Is Recovery of Large-Bodied Zooplankton after Nutrient Loading Reduction Hampered by Climate Warming? A Long-Term Study of Shallow Hypertrophic Lake Søbygaard, Denmark
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Sampling
2.3. Sample Analysis
2.4. Data Analysis
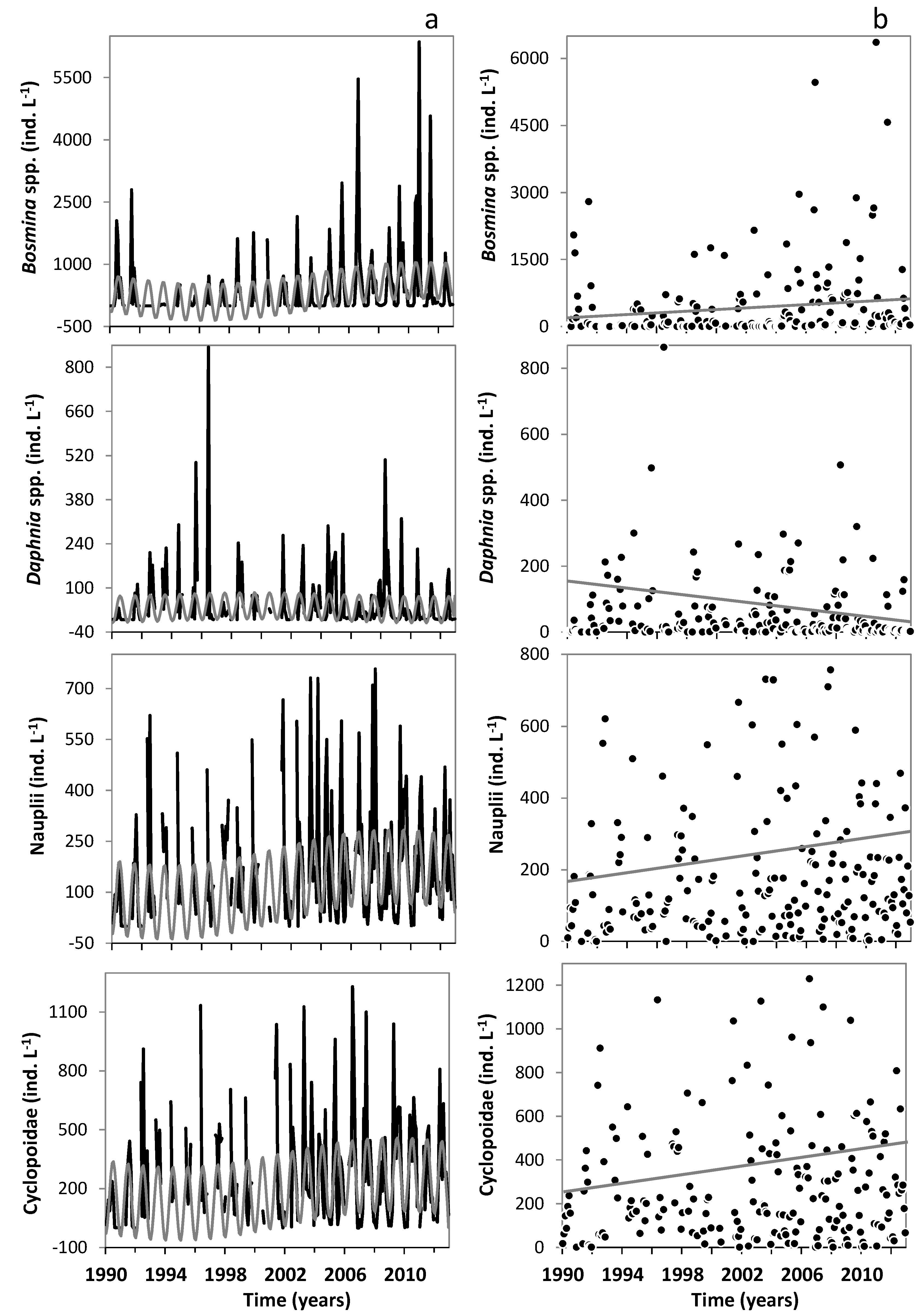
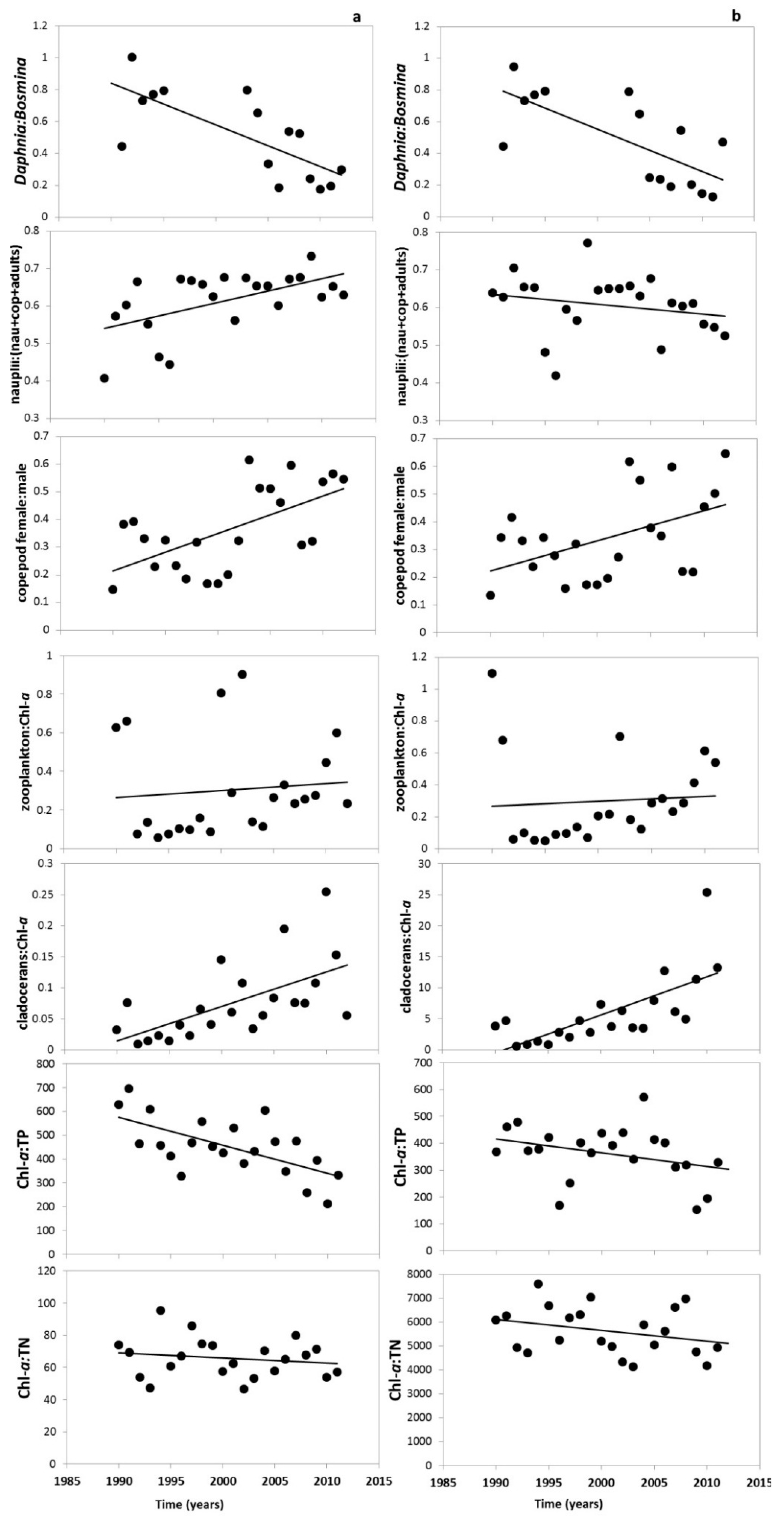
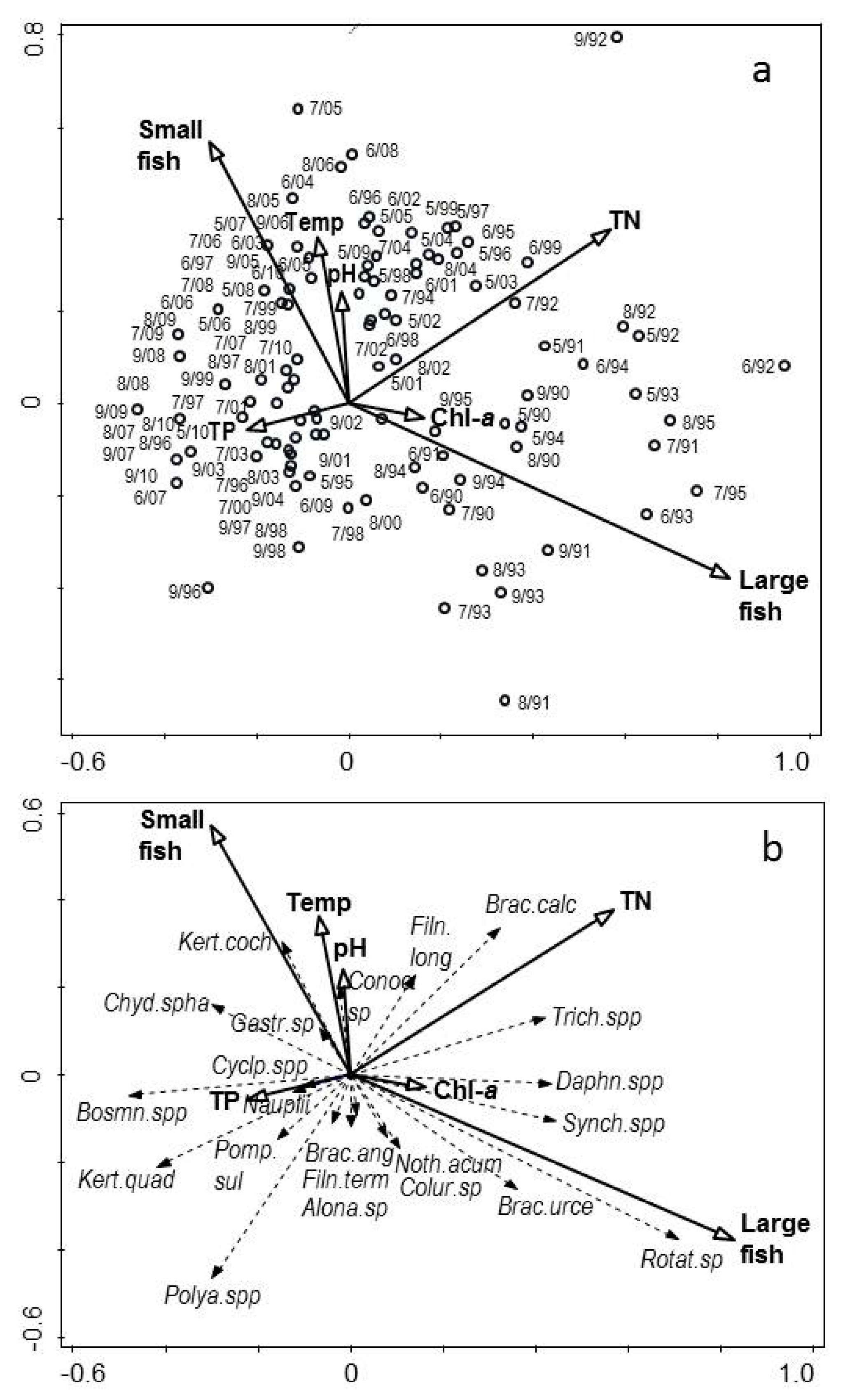
3. Results
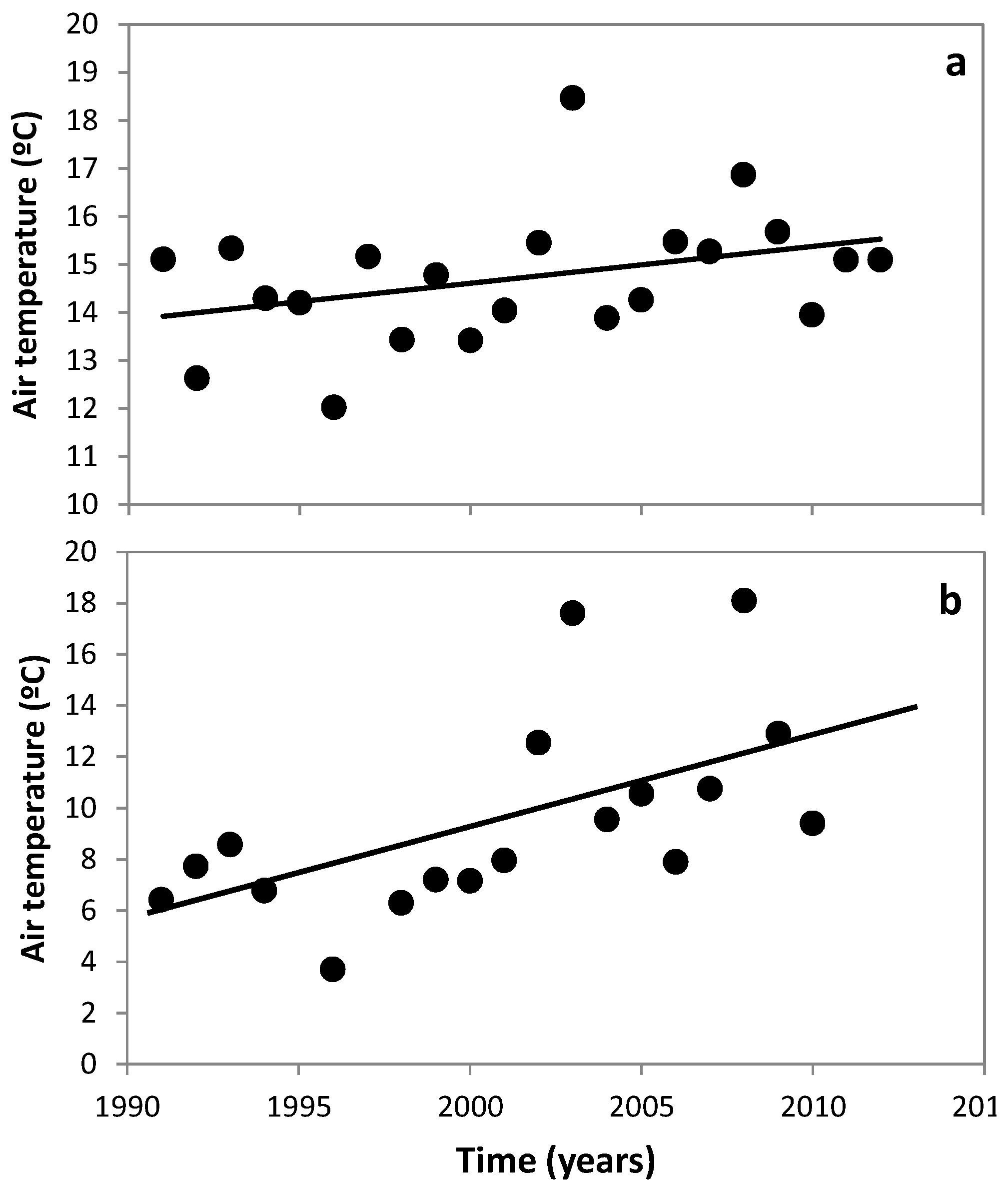
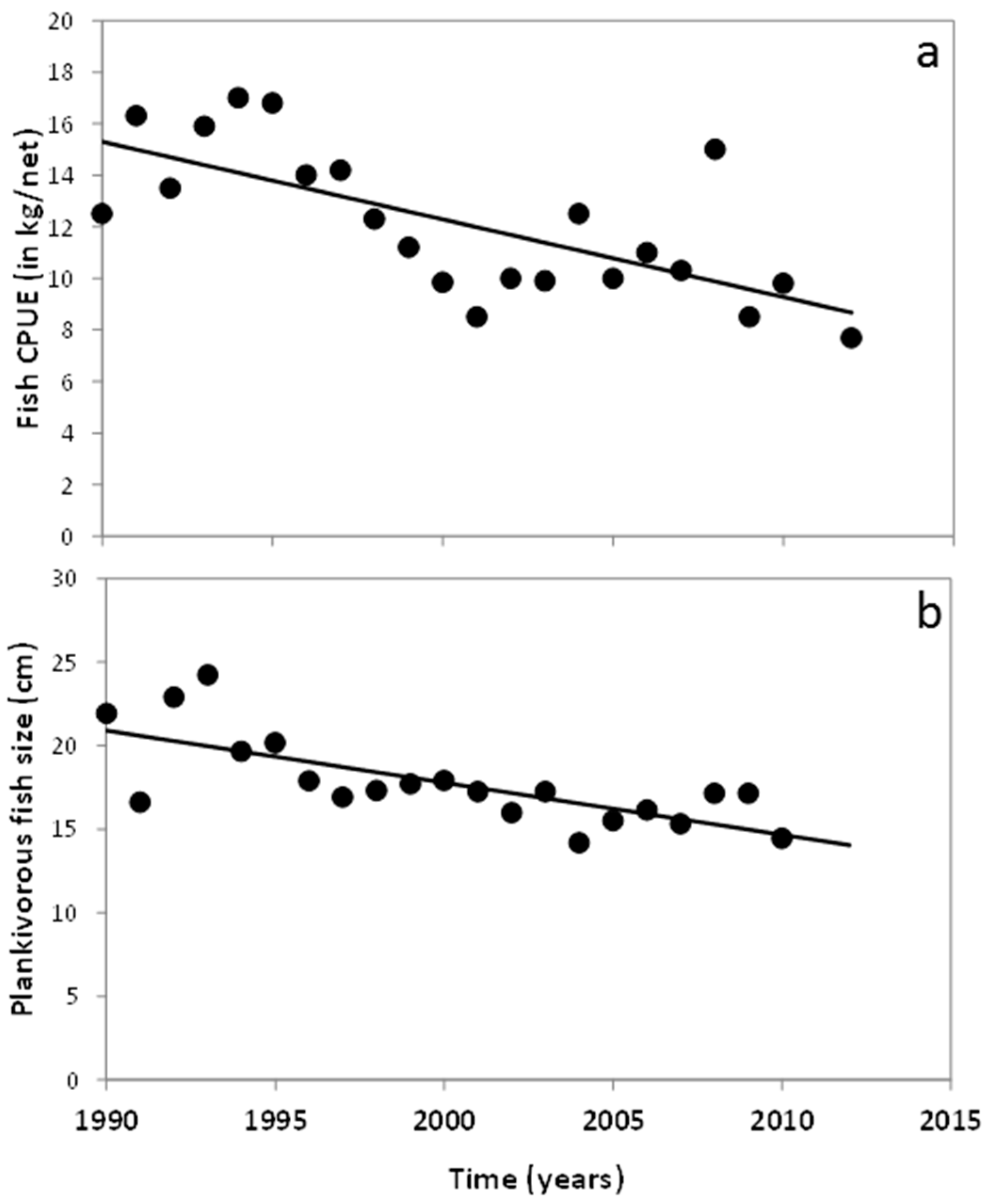
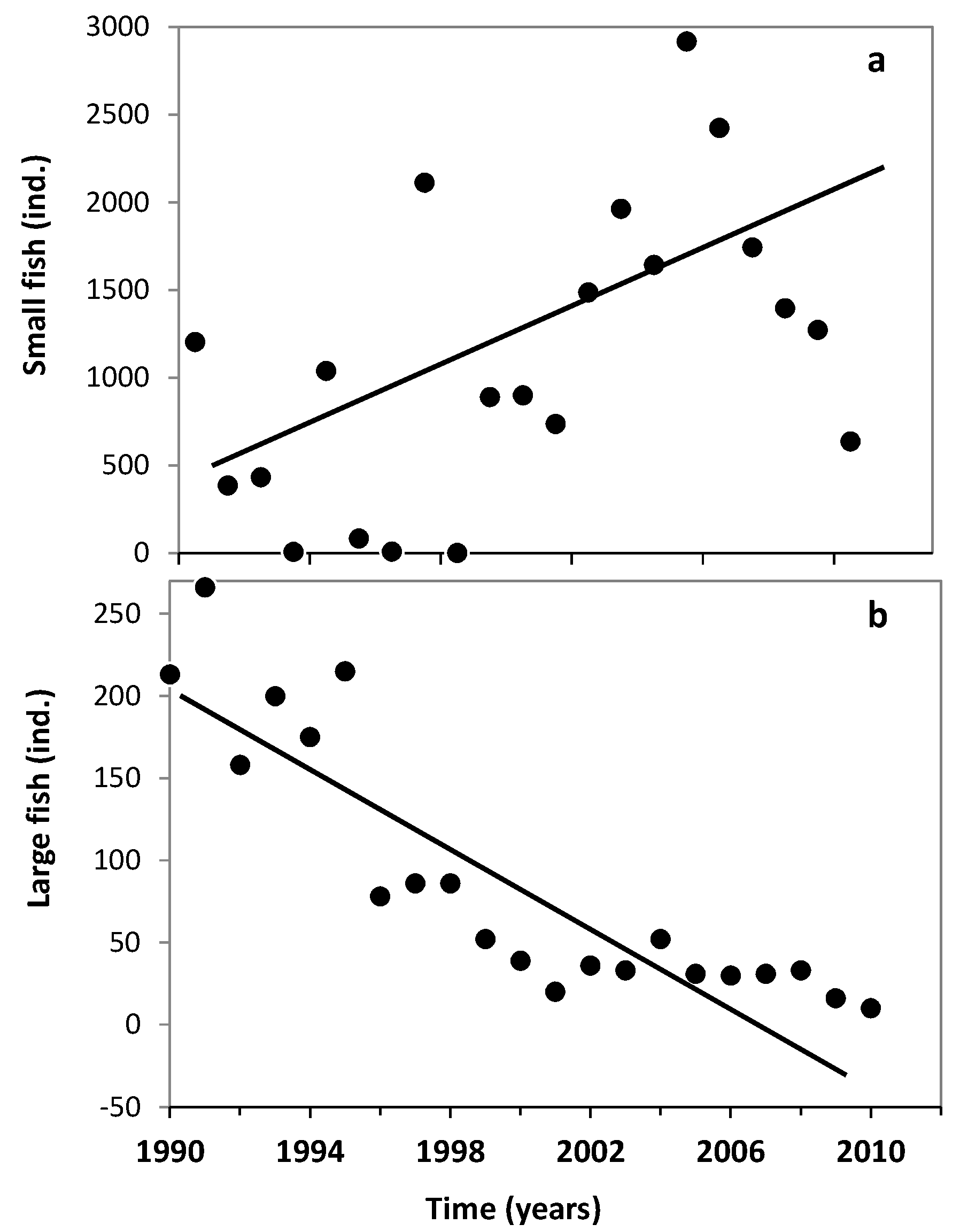
3.1. Changes in Temperature, Fish Assemblage and Their Influence on the Zooplankton Community
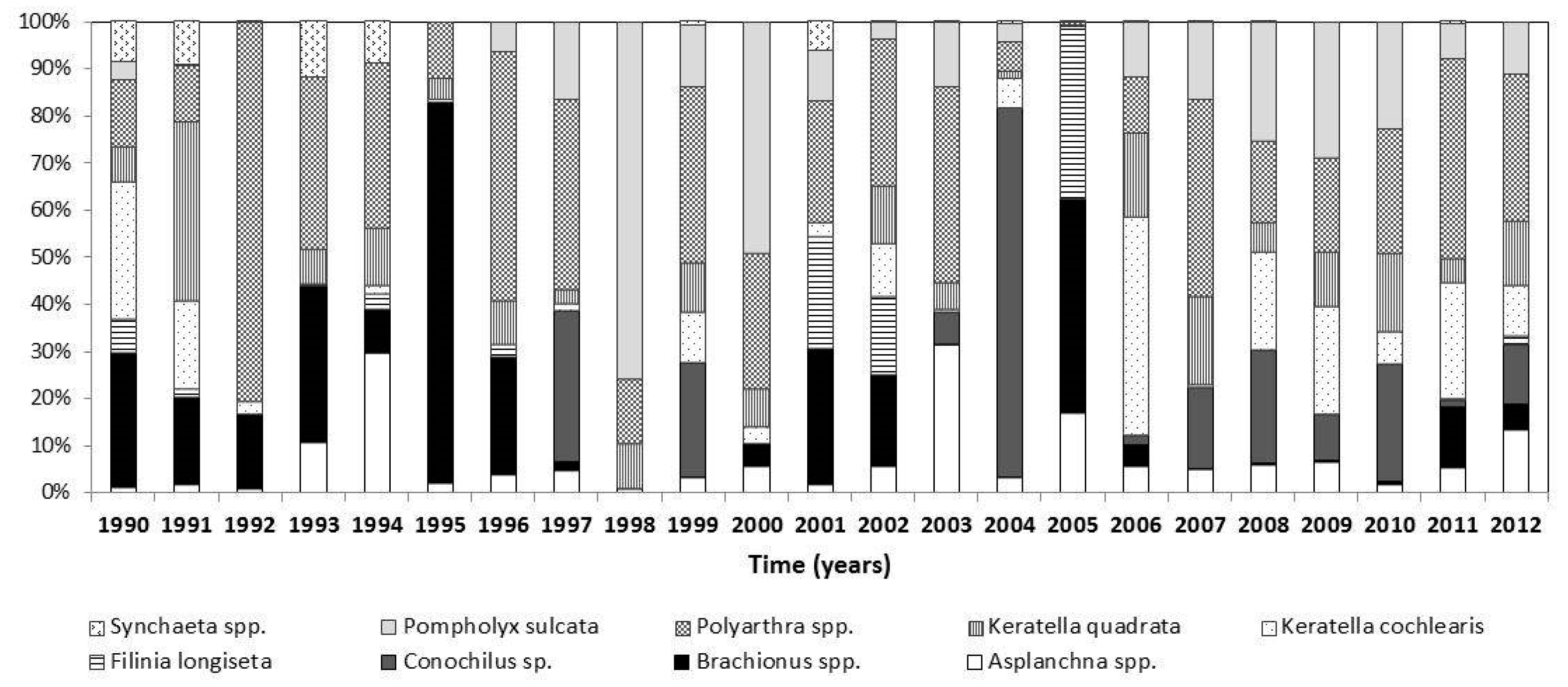
3.2. Changes in the Zooplankton Assemblage and Their Influence on Phytoplankton
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Sas, H. Lake Restoration by Reduction of Nutrient Loading: Expectations, Experiences, Extrapolations; Academia Verlag Richarz: St. Augustin, Germany, 1989. [Google Scholar]
- Marsden, M.W. Lake restoration by reducing external phosphorus loading: The influence of sediment phosphorus release. Freshw. Biol. 1989, 21, 139–162. [Google Scholar] [CrossRef]
- Benndorf, J. Possibilities and limits for controlling eutrophication by biomanipulation. Int. Rev. Gesamt. Hydrobiol. 1995, 80, 519–534. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Sandsten, H.; Møller, P.H. The restoration of a shallow lake by introducing Potamogeton spp. The impact of waterfowl grazing. Lakes Reserv. Res. Manag. 2003, 8, 177–187. [Google Scholar] [CrossRef]
- Søndergaard, M.; Liboriussen, L.; Pedersen, A.R.; Jeppesen, E. Lake restoration by fish removal: Long-term effects in 36 Danish lakes. Ecosystems 2008, 1, 1291–1305. [Google Scholar] [CrossRef]
- Köhler, J.; Hilt, S.; Adrian, R.; Nicklisch, A.; Kozerski, H.P.; Walz, N. Long-term response of a shallow, moderately flushed lake to reduced external phosphorus and nitrogen loading. Freshw. Biol. 2005, 50, 1639–1650. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Lauridsen, T.L.; Skov, C.; Van Nes, E.H.; Roijackers, R.; Portielje, R.O.B. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2007, 44, 1095–1105. