A Long-Term Study of Ecological Impacts of River Channelization on the Population of an Endangered Fish: Lessons Learned for Assessment and Restoration
Abstract
:1. Introduction
1.1. Learning by Doing
1.2. Challenges of Assessing Impacts on Populations
1.3. Challenges of Assessing Biological Effects of Fine Sediment
1.4. Challenges of Assessing Impacts on Endangered Species
1.5. Aim of This Paper
2. Case Study: The Roanoke River Flood Reduction Project
2.1. Background
2.1.1. Study Species
2.1.2. Study Area
2.1.3. Description of the Roanoke River Flood Reduction Project
2.2. Initial Conceptual Model and Monitoring Plan
2.3. Pre-Construction Monitoring Period
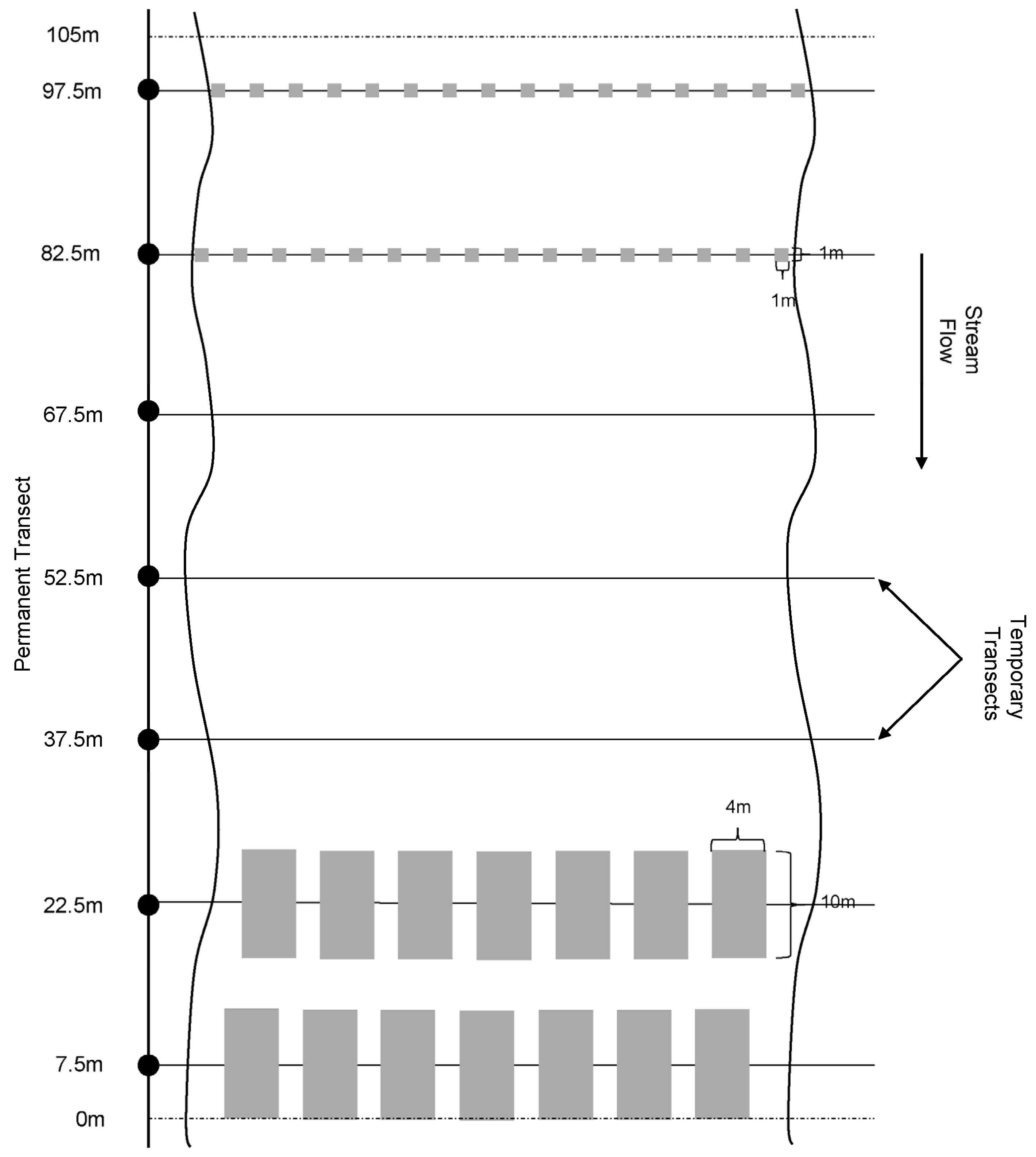
2.3.1. Description of Methods
2.3.2. Construction Delays
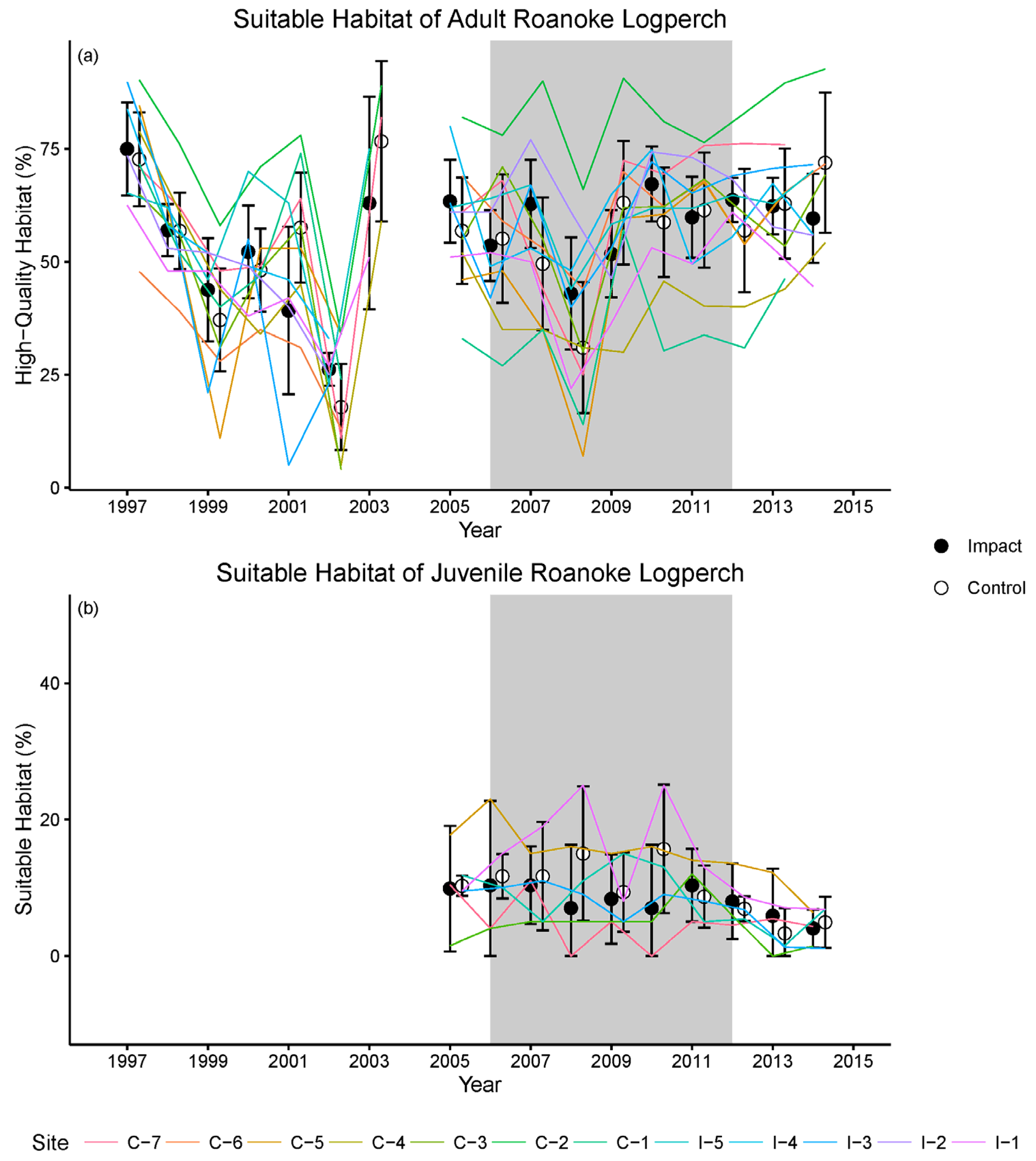
2.3.3. Results and Insights from Pre-Construction Monitoring
2.3.4. Other Opportunistic Data Collection
2.4. New Biological Opinion and Monitoring Plan
2.5. Construction and Post-Construction Monitoring Period
2.5.1. Description of New Methods
2.5.2. Results of Construction and Post-Construction Monitoring
2.6. Results and Power Analyses of BACI Tests for RRFRP Impacts
2.7. Applications of RRFRP Findings to P. rex Conservation
3. Key Lessons Learned
3.1. Lesson 1: Plan Ahead
3.2. Lesson 2: Develop Adaptable Conceptual and Analytical Models Early
3.3. Lesson 3: Recognize Limits of Study Scope
3.4. Lesson 4: Carefully Choose Analytical Frameworks and Tools
4. Conclusions: Robust Monitoring in the Real World?
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AM | Adaptive Management |
| ANOVA | Analysis of Variance |
| BACI | Before-After-Control-Impact |
| BO | Biological Opinion |
| ESA | Endangered Species Act |
| GLMM | Generalized Linear Mixed Model |
| ITS | Incidental Take Statement |
| RRFRP | Roanoke River Flood Reduction Project |
| USACE | United States Army Corps of Engineers |
| USFWS | United States Fish and Wildlife Service |
| USGS | United States Geological Survey |
| VA | Virginia |
Appendix
| Variable | Type | Hyp. | Modeled Levels | Description |
|---|---|---|---|---|
| Discharge | Continuous | 1–8 | - | Discharge at the time of sample. |
| Season | Factor | 1–8 | Fall | Samples taken in September–October. Summer samples are the reference category (i.e., intercept) |
