Soil CO2 Uptake in Deserts and Its Implications to the Groundwater Environment
Abstract
:1. Introduction
2. Materials and Methods
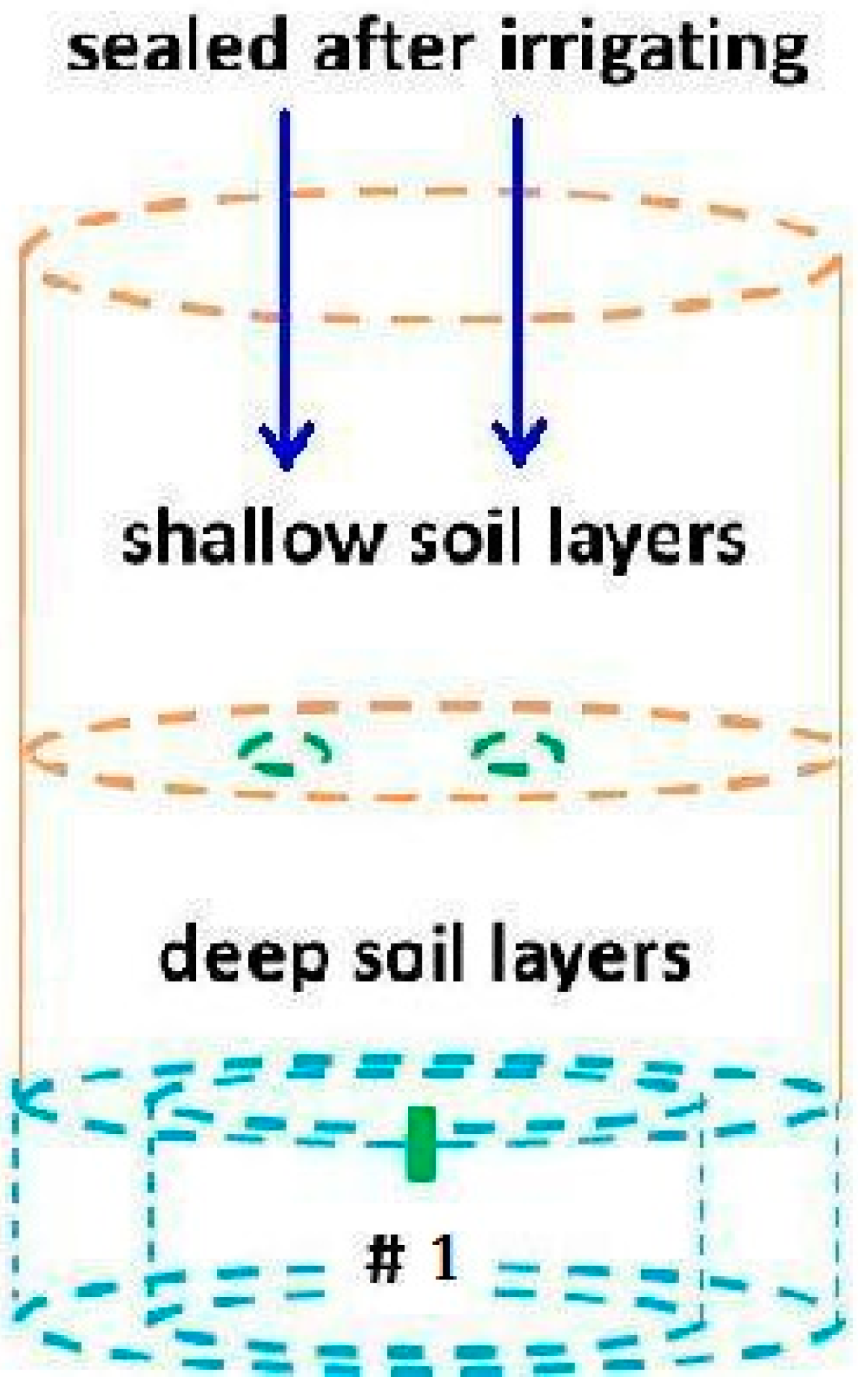
2.1. Data Sources
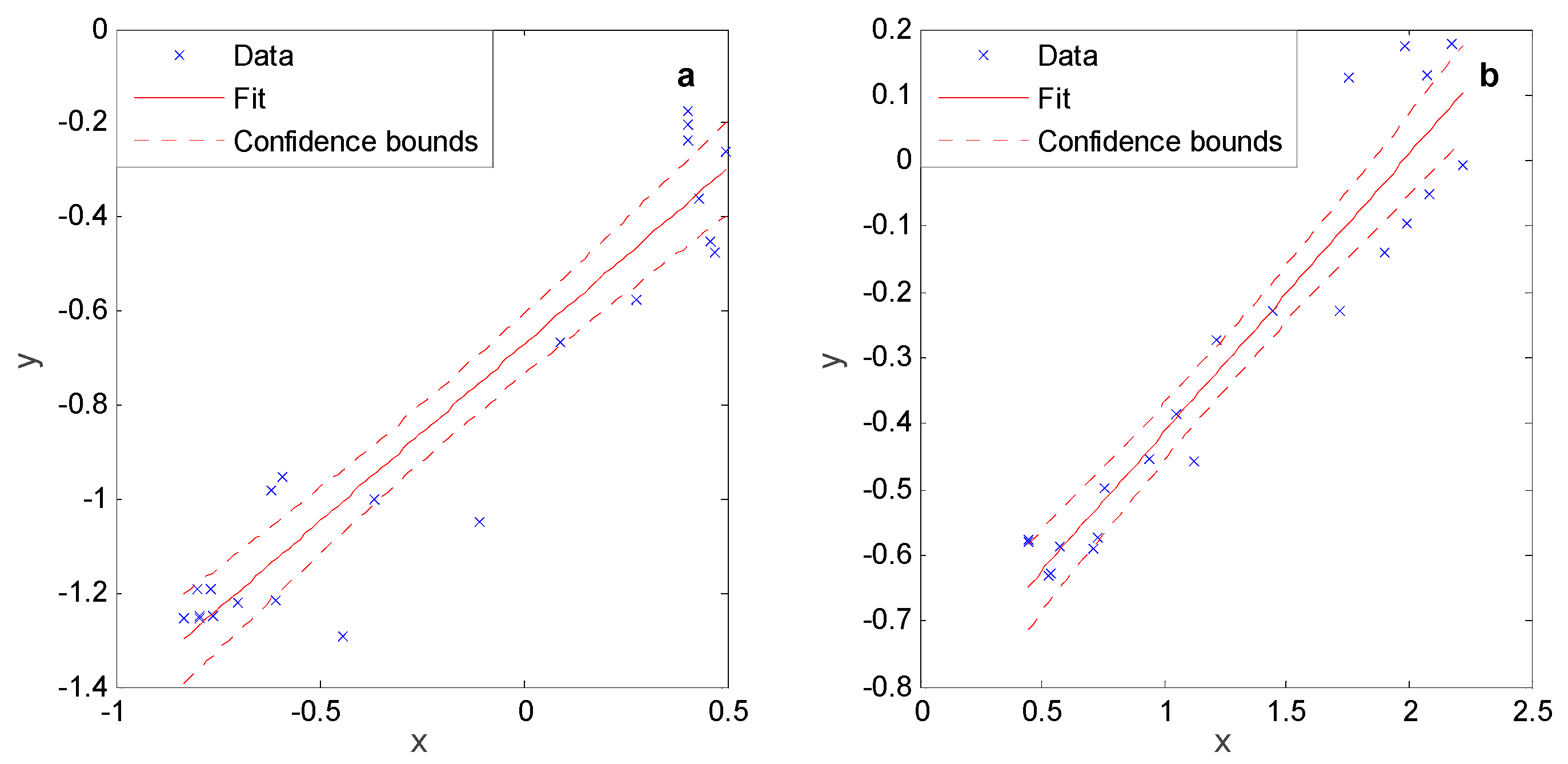
2.2. Modeling Approach
3. Results and Discussion
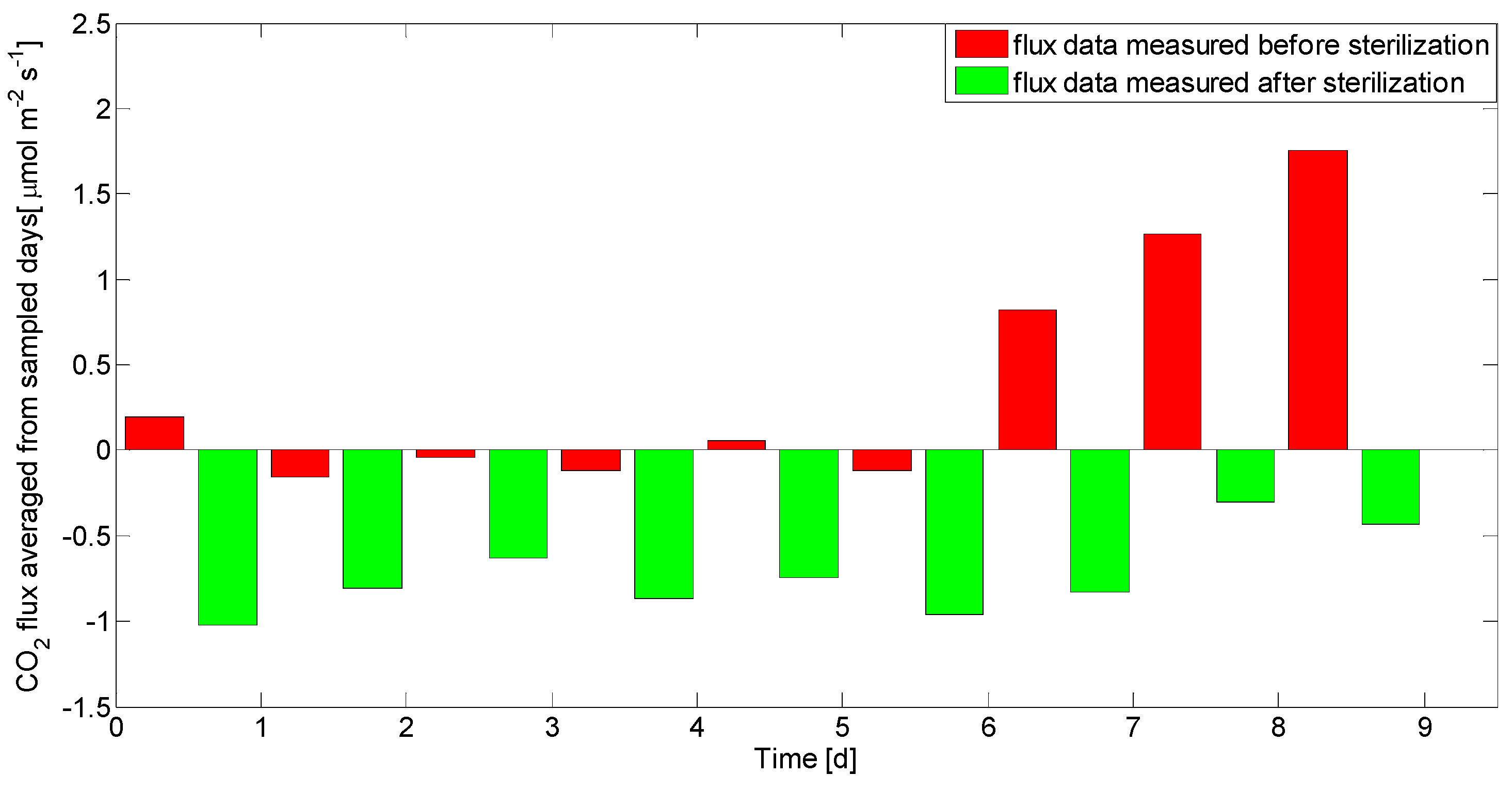
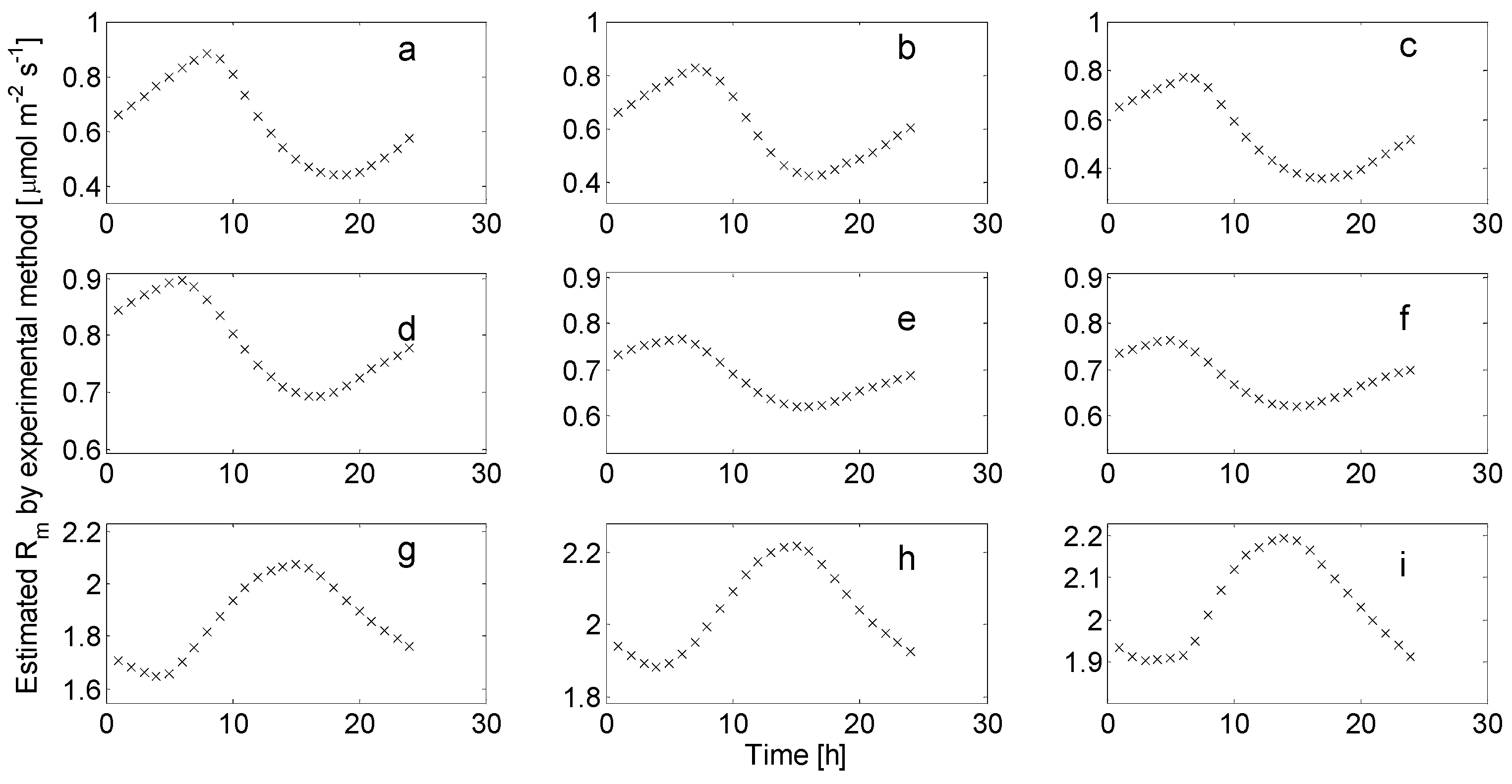
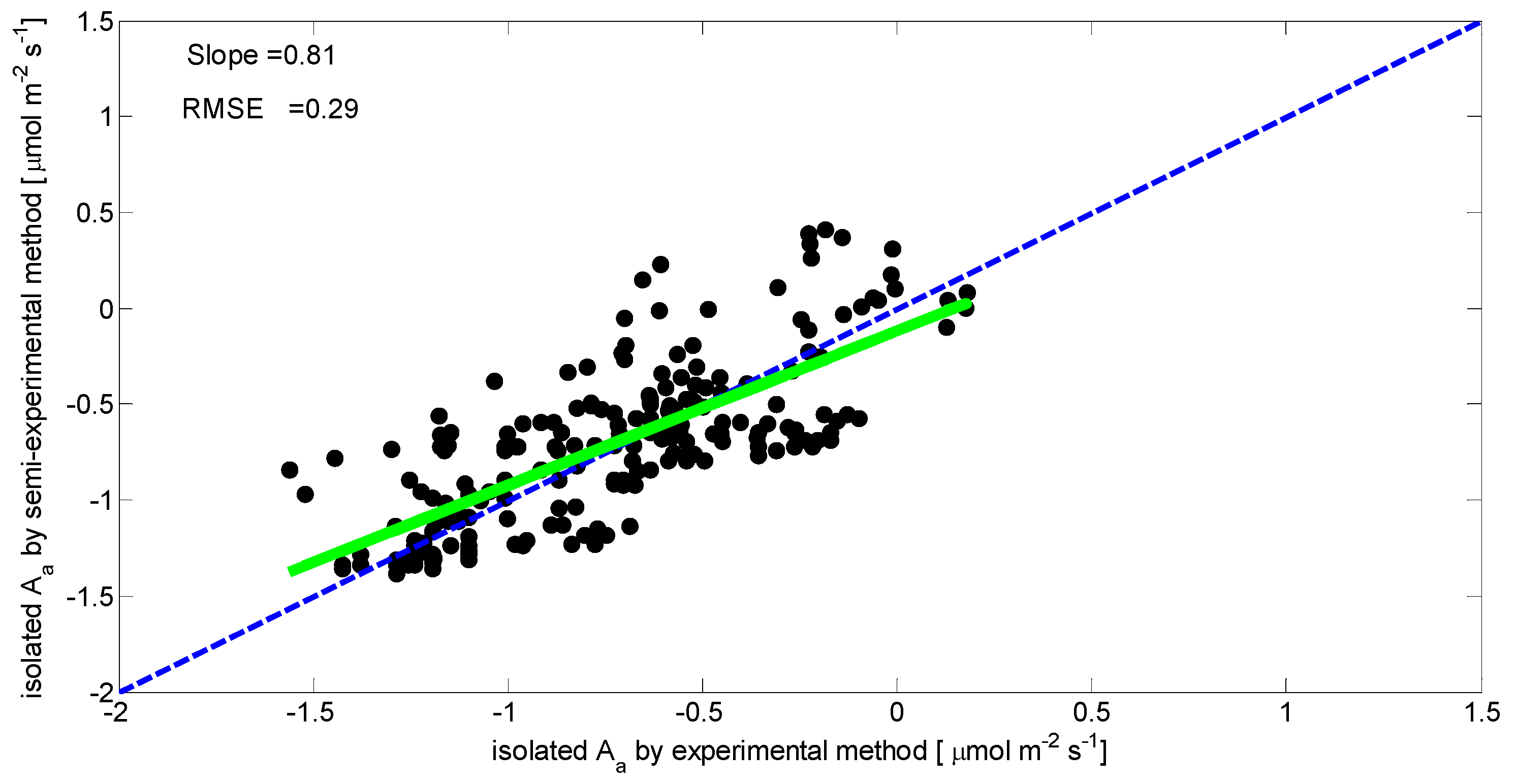
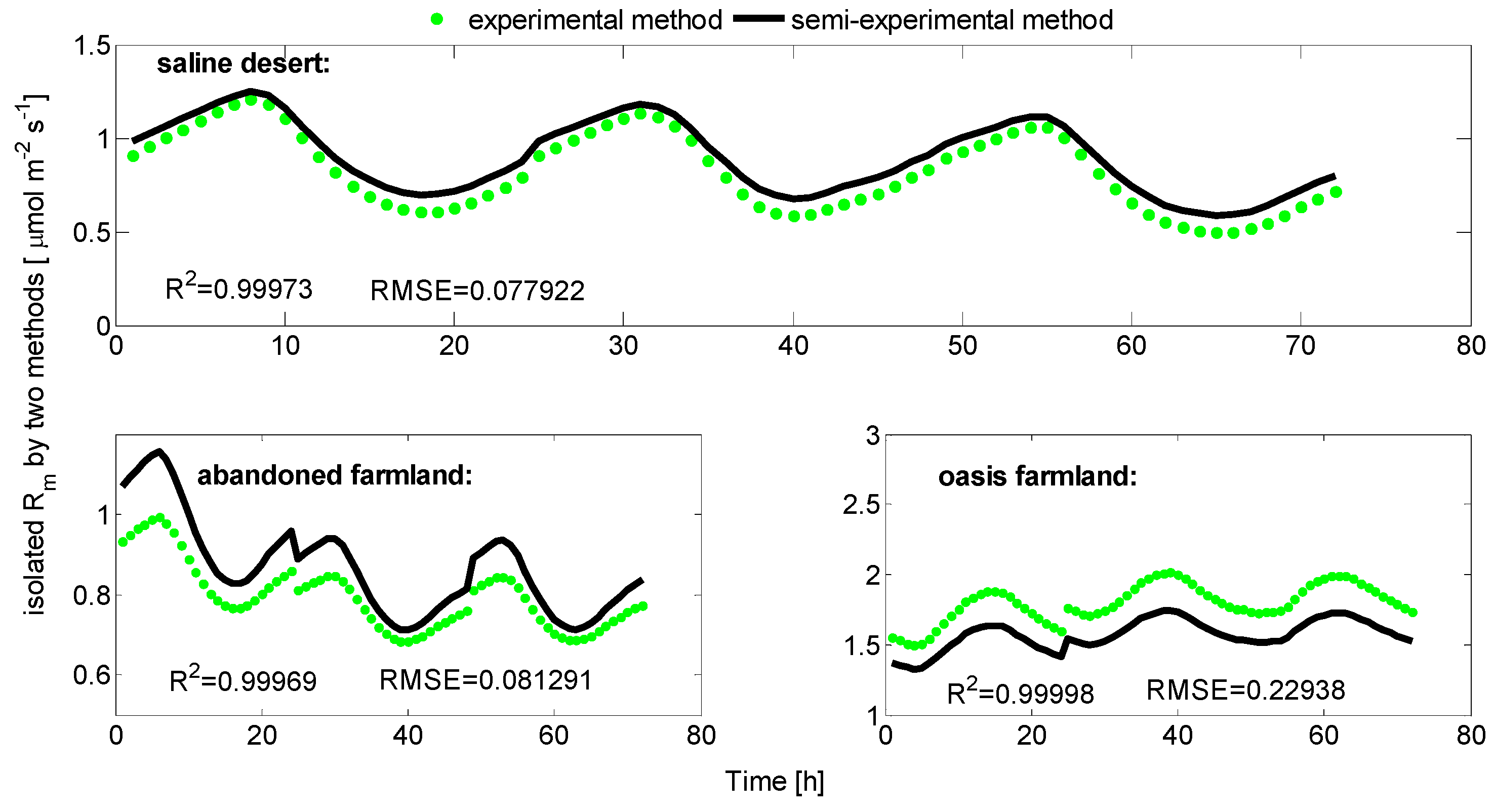
3.1. Separated Biotic and Abiotic Components of Soil CO2 Fluxes
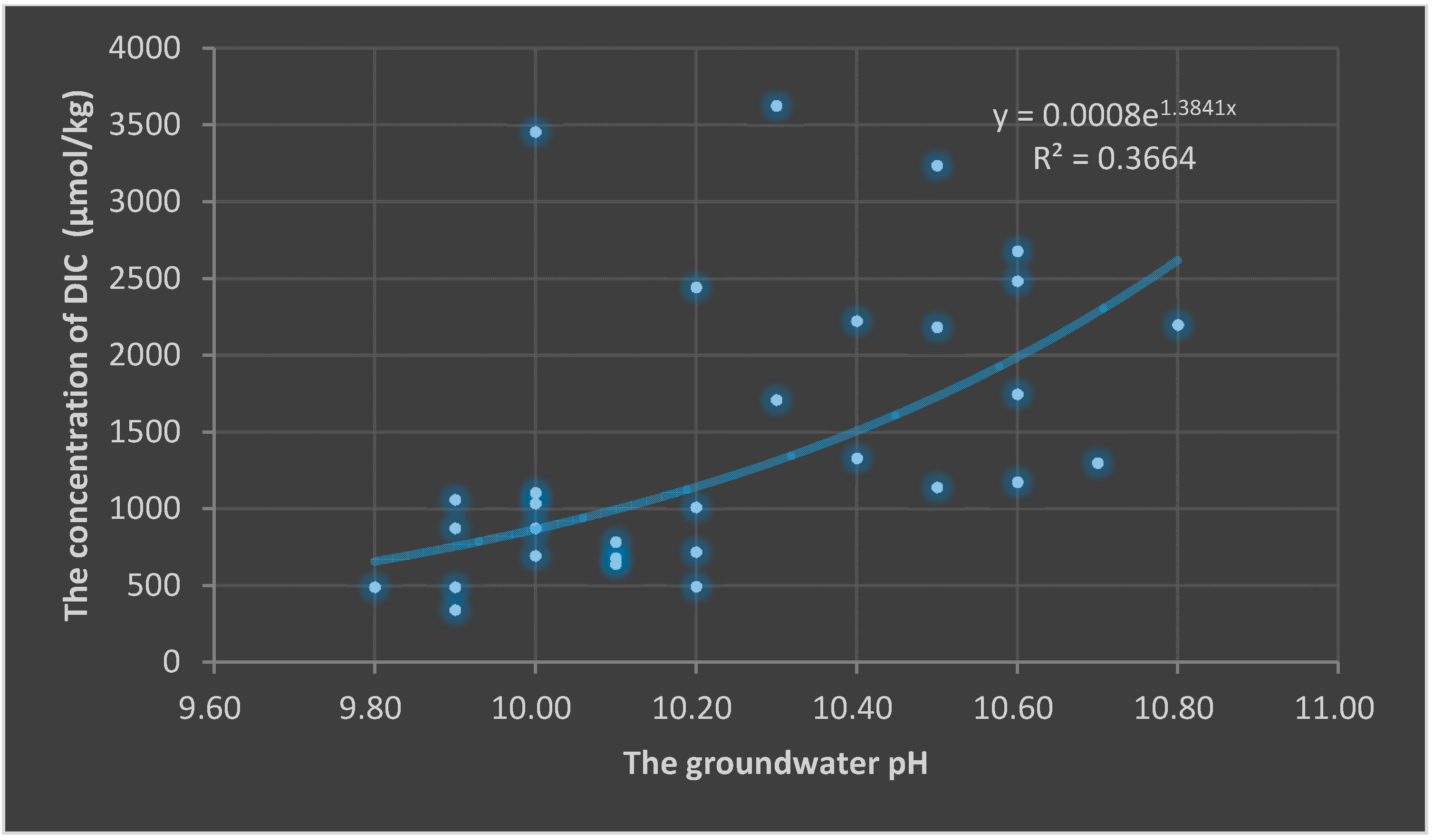
3.2. Implications of Soil CO2 Uptake to the Groundwater Environment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux insoil respiration and its relationship to vegetation and climate. Tellus 1992, 44, 81–99. [Google Scholar] [CrossRef]
- Hőgberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Bowling, D.R.; Pataki, D.E.; Randerson, J.T. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytol. 2008, 178, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Grace, J. Understanding and managing the global carbon cycle. J. Ecol. 2004, 92, 189–202. [Google Scholar] [CrossRef]
- Gregory, P.J. Roots, rhizosphere and soil: The route to a better understanding of soil science. Eur. J. Soil Sci. 2006, 57, 2–12. [Google Scholar] [CrossRef]
- Hendricks, J.J.; Nadelhoffer, K.J.; Aber, J.D. Assessing the role of fine roots in carbon and nutrient cycling. Trends Ecol. Evol. 1993, 8, 174–178. [Google Scholar] [CrossRef]
- Kowalski, A.S.; Serrano-Ortiz, P.; Janssens, I.A.; Sánchez-Moral, S.; Cuezva, S.; Domingo, F.; Were, A.; Alados-Arboledas, L. Can flux tower research neglect geochemical CO2 exchange? Agric. For. Meteorol. 2008, 148, 1045–1054. [Google Scholar] [CrossRef]
- Xie, J.X.; Li, Y.; Zhai, C.X.; Li, C.H.; Lan, Z.D. CO2 absorption by alkaline soils and its implication to the global carbon cycle. Environ. Geol. 2009, 56, 953–961. [Google Scholar] [CrossRef]