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E.; Møller, P.H. Seasonal dynamics in the concentrations and retention of phosphorus in shallow Danish lakes after reduced loading. Aquat. Ecosyst. Health. Manag. 2002, 5, 19–29. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Trolle, D.; Sondergaar, D.M.; Lauridsen, T.L.; Nielsen, A. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limnol. 2014, 73, 84–107. [Google Scholar] [CrossRef] [Green Version]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Meerhoff, M.; Clemente, J.M.; Teixeira-de Mello, F.; Iglesias, C.; Pedersen, C.R.; Jeppesen, E. Can warm climate-related structure of littoral predator assemblies weaken clear water state in shallow lakes? Glob. Chang. Biol. 2007, 13, 1888–1897. [Google Scholar] [CrossRef]
- Jeppesen, E.; Moss, B.; Bennion, H.; Carvalho, L.; De Meester, L.; Feuchtmayr, H.; Friberg, N.; Gessner, M.O.; Hefting, M.; Lauridsen, T.L.; et al. Interaction of Climate Change and Eutrophication. In Climate Change Impacts on Freshwater Ecosystems; Kernan, M., Moss, B., Battarbee, R., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 119–151. [Google Scholar]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful Cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Huber, V.; Wagner, C.; Gerten, D.; Adrian, R. To bloom or not to bloom: Contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Christoffersen, K.; Andersen, N.; Søndergaard, M.; Liboriussen, L.; Jeppesen, E. Implications of climate-enforced temperature increases on freshwater pico- and nanoplankton populations studied in artificial ponds during 16 months. Hydrobiologia 2006, 560, 259–266. [Google Scholar] [CrossRef]
- Özen, A.; Šorf, M.; Trochine, C.; Liboriussen, L.; Beklioglu, M.; Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppesen, E. Long-term effects of warming and nutrients on microbes and other plankton in mesocosms. Freshw. Biol. 2013, 58, 483–493. [Google Scholar] [CrossRef]
- Winder, M.; Schindler, D.E. Climatic effects on the phenology of lake processes. Glob. Chang. Biol. 2004, 10, 1844–1856. [Google Scholar] [CrossRef]
- Brucet, S.; Boix, D.; Quintana, X.D.; Jensen, E.; Nathansen, L.W.; Trochine, C.; Meerhoff, M.; Gascón, S.; Jeppesen, E. Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: Implications for effects of climate change. Limnol. Oceanogr. 2010, 55, 1697–1711. [Google Scholar] [CrossRef]