| Reach | Factor | 1–8 | I | Samples taken in the impact reach (I). Control reach (C) samples are the reference category. |
| Project Phase | Factor | 1 | Con. | Samples taken during the active construction project phase (2006–2011). Pre-construction (Pre Con.) samples (1997–2005) are the reference category. |
| Post Con. | Samples taken during the post-construction project phase (2012–2015). Pre-construction samples (1997–2005) are the reference category. | |||
| Continued Project Phase | Factor | 2 | Cont. Con. | Samples taken during the construction or post-construction project phase. Pre-construction samples are the reference category. |
| Lagged Project Phase | Factor | 3 | Lag Con. | Samples taken at least two years after the onset of construction to two years after the completion of construction (i.e., 2008–2013). Pre-construction samples and the first two years of construction samples are the reference category. |
| Lag Post Con. | Samples taken at least two years after the completion of construction (2014–2015). Pre-construction samples and the first two years of construction samples are the reference category. | |||
| Continued Lagged Project Phase | Factor | 4 | Cont. Lag Con. | Samples taken at least two years after the onset of construction through post-construction (i.e., 2008–2015). Pre-construction samples and the first two years of construction samples are the reference category. |
| Year | Continuous | - | - | Continuous variable representing the year of study. |
| Project Phase Slope | Interaction | 5 | Pre Con. × Year | Interaction between the Pre. Con level of Project Phase and Year. |
| Con. × Year | Interaction between the Con level of Project Phase and Year. | |||
| Post Con. × Year | Interaction between the Post Con level of Project Phase and Year. | |||
| Continued Project Phase Slope | Interaction | 6 | Pre Con. × Year | Interaction between the Pre. Con level of Continued Project Phase and Year. |
| Cont. Con. × Year | Interaction between the Cont. Con. level of Continued Project Phase and Year. | |||
| Lagged Project Phase Slope | Interaction | 7 | Lag Pre Con. × Year | Interaction between the Lag Pre Con. level of Lagged Project Phase and Year. |
| Lag Con. × Year | Interaction between the Lag Con. level of Lagged Project Phase and Year. | |||
