Influence of Humic Acid on the Transport and Deposition of Colloidal Silica under Different Hydrogeochemical Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colloidal Silica
2.2. Preparation of Porous Media
2.3. Electrolyte Solutions
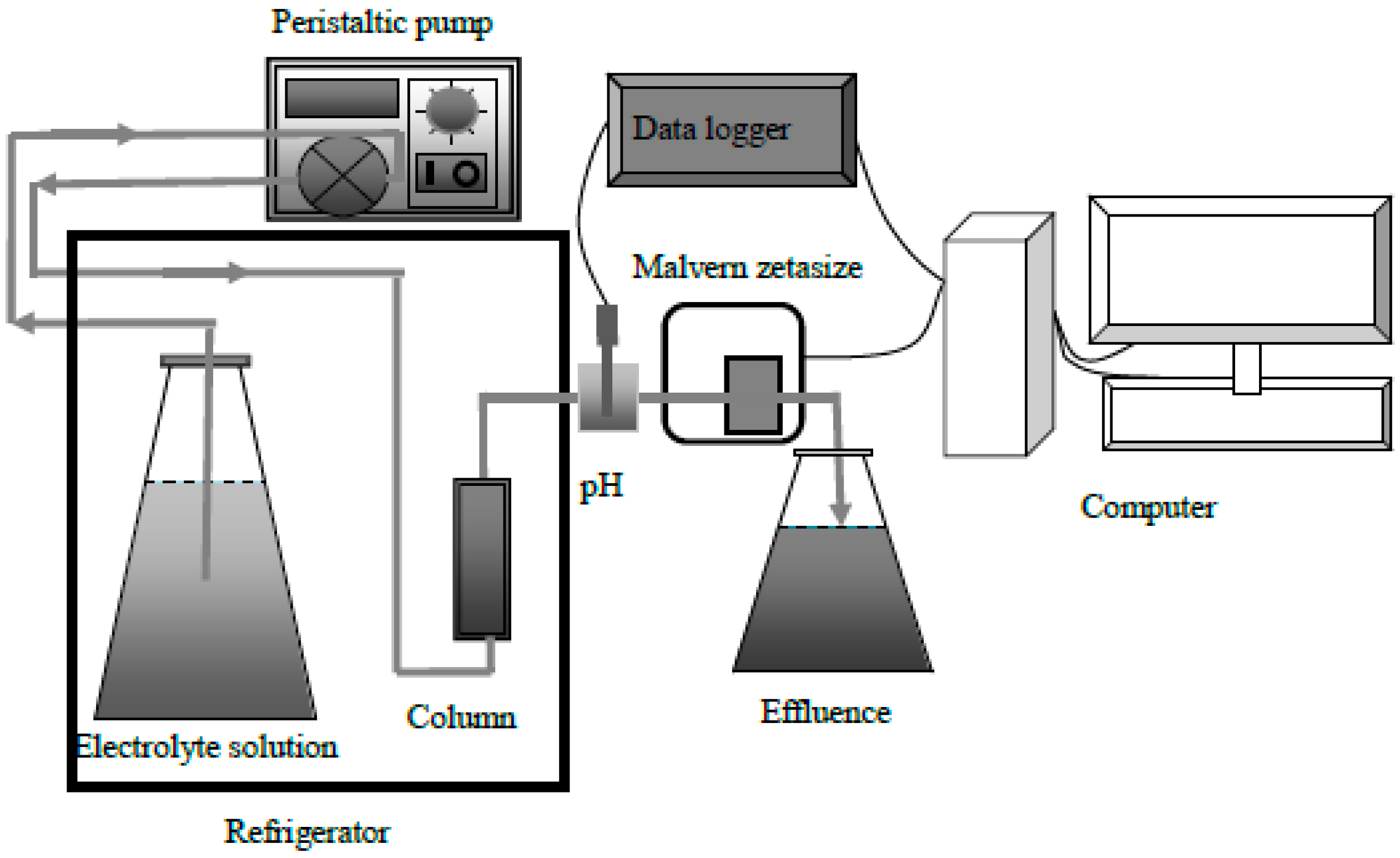
2.4. Column Experiments
- (1)
- Colloidal silica transport experiments in deionized water;
- (2)
- Colloidal silica transport experiments in the presence of monovalent ion (KCl, 0.01 mol/L);
- (3)
- Colloidal silica transport experiments in the presence of divalent ion (CaCl2, 0.0025 mol/L and 0.05 mol/L);
- (4)
- Colloidal silica transport in the presence of monovalent ion and divalent ion (KCl, 0.01 mol/L and CaCl2, 0.0025 mol/L).