- Emmrich, M.; Brucet, S.; Ritterbusch, D.; Mehner, T. Size spectra of lake fish assemblages: Responses along gradients of general environmental factors and intensity of lake-use. Freshw. Biol. 2011, 56, 2316–2333. [Google Scholar] [CrossRef]
- Basset, A.; Barbone, E.; Borja, A.; Brucet, S.; Pinna, M.; Quintana, X.D.; Reizopoulou, S.; Rosati, I.; Simboura, N. A benthic macroinvertebrate size spectra index for implementing the Water Framework Directive in coastal lagoons in Mediterranean and Black Sea ecoregions. Ecol. Indic. 2012, 12, 72–83. [Google Scholar] [CrossRef]
- Schweiger, O.; Settele, J.; Kudrna, O.; Klotz, S.; Kühn, I. Climate change can cause spatial mismatch of trophically interacting species. Ecology 2008, 89, 3472–3479. [Google Scholar] [CrossRef] [PubMed]
- Meerhoff, M.; Teixeira-de Mello, F.; Kruk, C.; Alonso, C.; Gonzalez Bergonzoni, I.; Pacheco, J.P.; Lacerot, G.; Arim, M.; Beklioglu, M.; Balmana, S.B. Environmental warming in shallow lakes: A review of potential changes in community structure as evidenced from space-for-time substitution approaches. Adv. Ecol. Res. 2012, 46, 259–349. [Google Scholar]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P.; et al. Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Teixeira de-Mello, F.; Meerhoff, M.; Pekcan-Hekim, Z.; Jeppesen, E. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshw. Biol. 2009, 54, 1202–1215. [Google Scholar] [CrossRef]
- Gonzalez-Bergonzoni, I.; Meerhoff, M.; Davidson, T.A.; Teixeira-de Mello, F.; Baattrup-Pedersen, A.; Jeppesen, E. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems 2012, 15, 492–503. [Google Scholar] [CrossRef]
- Brucet, S.; Pedron, S.; Mehner, T.; Lauridsen, T.L.; Argillier, C.; Winfield, I.J.; Volta, P.; Emmrich, M.; Hesthagen, T.; Holmgren, K.; et al. Fish diversity in European lakes: Geographical predictors dominate over anthropogenic pressures. Freshw. Biol. 2013, 58, 1779–1793. [Google Scholar] [CrossRef]
- Jackson, D.A.; Mandrak, N.E. Changing fish biodiversity: Predicting the loss of cyprind biodiversity due to global climate change. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 89–98. [Google Scholar]