| Lag Post Con. × Year | Interaction between the Lag Post Con. level of Lagged Project Phase and Year. | |||
| Continued Lagged Project Phase Slope | Interaction | 8 | Lag Pre Con. × Year | Interaction between the Lag Pre Con. level of Continued Lagged Phase and Year. |
| Cont. Lag Con. × Year | Interaction between the Cont. Lag Con. level of Continued Lagged Project Phase and Year. | |||
| Mean Effect | Interaction | 1 | I × Con. | Interaction between the I level of reach and the Con. level of Project Phase. |
| Continued Mean Effect | Interaction | 2 | I × Cont. Con. | Interaction between the I level of reach and the Cont. Con. level of Continued Project Phase. |
| Lagged Mean Effect | Interaction | 3 | I × Lag Con. | Interaction between the I level of reach and the Lag Con. level of Lagged Project Phase. |
| Continued Lagged Mean Effect | Interaction | 4 | I × Lag Cont. Con. | Interaction between the I reach and the Cont. Lag Con. level of Continued Lagged Project Phase. |
| Slope Effect | Interaction | 5 | I × Pre Con. × Year | Interaction between the I level of reach, the Pre Con. level of Project Phase, and the year. |
| I × Con. × Year | Interaction between the I level of reach, the Con. level of Project Phase, and the year. | |||
| I × Post Con. × Year | Interaction between the I level of reach, the Post Con. level of Project Phase, and the year. | |||
| Continued Slope Effect | Interaction | 6 | I × Pre Con. × Year | Interaction between the I level of reach, the Pre Con. level of Continued Project Phase, and the year. |
| I × Cont. Con. × Year | Interaction between the I level of reach, the Cont. Con. level of Continued Project Phase, and the year. | |||
| Lagged Slope Effect | Interaction | 7 | I × Lag Pre Con. × Year | Interaction between the I level of reach, the Lag Pre Con. level of Lagged Project Phase, and the year. |
| I × Lag Con. × Year | Interaction between the I level of reach, the Lag Con. level of Lagged Project Phase, and the year. | |||
| I × Lag Post Con. × Year | Interaction between the I level of reach, the Lag Post Con. level of Lagged Project Phase, and the year. | |||
| Continued Lagged Slope Effect | Interaction | 8 | I × Pre Con. × Year | Interaction between the I level of reach, the Lag Pre Con. level of Continued Lagged Project Phase, and the year. |