- Serrano-Ortiz, P.; Roland, M.; Sanchez-Moral, S.; Janssens, I.A.; Domingo, F.; Goddérise, Y.; Kowalski, A.S. Hidden, abiotic CO2 flows and gaseous reservoirs in the terrestrial carbon cycle: Review andperspectives. Agric. For. Meteorol. 2010, 150, 321–329. [Google Scholar] [CrossRef]
- Stone, R. Have desert researchers discovered a hidden loop inthe carbon cycle? Science 2008, 320, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Inglima, I.; Alberti, G.; Bertolini, T.; Vaccari, F.P.; Gioli, B.; Miglietta, F.; Cotrufo, M.F.; Peressotti, A. Precipitation pulses enhance respiration of mediterranean ecosystems: The balance between organic and inorganic components of increased soil CO2 efflux. Glob. Chang. Biol. 2009, 15, 1289–1301. [Google Scholar] [CrossRef]
- Baggs, E.M. Partitioning the components of soil respiration: A research challenge. Plant Soil 2006, 284, 1–5. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.F.; Luo, G.P.; Li, L.H.; Li, Y. Time lag between carbon dioxide influx to and efflux from bare saline-alkali soil detected by the explicit partitioning and reconciling of soil CO2, flux. Stoch. Environ. Res. Risk Assess. 2013, 27, 737–745. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Belnap, J.; Marion, G. On carbon sequestration in desert ecosystems. Glob. Chang. Biol. 2009, 15, 1488–1490. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V.; Cattanio, J.H.; Ackerman, I.L.; Carvalho, J.E.M. Effects of soil water content on soil respiration in forest and cattle pastures of eastern Amazonia. Biochemistry 2000, 48, 53–69. [Google Scholar]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Glob. Chang. Biol. 2010, 16, 3386–3406. [Google Scholar] [CrossRef]