- Balayla, D.; Lauridsen, T.L.; Søndergaard, M.; Jeppesen, E. Larger zooplankton in Danish lakes after cold winters: Are winter fish kills of importance? Hydrobiologia 2010, 646, 159–172. [Google Scholar] [CrossRef]
- Feuchtmayr, H.; Moran, R.; Hatton, K.; Connor, L.; Heyes, T.; Moss, B.; Harvey, I.; Atkinson, D. Global warming and eutrophication: Effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. J. Appl. Ecol. 2009, 46, 713–723. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Sakai, Y.; Ban, S.; Ishikawa, K.; Ishikawa, T.; Ichise, S.; Yamamura, N.; Kumagai, M. Eutrophication and warming effects on long-term variation of zooplankton in Lake Biwa. Biogeosciences 2011, 8, 1383–1399. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.L. Response of fish and plankton to nutrient loading reduction in eight shallow Danish lakes with special emphasis on seasonal dynamics. Freshw. Biol. 2005, 50, 1616–1627. [Google Scholar] [CrossRef]
- Romo, S.; Villena, M.J.; Sahuquillo, M.; Soria, J.M.; Gimenez, M.; Alfonso, T.; Miracle, M.R. Response of a shallow Mediterranean lake to nutrient diversion: Does it follow similar patterns as in northern shallow lakes? Freshw. Biol. 2005, 50, 1706–1717. [Google Scholar] [CrossRef]
- Straile, D.; Adrian, R. The North Atlantic Oscillation and plankton dynamics in two European lakes—Two variations on a general theme. Glob. Chang. Biol. 2000, 6, 663–670. [Google Scholar] [CrossRef]
- Lau, S.S.; Lane, S.N. Biological and chemical factors influencing shallow lake eutrophication: A long-term study. Sci. Tot. Environ. 2002, 288, 167–181. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Persson, L.; Diehl, S.; Johansson, L.; Andersson, G.; Hamrin, S.F. Shifts in fish communities along the productivity gradient of temperate lakes—Patterns and the importance of size-structured interactions. J. Fish Biol. 1991, 38, 281–293. [Google Scholar] [CrossRef]
- Mackey, A.P.; Berrie, A.D. The prediction of water temperatures in chalk streams from air temperatures. Hydrobiologia. 1991, 210, 183–189. [Google Scholar] [CrossRef]