| I × Cont. Con. × Year | Interaction between the I level of reach, the Cont. Lag Con. level of Continued Lagged Project Phase, and the year. |
| Variable | Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Null | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Intercept | −1.59 | −1.70 | −1.70 | −1.50 | −1.50 | −0.98 | −1.02 | −1.09 | −1.08 |
| Discharge | −0.16 | −0.15 | −0.16 | −0.16 | −0.16 | −0.20 | −0.20 | −0.20 | −0.20 |
| Season (Fall) | −0.07 | −0.06 | −0.06 | −0.06 | −0.07 | −0.10 | −0.11 | −0.09 | −0.10 |
| Reach (I) | 0.52 | 0.56 | 0.56 | 0.43 | 0.43 | 0.29 | 0.18 | 0.27 | 0.21 |
| Con. Phase | - | 0.39 | - | - | - | - | - | - | - |
| Cont. Con. Phase | - | - | 0.20 | - | - | - | - | - | - |
| Lag Con. Phase | - | - | - | −0.11 | - | - | - | - | - |
| Cont. Lag Con. Phase | - | - | - | - | −0.21 | - | - | - | - |
| Post Con. Phase | - | −0.17 | - | - | - | - | - | - | - |
| Lag Post Con. Phase | - | - | - | −0.62 | - | - | - | - | - |
| Pre Con. × Year | - | - | - | - | - | 0.68 | 0.64 | - | - |
| Lag Pre Con. × Year | - | - | - | - | - | - | - | 0.59 | 0.60 |
| Con. × Year | - | - | - | - | - | −0.78 | - | - | - |
| Cont. Con. × Year | - | - | - | - | - | - | −0.63 | - | - |
| Lag Con. × Year | - | - | - | - | - | - | - | −0.56 | - |
| Cont. Lag Con. × Year | - | - | - | - | - | - | - | - | −0.59 |
| Post Con. × Year | - | - | - | - | - | −0.63 | - | - | - |
| Lag Post Con. × Year | - | - | - | - | - | - | - | −0.63 | - |
| I × Con. | - | −0.31 | - | - | - | - | - | - | - |
| I × Cont. Con. | - | - | −0.07 | - | - | - | - | - | - |
| I × Lag Con. | - | - | - | 0.02 | - | - | - | - | - |
| I × Cont. Lag Con. | - | - | - | - | 0.21 | - | - | - | - |
| I × Post Con. | - | 0.37 | - | - | - | - | - | - | - |
| I × Lag Post Con. | - | - | - | 0.87 | - | - | - | - | - |
| I × Pre Con. × Year | - | - | - | - | - | −0.22 | −0.31 | - | - |
| I × Lag Pre Con. × Year | - | - | - | - | - | - | - | −0.25 | −0.29 |
| I × Con. × Year | - | - | - | - | - | 0.09 | - | - | - |
| I × Cont. Con. × Year | - | - | - | - | - | - | 0.49 | - | - |
| I × Lag Con. × Year | - | - | - | - | - | - | - | 0.26 | - |
| I × Cont. Lag Con. × Year | - | - | - | - | - | - | - | - | 0.48 |
| I × Post Con. × Year | - | - | - | - | - | 0.47 | - | - | - |