- Wang, X.H.; Piao, S.L.; Ciais, P.; Janssens, I.A.; Reichstein, M.; Peng, S.; Wang, T. Are ecological gradients in seasonal Q10 of soil respiration explained by climate or by vegetation seasonality? Soil Biol. Biochem. 2010, 42, 1728–1734. [Google Scholar] [CrossRef]
- Yuste, J.C.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency oncarbon inputs, soil temperature and moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef]
- Arora, B.; Spycher, N.F.; Steefel, C.I.; Molins, S.; Bill, M.; Conrad, M.E.; Dong, W.; Faybishenko, B.; Tokunaga, T.K.; Wan, J.; et al. Influence of hydrological, biogeochemical and temperature transients on subsurface carbon fluxes in a flood plain environment. Biogeochemistry 2016, 127, 367–396. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.F. On the apparent CO2 absorption by alkaline soils. Biogeosci. Discuss. 2014, 11, 2665–2683. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Tol, C.; Luo, G.; Su, Z. Growing season net ecosystem CO2 exchange of two desert ecosystems with alkaline soils in Kazakhstan. Ecol. Evol. 2014, 4, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Dong, Y.S.; Wang, Y.Q.; Wei, X.R.; Wang, Y.F.; Cui, B.L.; Zhou, W.J. Natural vegetation restoration is more beneficial to soil surface organic and inorganic carbon sequestration than tree plantation on the Loess Plateau of China. Sci. Total Environ. 2014, 485–486, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Chen, X.; Luo, G.P.; Li, L.H. Modeling the contribution of abiotic exchange to CO2 flux in alkaline soils of arid areas. J. Arid Land 2014, 6, 27–36. [Google Scholar] [CrossRef]