- Livingstone, D.M.; Lotter, A.F. The relationship between air and water temperatures in lakes of the Swiss Plateau: A case study with paleolimnological implications. J. Paleolimnol. 1998, 19, 181–198. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Mortensen, E.; Hansen, A.; Jørgensen, T. Cascading trophic interactions from fish to bacteria and nutrients after reduced sewage loading: An 18-year study of a shallow hypertrophic lake. Ecosystems 1998, 1, 250–267. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Koroleff, F. Determination of total phosphorus in natural waters by means of persulphate oxidation. Interlab. Rep. Cons. Int. Explor Mer. 1970, 3, 19–22. [Google Scholar]
- Solórzano, L.; Sharp, J.H. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol. Oceanogr. 1980, 25, 754–758. [Google Scholar] [CrossRef]
- Jespersen, A.M.; Christoffersen, K. Measurements of chlorophyll a from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 1987, 109, 445–454. [Google Scholar]
- Ruttner-Kolisko, A. Suggestions for biomass calculations of plankton rotifers. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1977, 8, 71–76. [Google Scholar]
- Koste, W. Rotatoria. Die RaÈdertiere Mitteleuropas; Borntraeger: Berlin, Germany, 1978; Volume 2, p. 234. [Google Scholar]
- Smirnov, N.N. Cladocera: The Chydorinae and Sayciinae (Chydoridae) of the World; SPB Academic Publishing: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Ueda, H.; Reid, J.W. (Eds.) Copepoda: Cyclopoida. Genera Mesocyclops and Thermocyclops. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 20; Backhuys Publishers: Leiden, The Netherlands, 2003.
- Bottrell, H.H.; Duncan, A.; Gliwicz, Z.M.; Grygierek, E.; Herzig, A.; Hillbricht-Ilkows, A. A review of some problems in zooplankton production studies. Norw. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Culver, D.A.; Bouchete, M.M.; Bean, D.J.; Fletcher, J.W. Biomass of freshwater crustacean zooplankton from length-weight regressions. Can. J. Fish. Aquat. Sci. 1985, 42, 1380–1390. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1964. [Google Scholar]
- Hadfield, J.D. MCMC methods for Multi-response Generalised Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Jackman, S. pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory, Stanford University; R Package Version 1.4.9; Department of Political Science, Stanford University: Stanford, CA, USA, 2015. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. Canoco for Windows Version 4.5; Centre for Biometry Wageningen: Wageningen, The Netherlands, 2002. [Google Scholar]