2.5. Mathematical Model
3. Results and Discussion
3.1. Characterization of Colloidal Silica
3.2. Colloidal Silica Transport Experiment
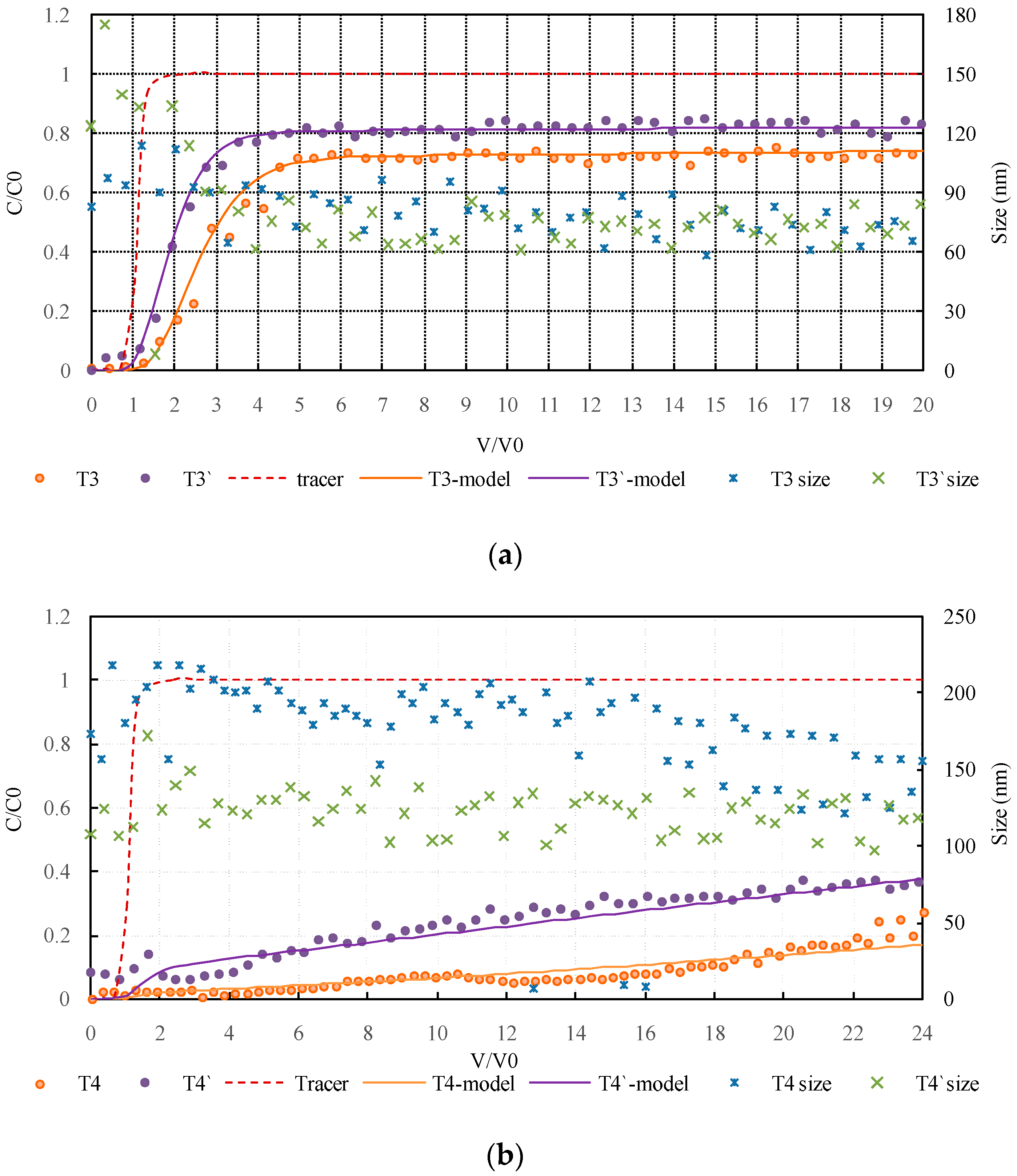
3.2.1. Influence of Hydrogeochemical Conditions on Colloidal Silica Transport during MAR
3.2.2. Influence of HA on Colloidal Silica Transport in Monovalent Ion Solution during MAR
3.2.3. Influence of HA on Colloidal Silica Transport in Divalent Ion Solution during MAR
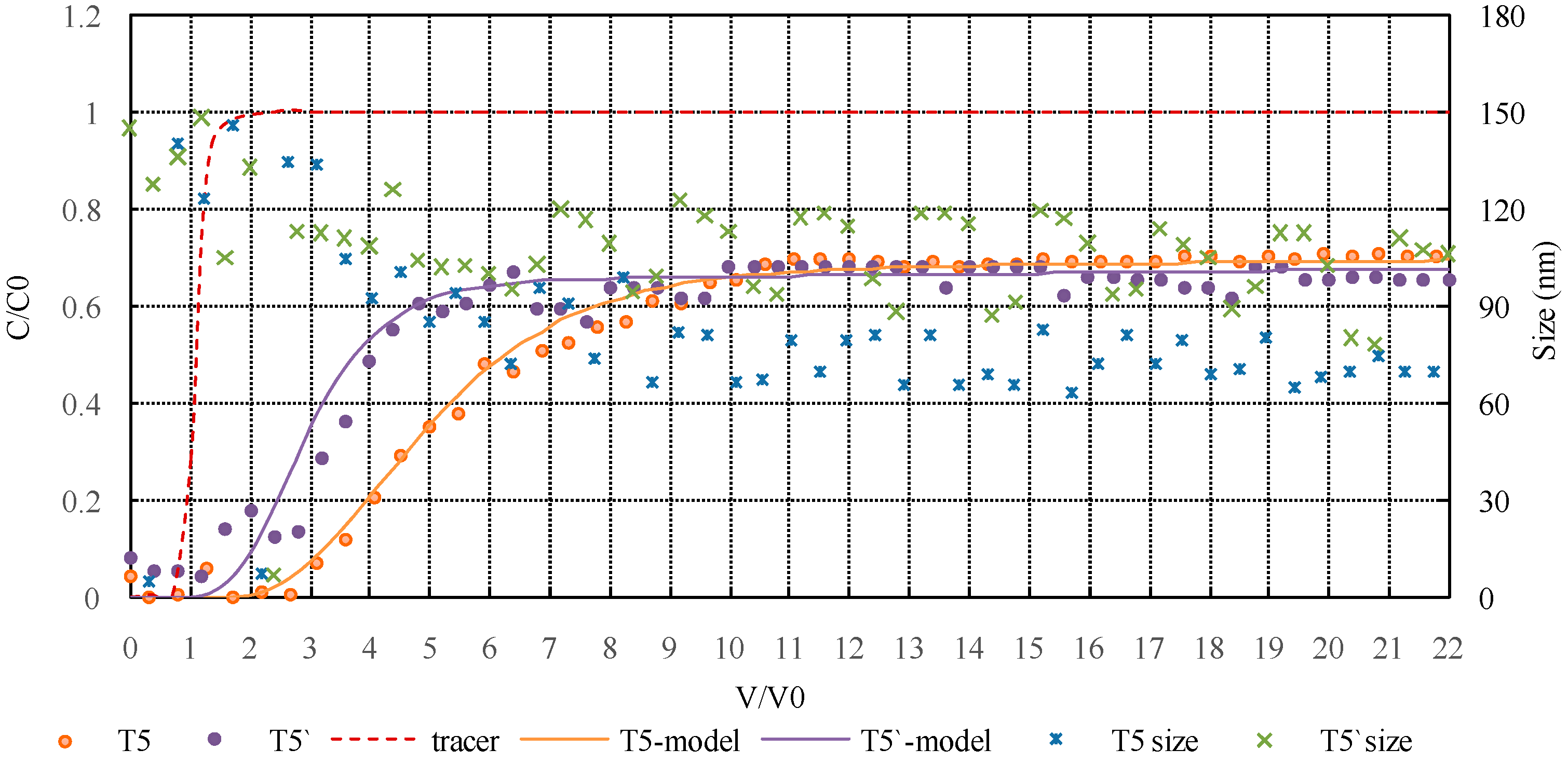
3.2.4. Influence of HA on Colloidal Silica Transport in Mixed (Monovalent Ion and Divalent Ion) Solution during MAR
3.3. Modeling Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, W.J.; Huan, Y.; Yu, X.P.; Liu, D.; Zhou, J.J. Multi-component transport and transformation in deep confined aquifer during groundwater artificial recharge. J. Environ. Manag. 2015, 152, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.K.; Sharma, S.K.; Lekkerkerker-Teunissen, K.; Amy, G.L. Occurrence and fate of bulk organic matter and pharmaceutically active compounds in managed aquifer recharge: A review. Water Res. 2011, 45, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.A.; Torkzaban, S.; Leij, F.; Simunek, J. Equilibrium and kinetic models for colloid release under transient solution chemistry conditions. J. Contam. Hydrol. 2015, 181, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Masciopinto, C. Management of aquifer recharge in lebanon by removing seawater intrusion from coastal aquifers. J. Environ. Manag. 2013, 130, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar]
- Vendelboe, A.L.; Moldrup, P.; Schjonning, P.; Oyedele, D.J.; Jin, Y.; Scow, K.M.; de Jonge, L.W. Colloid release from soil aggregates: Application of laser diffraction. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef]