- Raggio, J.; Pintado, A.; Vivas, M.; Sancho, L.G.; Büdel, B.; Colesie, C.; Weber, B.; Schroeter, B.; Lázaro, R.; Green, T.G.A. Continuous chlorophyll fluorescence, gas exchange and microclimate monitoring in a natural soil crust habitat in Tabernas badlands, Almería, Spain: Progressing towards a model to understand productivity. Biodivers. Conserv. 2014, 23, 1809–1826. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Tang, L.S.; Lan, Z.D.; Li, Y. A downward CO2 flux seems to have nowhere to go. Biogeoscience 2014, 11, 6251–6262. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Novara, A.; Gristina, L.; Badalucco, L. Soil carbon dynamics as affected by long-term contrasting cropping systems and tillages under semiarid Mediterranean climate. Appl. Soil Ecol. 2014, 73, 140–147. [Google Scholar] [CrossRef]
- Liu, J.; Feng, W.; Zhang, Y.; Jia, X.; Wu, B.; Qin, S.G.; Fa, K.Y.; Lai, Z.R. Abiotic CO2, Exchange between soil and atmosphere and its response to temperature. Environ. Earth Sci. 2015, 73, 2463–2471. [Google Scholar] [CrossRef]
- Lu, N.; Liu, X.R.; Du, Z.L.; Wang, Y.D.; Zhang, Q.Z. Effect of biochar on soil respiration in the maize growing season after 5 years of consecutive application. Soil Res. 2014, 52, 505–512. [Google Scholar] [CrossRef]
- Wang, X.J.; Xu, M.G.; Wang, J.P.; Zhang, W.J.; Yang, X.Y.; Huang, S.M.; Liu, H. Fertilization enhancing carbon sequestration as carbonate in arid cropland: Assessments of long-term experiments in northern China. Plant Soil 2014, 380, 89–100. [Google Scholar] [CrossRef]
- Fa, K.Y.; Liu, J.B.; Zhang, Y.Q.; Wu, B.; Qin, S.G.; Feng, W.; Lai, Z.R. CO2, absorption of sandy soil induced by rainfall pulses in a desert ecosystem. Hydrol. Process. 2014, 29, 2043–2051. [Google Scholar] [CrossRef]
- Wang, J.P.; Wang, X.J.; Zhang, J.; Zhao, C.Y. Soil organic and inorganic carbon and stable carbon isotopes in the Yanqi Basin of northwestern China. Eur. J. Soil Sci. 2015, 66, 95–103. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.J.; Wang, J.P.; Wang, W.X. Carbon and Nitrogen Contents in Typical Plants and Soil Profiles in Yanqi Basin of Northwest China. J. Integr. Agric. 