- Phillips, G.; Kelly, A.; Pitt, J.A.; Sanderson, R.; Taylor, E. The recovery of a very shallow eutrophic lake, 20 years after the control of effluent derived phosphorus. Freshw. Biol. 2005, 50, 1628–1638. [Google Scholar] [CrossRef]
- Mehner, T.; Benndorf, J.; Kasprzak, P.; Koschel, R. Biomanipulation of lake ecosystems: Successful applications and expanding complexity in the underlying science. Freshw. Biol. 2002, 4, 2453–2465. [Google Scholar] [CrossRef]
- Molinero, J.C.; Anneville, O.; Souissi, S.; Balvay, G.; Gerdeaux, D. Anthropogenic and climate forcing on the long-term changes of planktonic rotifers in Lake Geneva, Europe. J. Plankton Res. 2006, 28, 287–296. [Google Scholar] [CrossRef]
- Seebens, H.; Straile, D.; Hoegg, R.; Stich, H.B.; Einsle, U. Population dynamics of a freshwater calanoid copepod: Complex responses to changes in trophic status and climate variability. Limnol. Oceanogr. 2007, 52, 2364–2372. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, body size and composition of the plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Emmrich, M.; Pédron, S.; Brucet, S.; Winfield, I.J.; Jeppesen, E.; Volta, P.; Argillier, C.; Lauridsen, T.L.; Holmgren, K.; Hesthagen, H.; et al. Geographical patterns of size structure of European lake fish communities along abiotic and biotic gradients. J. Biogeogr. 2014, 41, 2221–2233. [Google Scholar] [CrossRef] [Green Version]
- Šorf, M.; Davidson, T.A.; Brucet, S.; Menezes, R.F.; Søndergaard, M.; Lauridsen, T.L.F.; Liboriussen, L.; Jeppesen, E. Zooplankton response to climate warming: A mesocosm experiment at contrasting temperatures and nutrient levels. Hydrobiologia 2015, 742, 185–203. [Google Scholar] [CrossRef]
- Havens, K.E.; Elia, A.C.; Taticchi, M.I.; Fulton, R.S. Zooplankton–phytoplankton relationships in shallow subtropical versus temperate lakes Apopka (Florida, USA) and Trasimeno (Umbria, Italy). Hydrobiologia 2009, 628, 165–175. [Google Scholar] [CrossRef]
