Kennedy, H.; Alongi, D.M.; Karim, A.; Chen, G.; Chmura, G.L.; Crooks, S. Chapter 4 coastal wetlands. In 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Lund, M.; Lafleur, P.M.; Roulet, N.T.; Lindroth, A.; Christensen, T.R.; Aurela, M. Variability in exchange of CO2, across 12 northern peatland and tundra sites. Glob. Chang. Biol. 2010, 16, 2436–2448. [Google Scholar] [CrossRef]
- Kerkhoff, A.; Enquist, B.; Elser, J.; Fagan, W. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob. Ecol. Biogeogr. 2005, 14, 585–598. [Google Scholar] [CrossRef]
- Staehr, P.A.; Sand-Jensen, K. Seasonal changes in temperature and nutrient control of photosynthesis, respiration and growth of natural phytoplankton communities. Fresh Water Biol. 2006, 51, 249–262. [Google Scholar] [CrossRef]
- Flanagan, L.B. Stimulation of both photosynthesis and respiration in response to warmer and drier conditions in a boreal peatland ecosystem. Glob. Chang. Biol. 2011, 17, 2271–2287. [Google Scholar] [CrossRef]
- Gedan, K.B.; Bertness, M.D. How will warming affect the salt marsh foundation species Spartina patens and its ecological role? Oecologia 2010, 164, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.L.; Blum, L.K. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosciences 2011, 8, 987–993. [Google Scholar] [CrossRef]
- Charles, H.; Dukes, J.S. Effects of warming and altered precipitation on plant and nutrient dynamics of a New England salt marsh. Ecol. Appl. Publ. Ecol. Soc. Am. 2009, 19, 1758. [Google Scholar] [CrossRef]
- Anna, M.A.; Jianjian, L.U. The Progress of research on carbon flux in wetland ecosystems. Wetl. Sci. 2008, 6, 116–123. [Google Scholar]
- Zhu, X.; Wang, S.; Zhang, C. Responses of different ecotypes of reed growing in the Hexi corridor to natural drought and salinity. Plant Physiol. J. 2003, 39, 371–376. [Google Scholar]
- Saenger, C.; Cronin, T.; Thunell, R.; Vann, C. Modelling river discharge and precipitation from estuarine salinity in the northern Chesapeake Bay: Application to Holocene palaeoclimate. Holocene 2006, 16, 467–477. [Google Scholar] [CrossRef]
- Vretare, V.; Weisner, S.E.B.; Strand, J.A.; Granéli, W. Phenotypic plasticity in Phragmites australis as a functional response to water depth. Aquat. Bot. 2001, 69, 127–145. [Google Scholar] [CrossRef]
- Zhao, K.; Li, J. Effects of salinity on the contents of osmotica of monocotyledenous halophytes and their contribution to osmotic adjustment. Acta Bot. Sin. 2009, 41, 1287–1292. [Google Scholar]
- Zhang, S.; Guo, C.; Su, F. Effect of salinity on the growth of reed. J. Shenyang Agric. Univ. 2008, 39, 65–68. [Google Scholar]
- Weston, N.B.; Vile, M.A.; Neubauer, S.C.; Velinsky, D.J. Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 2011, 102, 135–151. [Google Scholar] [CrossRef]
- Bubier, J.; Crill, P.; Mosedale, A.; Frolking, S.; Linder, E. Peatland responses to varying interannual misture conditions as measured by automatic CO2 chambers. Glob. Biogeochem. Cycles 2003, 17, 1066–1081. [Google Scholar] [CrossRef]
- Chen, G. Study on Marsh in Sanjiang Plain; Science Press: Beijing, China, 1996; pp. 165–168. [Google Scholar]
- Minchinton, T.E. Disturbance by wrack facilitates spread of Phragmites australis in a coastal marsh. J. Exp. Mar. Biol. Ecol. 2002, 281, 89–107. [Google Scholar] [CrossRef]