2014, 13, 648–656. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Houghton, R.A.; Tang, L.S. Hidden carbon sink beneath desert. Geophys. Res. Lett. 2015, 42, 5880–5887. [Google Scholar] [CrossRef]
- Verburg, P.S.J.; Kapitzke, S.E.; Stevenson, B.A.; Bisiaux, M. Carbon allocation in Larrea tridentata plant-soil systems as affected by elevated soil moisture and N availability. Plant Soil 2014, 378, 227–238. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Rivas, J.; Paulo, Ú.; Ruas, F.; Carvalho, F. Sustainable treatment of different high-strength cheese whey wastewaters: An innovative approach for atmospheric CO2, mitigation and fertilizer production. Environ. Sci. Pollut. 2016, 23, 13062–13075. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Liu, R. The abiotic contribution to total CO2 flux for soils in arid zone. Biogeosci. Discuss. 2015, 12, 11217–11244. [Google Scholar] [CrossRef]
- Liu, B.; Jin, H.; Sun, Z.; Zhao, S. Geochemical weathering of aeolian sand and its palaeoclimatic implications in the Mu Us Desert, northern China, since the Late Holocene. J. Arid Land 2016, 8, 647–659. [Google Scholar] [CrossRef]
- Wen, Q.; Dong, Z. Geomorphologic patterns of dune networks in the Tengger Desert, China. J. Arid Land 2016, 8, 660–669. [Google Scholar] [CrossRef]
- Li, J.Y.; Dong, Z.B.; Qian, G.Q.; Zhang, Z.C.; Luo, W.Y.; Lu, J.F.; Wang, M. Pattern analysis of a linear dune field on the northern margin of Qarhan Salt Lake, northwestern China. J. Arid Land 2016, 8, 670–680. [Google Scholar] [CrossRef]