- Eckert, B.; Walz, N. Zooplankton succession and thermal stratification in the polymictic shallow Müggelsee (Berlin, Germany): A case for the intermediate disturbance hypothesis? Hydrobiologia 1998, 337, 199–206. [Google Scholar] [CrossRef]
- Pieczyńska, E.; Kołodziejczyk, A.; Rybak, J.I. The responses of littoral invertebrates to eutrophication-linked changes in plant communities. Hydrobiologia 1998, 391, 9–21. [Google Scholar] [CrossRef]
- Rolighed, J.; Trolle, D.; Søndergaard, M.; Bjerring, R.; Janse, J.H.; Mooij, W.M.; Jeppesen, E. Climate change makes recovery from eutrophication more difficult—A PCLake model study of shallow Danish Lake Søbygaard. 2016; submitted. [Google Scholar]
- Fryer, G. Evolution and adaptive radiation in the Chydoridae (Crustacea: Cladocera): A study in comparative functional morphology and ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1968, 254, 221–384. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hakala, T.; Saesmaa, S.; Viitasalo, M. Prey selection by the larvae of three species of littoral fishes on natural zooplankton assemblages. Aquat. Ecol. 2007, 41, 85–94. [Google Scholar] [CrossRef]
- Mahjoub, M.S.; Souissi, S.; Michalec, F.G.; Schmitt, F.G.; Hwang, J.S. Swimming kinematics of Eurytemora affinis (Copepoda, Calanoida) reproductive stages and differential vulnerability to predation of larval Dicentrarchus labrax (Teleostei, Perciformes). J. Plankton Res. 2011. [Google Scholar] [CrossRef]
- Kratina, P.; Greig, H.S.; Thompson, P.L.; Carvalho-Pereira, T.S.; Shurin, J.B. Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 2012, 93, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.I.; Piehler, M.F.; Leech, D.M.; Anton, A.; Bruno, J.F. Warming and resource availability shift food web structure and metabolism. PLoS Biol. 2009, 7, e1000178. [Google Scholar]
- Shurin, J.B. Interactive effects of predation and dispersal on zooplankton communities. Ecology 2001, 82, 3404–3416. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development (OECD). Eutrophication of Waters. Monitoring, Assessment and Control; OECD: Paris, France, 1982. [Google Scholar]

| Taxa | Contribution % | |
|---|---|---|
| among Years | between Decades | |
| CLADOCERA | ||
| Bosmina spp. | 16.15 | 15.32 |
| Daphnia spp. | 3.083 | 3.083 |
| COPEPODA | ||
| Cyclopoidae spp. | 7.27 | 7.196 |
| Nauplii | 5.153 | 5.161 |