- Robroek, B.J.M.; Schouten, M.G.C.; Limpens, J.; Berendse, F.; Poorter, H. Interactive effects of water table and precipitation on net CO2 assimilation of three co-occurring sphagnum mosses differing in distribution above the water table. Glob. Chang. Biol. 2009, 15, 680–691. [Google Scholar] [CrossRef]
- Daulat, W.E.; Clymo, R.S. Effects of temperature and watertable on the efflux of methane from peatland surface cores. Atmos. Environ. 1998, 32, 3207–3218. [Google Scholar] [CrossRef]
- Frolking, S.; Roulet, N.T. Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions. Glob. Chang. Biol. 2010, 13, 1079–1088. [Google Scholar] [CrossRef]
- Heinsch, F.A.; Heilman, J.L.; Mcinnes, K.J.; Cobos, D.R.; Zuberer, D.A. Carbon dioxide exchange in a high marsh on the Texas Gulf Coast: Effects of freshwater availability. Agric. For. Meteorol. 2004, 125, 159–172. [Google Scholar] [CrossRef]
- Fenner, N.; Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 2011, 4, 895–900. [Google Scholar] [CrossRef]
- Han, G.; Yang, L.; Yu, J.; Wang, G.; Mao, P.; Gao, Y. Environmental Controls on Net Ecosystem CO2 Exchange Over a Reed (Phragmites australis) Wetland in the Yellow River Delta, China. Estuaries Coasts 2013, 36, 401–413. [Google Scholar] [CrossRef]
- Thomas, K.L.; Benstead, J.; Davies, K.L.; Lloyd, D. Role of wetland plants in the diurnal control of CH4 and CO2 fluxes in peat. Soil Biol. Biochem. 1996, 28, 17–23. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, X. Carbon storage and fixation by a typical wetland vegetation in Changjiang River Estuary—A case study of Phragmites australis in east beach of Chongming Island. Chin. J. Eco-Agric. 2008, 16, 269–272. [Google Scholar] [CrossRef]
- Li, B.; Liu, C.-Q.; Wang, J.-X.; Zhang, Y.-J. Carbon Storage and Fixation Function by Phragmites australis, a Typical Vegetation in Baiyangdian Lake. J. Agro-Environ. Sci. 2009, 28, 2603–2607. [Google Scholar]
- Qi, Y. Study on the allocation scheme of multi-source ecological water supply of Panjin reed wetland. Water Resour. Hydropower Northeast China 2015, 33, 19–21. [Google Scholar]
- Sagerfors, J.; Lindroth, A.; Grelle, A.; Klemedtsson, L.; Weslien, P.; Nilsson, M. Annual CO2 exchange between a nutrient-poor, minerotrophic, boreal mire and the atmosphere. J. Geophys. Res. 2008, 113, G01001. [Google Scholar] [CrossRef]
- Lv, X. Wetland Ecosystem Observation Method; China Environmental Science Press: Beijing, China, 2005; pp. 94–99. [Google Scholar]
- Krishnan, A.; Williams, L.J.; Mcintosh, A.R.; Abdi, H. Partial least squares (PLS) methods for neuroimaging: A tutorial and review. Neuroimage 2011, 56, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Giuseppe, P.; Paolo, P.; Hans-Dieter, Z. Performance of PLS regression coefficients in selecting variables for each response of a multivariate PLS for omics-type data. Adv. Appl. Bioinform. Chem. 2009, 2, 57–70. [Google Scholar]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis; Prentice Hall: New Jersey, NJ, USA, 2002. [Google Scholar]
- Sagerfors, J. Land-Atmosphere Exchange of CO2, Water and Energy at a Boreal Minerotrophic Mire. Ph.D. Thesis, Swedish University of Agricultural Sciences, Umea, Sweden, January 2007. [Google Scholar]
- Peichl, M.; Sonnentag, O.; Nilsson, M.B. Bringing color into the picture: Using digital repeat photography to investigate phenology controls of the carbon dioxide exchange in a boreal mire. Ecosystems 2014, 18, 115–131. [Google Scholar] [CrossRef]