- Zhang, F.; Bai, L.; Li, L.H.; Wang, Q. Sensitivity of runoff to climatic variability in the northern and southern slopes of the Middle Tianshan Mountains, China. J. Arid Land 2016, 8, 681–693. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, X.; Hu, B.X.; Zhou, Y.X.; Hao, C.B.; Wan, L. Groundwater contributions in water-salt balances of the lakes in the Badain Jaran Desert, China. J. Arid Land 2016, 8, 694–706. [Google Scholar] [CrossRef]
- Jia, W.R.; Zhang, C.L.; Li, S.Y.; Wang, H.F.; Ma, X.X.; Wang, N.B. Grain size distribution at four developmental stages of crescent dunes in the hinterland of the Taklimakan Desert, China. J. Arid Land 2016, 8, 722–733. [Google Scholar] [CrossRef]
- Song, X.D.; Zhang, G.L.; Liu, F.; Li, D.C.; Zhao, Y.G.; Yang, J.L. Modeling spatio-temporal distribution of soil moisture by deep learning-based cellular automata model. J. Arid Land 2016, 8, 734–748. [Google Scholar] [CrossRef]
- Wang, P.Y.; Li, Z.Q.; Huai, B.J.; Wang, W.B.; Li, H.L.; Wang, L. Spatial variability of glacial changes and their effects on water resources in the Chinese Tianshan Mountains during the last five decades. J. Arid Land 2015, 7, 717–727. [Google Scholar] [CrossRef]
- Yahiaoui, I.; Douaoui, A.; Zhang, Q.; Ziane, A. Soil salinity prediction in the Lower Cheliff plain(Algeria) based on remote sensing and topographic feature analysis. J. Arid Land 2015, 7, 794–805. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Gan, Z. Changes in soil organic carbon and nitrogen after 26 years of farmland management on the Loess Plateau of China. J. Arid Land 2015, 7, 806–813. [Google Scholar] [CrossRef]
- Lundberg, A. Surface water-groundwater interaction. Theriogenology 2016, 85, 543–546. [Google Scholar]
- Baram, S.; Couvreur, V.; Harter, T.; Read, M.; Brown, P.H.; Hopmans, J.W.; Smart, D.R. Assessment of orchard N losses to groundwater with a vadose zone monitoring network. Agric. Water Manag. 2016, 172, 83–95. [Google Scholar] [CrossRef]