- Baumann, T.; Fruhstorfer, P.; Klein, T.; Niessner, R. Colloid and heavy metal transport at landfill sites in direct contact with groundwater. Water Res. 2006, 40, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, T.; Wu, Y. Distinct roles of illite colloid and humic acid in mediating arsenate transport in water-saturated sand columns. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Borkovec, M.; Grolimund, D.; Elimelech, M. Mobile subsurface colloids and their role in contaminant transport. Adv. Agron. 1999, 66, 121–193. [Google Scholar]
- Bob, M.M.; Walker, H.W. Effect of natural organic coatings on the polymer-induced coagulation of colloidal particles. Colloids Surf. A 2001, 177, 215–222. [Google Scholar] [CrossRef]
- Franchi, A.; O’Melia, C.R. Effects of natural organic matter and solution chemistry on the deposition and reentrainment of colloids in porous media. Environ. Sci. Technol. 2003, 37, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Harbour, P.J.; Dixon, D.R.; Scales, P.J. The role of natural organic matter in suspension stability: Electrokinetic-rheology relationships. Colloids Surf. A 2007, 295, 38–48. [Google Scholar] [CrossRef]
- Laegdsmand, M.; de Jonge, L.W.; Moldrup, P. Leaching of colloids and dissolved organic matter from columns packed with natural soil aggregates. Soil Sci. 2005, 170, 13–27. [Google Scholar] [CrossRef]
- Bekhit, H.M.; Hassan, A.E.; Harris-Burr, R.; Papelis, C. Experimental and numerical investigations of effects of silica colloids on transport of strontium in saturated sand columns. Environ. Sci. Technol. 2006, 40, 5402–5408. [Google Scholar] [CrossRef] [PubMed]
- Saiers, J.E.; Hornberger, G.M. The influence of ionic strength on the facilitated transport of cesium by kaolinite colloids. Water Resour. Res. 1999, 35, 1713–1727. [Google Scholar] [CrossRef]
- Kearns, P.; Gonzalez, M.; Oki, N.; Lee, K.; Rodriguez, F. The safety of nanotechnologies at the oecd. In Nanomaterials: Risks and Benefits; Linkov, I., Steevens, J., Eds.; Springer: West Berlin, Germany, 2009; pp. 351–358. [Google Scholar]