- Sun, B.; Xie, J.C.; Wang, N.; Li, S.-Q.; Li, C.-J. Effect of reeds on salt enrichment and improvement of saline-alkali land. J. Soil Water Conserv. 2012, 26, 92–101. [Google Scholar]
- Hair, J.; Black, W.; Babin, B.; Anderson, R. Multivariate Data Analysis: Pearson New International Edition PDF eBook; Pearson: Zug, Switzerland, 2013; Volume 3, pp. 128–134. [Google Scholar]
- Li, Y.; Cui, L.; Pan, X.; Ning, Y.; Li, W.; Kang, X. Spatial distribution of plant diversity and functional groups in the Liaohe estuary. Biodivers. Sci. 2015, 23, 471–478. [Google Scholar] [CrossRef]
- Kang, X.M.; Cui, L.J.; Yue, X.L.; Li, W.; Zhang, M.Y.; Zhao, X.S.; Hao, Y.B.; Lei, Y.R.; Gao, Q. Valuation of Air Regulation by the Reed (Phragmites australis) Wetland in the Yellow River Delta. Wetl. Sci. Manag. 2015, 11, 23–25. [Google Scholar]





| Depth (cm) | pH | Organic Matter (g·kg−1) | Total N (g·kg−1) | Nitrate N (mg·kg−1) | Ammonium N (mg·kg−1) | Alkali-Hydrolysis N (mg·kg−1) | Total P (mg·kg−1) | Available P (mg·kg−1) | Total K (g·kg−1) | Available K (g·kg−1) | C/N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 | 8.80 ± 0.2 | 19.4 ± 10 | 1.55 ± 0.1 | 4.21 ± 1.4 | 14.75 ± 6.0 | 67.2 ± 26 | 35 ± 6 | 14.50 ± 0.7 | 2.86 ± 0.3 | 0.75 ± 0.05 | 6.8 ± 1.6 |
| 20–40 | 9.27 ± 0.1 | 9.8 ± 3 | 1.65 ± 0.1 | 6.24 ± 0.9 | 4.33 ± 0.5 | 30.8 ± 14 | 22.62 ± 1.4 | 0.83 ± 0.32 |
| Year | Reed Wetland (ppm) | Standard Deviation SD |
|---|---|---|
| 2012 | 375.14 | 24.67 |
| 2013 | 366.78 | 21.76 |
| 2014 | 383.65 | 11.90 |
| 2015 | 397.84 | 14.77 |
| Year | Ta (°C) | PPT (mm) | RH (%) | Sunshine Duration (h) | WS (m·s−1) |
|---|---|---|---|---|---|
| 2012 | 8.88 | 999 | 68.13 | 2688.2 | 3.03 |
| 2013 | 9.48 | 499.8 | 69.22 | 2534.8 | 3.22 |
| 2014 | 9.71 | 350.4 | 60.32 | 2525.9 | 3.20 |
| 2015 | 10.26 | 447.1 | 63.67 | 2326.8 | 3.45 |
| 1961–2010 | 8.92 ± 0.73(±SD) | 611.2 ± 158.3(±SD) | 65.97 ± 2.47(±SD) | 2697.6 ± 192.3(±SD) | 3.93 ± 0.65(±SD) |
| Factors | Tolerance | VIF |
|---|---|---|
| t1 | 0.150 | 6.673 |
| t2 | 0.017 | 57.506 |
| t3 | 0.017 | 59.054 |
| t4 | 0.405 | 2.471 |
| t5 | 0.104 | 9.629 |
| t6 | 0.451 | 2.215 |
| t7 | 0.087 | 11.540 |
| t8 | 0.361 | 2.770 |
| t9 | 0.070 | 14.303 |
| t10 | 0.274 | 3.653 |
| Factor | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|
| The first group (Total factors) | 0.567 | 0.647 | 0.629 |
| The second group (Tair, P, RH) | 0.9 | 0.621 | 0.574 |
| The third group (WTL, SAL, TSoil) | 0.9 | 0.673 | 0.661 |
| The fourth group (H, Biomass) | 0.907 | 0.162 | 0.091 |
| The fifth group (Tair, P, RH, WTL, SAL, Tsoil, Biomass) | 0.885 | 0.781 | 0.711 |
| The sixth group (Tair, P, RH, WTL, SAL, Biomass) | 0.0898 | 0.781 | 0.725 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; Yu, W.; Zhou, L.; Liang, C. Atmospheric and Surface-Condition Effects on CO2 Exchange in the Liaohe Delta Wetland, China. Water 2017, 9, 806. https://doi.org/10.3390/w9100806
Jia Q, Yu W, Zhou L, Liang C. Atmospheric and Surface-Condition Effects on CO2 Exchange in the Liaohe Delta Wetland, China. Water. 2017; 9(10):806. https://doi.org/10.3390/w9100806
Chicago/Turabian StyleJia, Qingyu, Wenying Yu, Li Zhou, and Chenghua Liang. 2017. "Atmospheric and Surface-Condition Effects on CO2 Exchange in the Liaohe Delta Wetland, China" Water 9, no. 10: 806. https://doi.org/10.3390/w9100806