| ROTIFERA | ||
| Polyarthra spp. | 7.065 | 6.862 |
| Keratella cochlearis | 4.216 | 4.167 |
| Keratella quadrata | 3.251 | 3.257 |
| Pompholyx sulcata | 4.143 | 4.056 |
| Conochilus sp. | 3.452 | 3.229 |
| Brachionus calyciflorus | 2.468 | 2.478 |
| Rotatoria sp. 1 | 4.108 | 4.623 |
| Asplanchna spp. | 2.39 | 2.325 |
| Estimate | SE | ppscl | log-likelihood | |
|---|---|---|---|---|
| Bosmina spp. | ||||
| intercept | 73130 | 0.0063 | <0.001 | −27,270 |
| large fish | −0.0071 | 0.0000 | <0.001 | |
| Daphnia spp. | ||||
| intercept | 44700 | 0.0443 | <0.001 | −1291 |
| small fish | −0.0005 | <0.0001 | <0.001 | |
| Chl-a | 0.0010 | 0.0002 | <0.001 |
| post.mean | l-95% CI | u-95% CI | pMCMC | DIC | |
|---|---|---|---|---|---|
| Chl-a (all period) | |||||
| intercept | 3.4927 | 3.0223 | 4.0609 | <0.001 | 1479 |
| Daphnia spp. | −0.0015 | −0.0025 | −0.0003 | 0.004 | |
| TN | 0.0034 | 0.0019 | 0.0049 | <0.001 | |
| TP | 0.0075 | 0.0029 | 0.0125 | 0.004 | |
| Chl-a (spring-summer) | |||||
| intercept | 3.6550 | 3.3707 | 3.9376 | <0.001 | 958 |
| Daphnia spp. | −0.0014 | −0.0021 | −0.0007 | <0.001 | |
| TN | 0.0056 | 0.0039 | 0.0072 | <0.001 | |
| TP | 0.0066 | 0.0031 | 0.0094 | <0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, M.F.; Devercelli, M.; Brucet, S.; Lauridsen, T.L.; Søndergaard, M.; Jeppesen, E. Is Recovery of Large-Bodied Zooplankton after Nutrient Loading Reduction Hampered by Climate Warming? A Long-Term Study of Shallow Hypertrophic Lake Søbygaard, Denmark. Water 2016, 8, 341. https://doi.org/10.3390/w8080341
Gutierrez MF, Devercelli M, Brucet S, Lauridsen TL, Søndergaard M, Jeppesen E. Is Recovery of Large-Bodied Zooplankton after Nutrient Loading Reduction Hampered by Climate Warming? A Long-Term Study of Shallow Hypertrophic Lake Søbygaard, Denmark. Water. 2016; 8(8):341. https://doi.org/10.3390/w8080341
Chicago/Turabian StyleGutierrez, María Florencia, Melina Devercelli, Sandra Brucet, Torben L. Lauridsen, Martin Søndergaard, and Erik Jeppesen. 2016. "Is Recovery of Large-Bodied Zooplankton after Nutrient Loading Reduction Hampered by Climate Warming? A Long-Term Study of Shallow Hypertrophic Lake Søbygaard, Denmark" Water 8, no. 8: 341. https://doi.org/10.3390/w8080341