- Kurunc, A.; Ersahin, S.; Sonmez, N.K.; Kaman, H.; Uz, I.; Uz, B.Y.; Aslan, G.E. Seasonal changes of spatial variation of some groundwater quality variables in a large irrigated coastal Mediterranean region of Turkey. Sci. Total Environ. 2016, 554–555, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.A.; Koike, K.; Mashaly, H.A.; Gergis, F. Spatio-temporal trends and change factors of groundwater quality in an arid area with peat rich aquifers: Emergence of water environmental problems in Tanta District, Egypt. J. Arid Environ. 2016, 124, 360–376. [Google Scholar] [CrossRef]
- Pawar, R.J.; Bromhal, G.S.; Chu, S.; Dilmore, R.M.; Oldenburg, C.M.; Stauffer, P.H.; Zhang, Y.Q.; Guthrie, G.D. The National Risk Assessment Partnership’s integrated assessment model for carbon storage: A tool to support decision making amidst uncertainty. Int. J. Greenh. Gas Control 2016, 52, 175–189. [Google Scholar] [CrossRef]
- Liu, F.; Huang, G.; Sun, J.; Jing, J.H.; Zhang, Y. A New Evaluation Method for Groundwater Quality Applied in Guangzhou Region, China: Using Fuzzy Method Combining Toxicity Index. Water Environ. Res. 2016, 88, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, A.L.; Barnes, C.J.; Macumber, P.G.; Olley, J.M. A stable isotope investigation of groundwater-surface water interactions at Lake Tyrrell, Victoria, Australia. Chem. Geol. 2016, 96, 19–32. [Google Scholar] [CrossRef]
- Rawling, G.C.; Newton, B.T. Quantity and location of groundwater recharge in the Sacramento Mountains, south-central New Mexico (USA), and their relation to the adjacent Roswell Artesian Basin. Hydrogeol. J. 2016, 24, 1–30. [Google Scholar] [CrossRef]
- Jayalath, N.; Mosley, L.M.; Fitzpatrick, R.W.; Marschner, P. Addition of organic matter influences pH changes in reduced and oxidised acid sulfate soils. Geoderma 2016, 262, 125–132. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.F.; Luo, G.P.; Ye, H. Can soil respiration estimate neglect the contribution of abiotic exchange? J. Arid Land 2014, 6, 129–135. [Google Scholar] [CrossRef]