- Vitorge, E.; Szenknect, S.; Martins, J.M.F.; Barthes, V.; Auger, A.; Renard, O.; Gaudet, J.P. Comparison of three labeled silica nanoparticles used as tracers in transport experiments in porous media, Part I: Syntheses and characterizations. Environ. Pollut. 2014, 184, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, W.J.; Li, S.; Zhou, J.J.; Liu, D. Transport of colloidal silicon through saturated porous media under different hydrogeochemicaland hydrodynamic conditions during managed aquifer recharge. Water 2016, 8, 555. [Google Scholar] [CrossRef]
- Saleh, N.B.; Pfefferle, L.D.; Elimelech, M. Influence of biomacromolecules and humic acid on the aggregation kinetics of single-walled carbon nanotubes. Environ. Sci. Technol. 2010, 44, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Elimelech, M. Interaction of fullerene (C60) nanoparticles with humic acid and alginate coated silica surfaces: Measurements, mechanisms, and environmental implications. Environ. Sci. Technol. 2008, 42, 7607–7614. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Jiang, X.J.; Tong, M.P.; Kim, H. Influence of natural organic matter on the transport and deposition of zinc oxide nanoparticles in saturated porous media. J. Colloid Interface Sci. 2012, 386, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, J.J.; Zhang, W.J.; Huan, Y.; Yu, X.P.; Li, F.L.; Chen, X.Q. Column experiments to investigate transport of colloidal humic acid through porous media during managed aquifer recharge. Hydrol. J. 2016. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Transport of biocolloids in water saturated columns packed with sand: Effect of grain size and pore water velocity. J. Contam. Hydrol. 2012, 129, 301–314. [Google Scholar] [CrossRef]