- Hildenbrand, Z.L.; Carlton, D.D.; Fontenot, B.E.; Meik, J.M.; Walton, J.L.; Thacker, J.B.; Korlie, S.; Shelor, C.P.; Kadjo, A.F.; Clark, A.; et al. Temporal variation in groundwater quality in the Permian Basin of Texas, a region of increasing unconventional oil and gas development. Sci. Total Environ. 2016, 562, 906–913. [Google Scholar] [CrossRef] [PubMed]



| Study Sites | Parameters Applied in Semi-Experimental Method | |
|---|---|---|
| Saline desert | β0 = 0.5284 | β1 = −0.8975 |
| Abandoned farmland | β0 = 0.5284 | β1 = −0.8975 |
| Oasis farmland | β0 = 0.4266 | β1 = −0.8330 |
| Study Sites | Semi-Experimental Method | Experimental Method |
|---|---|---|
| Saline desert | 0.53200 | 0.58711 |
| Abandoned farmland | 0.81835 | 0.77012 |
| Oasis farmland | 1.21009 | 1.19123 |
| Flux Components | Slope | RMSE |
|---|---|---|
| Alkaline absorption | 0.80630 | 0.29089 |
| Microbial respiration | 0.71842 | 0.14753 |
| Reconciled soil CO2 flux | 0.91428 | 0.25061 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, X.; Zheng, H.; Yu, R.; Qian, J.; Zhang, Y.; Yu, J. Soil CO2 Uptake in Deserts and Its Implications to the Groundwater Environment. Water 2016, 8, 379. https://doi.org/10.3390/w8090379
Wang W, Chen X, Zheng H, Yu R, Qian J, Zhang Y, Yu J. Soil CO2 Uptake in Deserts and Its Implications to the Groundwater Environment. Water. 2016; 8(9):379. https://doi.org/10.3390/w8090379
Chicago/Turabian StyleWang, Wenfeng, Xi Chen, Hongwei Zheng, Ruide Yu, Jing Qian, Yifan Zhang, and Jianjun Yu. 2016. "Soil CO2 Uptake in Deserts and Its Implications to the Groundwater Environment" Water 8, no. 9: 379. https://doi.org/10.3390/w8090379