- Hu, J.D.; Zevi, Y.; Kou, X.M.; Xiao, J.; Wang, X.J.; Jin, Y. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci. Total Environ. 2010, 408, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lin, D.H.; Xing, B.S. Interactions of humic acid with nanosized inorganic oxides. Langmuir 2009, 25, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Tong, M.P.; Lu, R.Q.; Kim, H. Transport and deposition of ZnO nanoparticles in saturated porous media. Colloids Surf. A 2012, 401, 29–37. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A.; Vanderzalm, J.L.; Patterson, B.M.; Harris, B.; Prommer, H. Colloid release and clogging in porous media: Effects of solution ionic strength and flow velocity. J. Contam. Hydrol. 2015, 181, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Luo, L.; Zhang, S.Z. Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano- and micro-scales. Colloids Surf. A 2011, 384, 126–130. [Google Scholar] [CrossRef]
- Chen, G.X.; Liu, X.Y.; Su, C.M. Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ. Sci. Technol. 2012, 46, 7142–7150. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, T.; Dror, I.; Berkowitz, B. Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 2010, 81, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Thio, B.J.R.; Zhou, D.X.; Keller, A.A. Influence of natural organic matter on the aggregation and deposition of titanium dioxide nanoparticles. J. Hazard. Mater. 2011, 189, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Li, K.G.; Chen, Y.S. Effect of natural organic matter on the aggregation kinetics of CeO2 nanoparticles in KCl and CaCl2 solutions: Measurements and modeling. J. Hazard. Mater. 2012, 209, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; University of Birmingham, Birmingham, England. The mobility of colloids in red sandstone: The effect of flow rate and ionic strength. Unpublished work. 2008. [Google Scholar]
- Elimelech, M.; Nagai, M.; Ko, C.H.; Ryan, J.N. Relative insignificance of mineral grain zeta potential to colloid transport in geochemically heterogeneous porous media. Environ. Sci. Technol. 2000, 34, 2143–2148. [Google Scholar] [CrossRef]
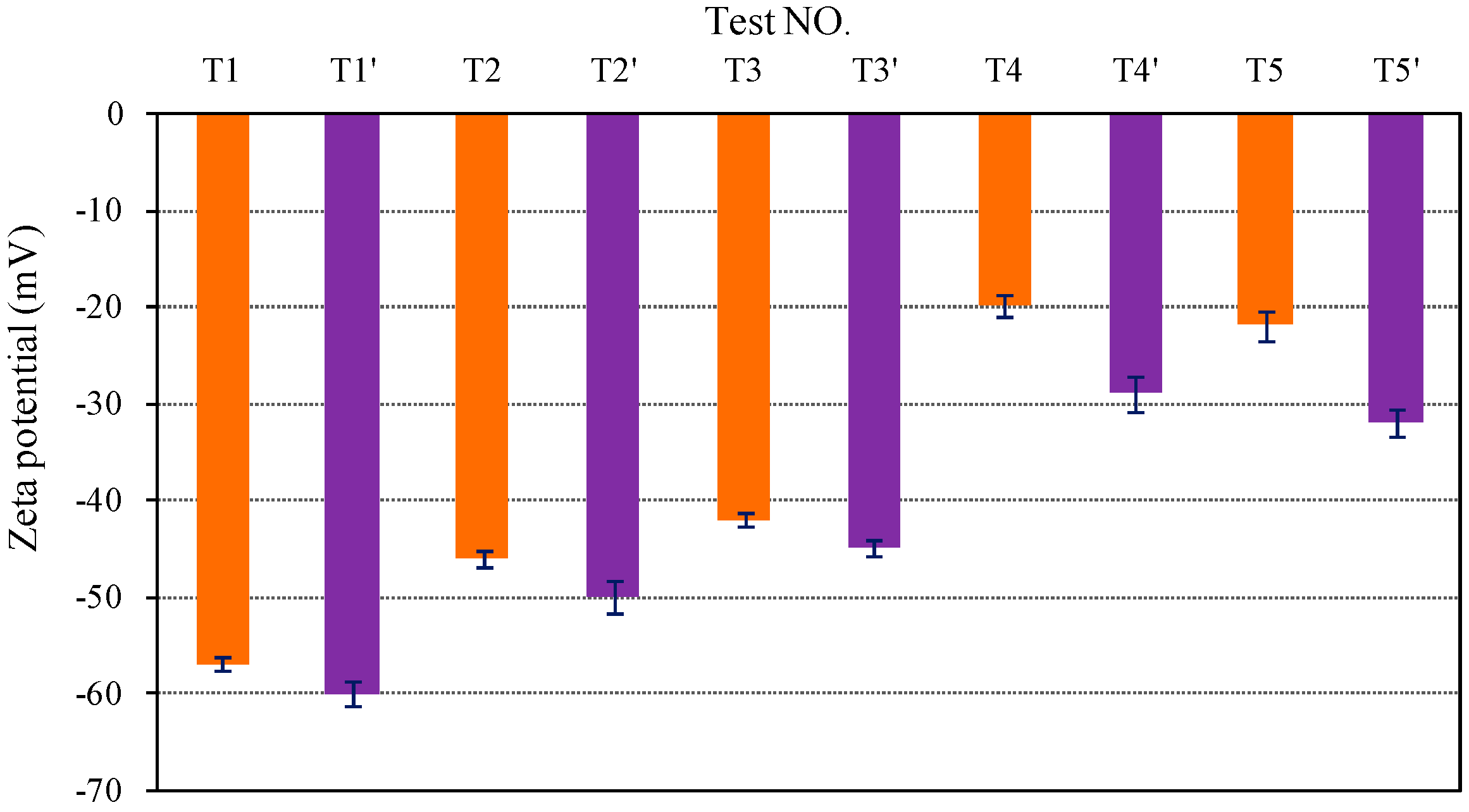



| Group | TEST NO. | SiO2 (ppm) | HA (ppm) | KCl (mol/L) | CaCl2 (mol/L) | PH | IS (M) | Peak (%) | R |
|---|---|---|---|---|---|---|---|---|---|
| 1 | T1 | 10 | 0 | 0 | 0 | 6~7 | <0.0005 | 100 | 1 |
| 2 | T2 | 10 | 0 | 0.01 | 0 | 6~7 | 0.013 | 100 | 1.5 |
| 3 | T3 | 10 | 0 | 0 | 0.0025 | 6~7 | 0.0093 | 70 | 2.3 |
| T4 | 10 | 0 | 0 | 0.005 | 6~7 | 0.013 | 32 | 15 | |
| 4 | T5 | 10 | 0 | 0.01 | 0.0025 | 6~7 | 0.0225 | 69 | 4.6 |
| 1 | T1’ | 10 | 10 | 0 | 0 | 6~7 | <0.0005 | 100 | 1 |
| 2 | T2’ | 10 | 10 | 0.01 | 0 | 6~7 | 0.013 | 100 | 1.25 |
| 3 | T3’ | 10 | 10 | 0 | 0.0025 | 6~7 | 0.0093 | 85 | 2 |
| T4’ | 10 | 10 | 0 | 0.005 | 6~7 | 0.013 | 38 | 8 | |
| 4 | T5’ | 10 | 10 | 0.01 | 0.0025 | 6~7 | 0.0225 | 68 | 3 |
| Test | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| kd (s−1) | 1.83 × 10−9 | 9.55 × 10−9 | 1.84 × 10−4 | 3.64 × 10−4 | 2.41 × 10−4 |
| Test | T1’ | T2’ | T3’ | T4’ | T5’ |
| kd (s−1) | 1.52 × 10−9 | 7.64 × 10−9 | 1.06 × 10−4 | 3.52 × 10−4 | 1.88 × 10−4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, W.; Liu, D.; Wang, Z.; Li, S. Influence of Humic Acid on the Transport and Deposition of Colloidal Silica under Different Hydrogeochemical Conditions. Water 2017, 9, 10. https://doi.org/10.3390/w9010010
Zhou J, Zhang W, Liu D, Wang Z, Li S. Influence of Humic Acid on the Transport and Deposition of Colloidal Silica under Different Hydrogeochemical Conditions. Water. 2017; 9(1):10. https://doi.org/10.3390/w9010010
Chicago/Turabian StyleZhou, Jingjing, Wenjing Zhang, Dan Liu, Zhuo Wang, and Shuo Li. 2017. "Influence of Humic Acid on the Transport and Deposition of Colloidal Silica under Different Hydrogeochemical Conditions" Water 9, no. 1: 10. https://doi.org/10.3390/w9010010