| I × Lag Post Con. × Year | - | - | - | - | - | - | - | 0.63 | - |
| Model Performance | |||||||||
| ℓ | −925.6 | −915.0 | −924.6 | −916.3 | −923.8 | −907.6 | −910.8 | −905.8 | −908.7 |
| Δi | 27.64 | 14.44 | 29.52 | 16.98 | 30.00 | 3.65 | 5.88 | 0.000 | 1.82 |
| wi | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.097 | 0.033 | 0.618 | 0.249 |
References
- Malmqvist, B.; Rundle, S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002, 29, 134–153. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Zawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Closs, G.P.; Angermeier, P.L.; Darwall, W.R.T.; Balcombe, S.R. Why are freshwater fish so threatened? In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J., Eds.; Cambridge University Press: London, UK, 2015; pp. 37–75. [Google Scholar]
- Bash, J.S.; Ryan, C.M. Stream Restoration and Enhancement Projects: Is Anyone Monitoring? Environ. Manag. 2002, 29, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Downs, P.W.; Kondolf, G.M. Post-project appraisals in adaptive management of river channel restoration. Environ. Manag. 2002, 29, 477–496. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A.; Allan, J.D.; The National River Restoration Science Synthesis Working Group. Restoration of U.S. Rivers: A national synthesis. Science 2005, 308, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Bernhardt, E.; Allan, J.D.; The National River Restoration Science Synthesis Working Group. Standards for ecologically successful river restoration. J. Appl. Ecol. 2005, 42, 208–217. [Google Scholar] [CrossRef]
- Alexander, A.; Alexander, G.; Allan, J.D. Ecological success in stream restoration: Case studies from the Midwestern United States. Environ. Manag. 2007, 40, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E.; Sudduth, E.; Palmer, M.; Allan, J.; Meyer, J.; Alexander, G.; Follastad Shah, J.; Hassett, B.; Jenkinson, R.; Lave, R.; et al. Restoring rivers one reach at a time: Results from a survey of US river restoration practitioners. Restor. Ecol. 2007, 15, 482–493. [Google Scholar] [CrossRef]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshw. Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Feld, C.K.; Birk, S.; Bradley, D.C.; Hering, D.; Kail, J.; Marzin, A.; Melcher, A.; Nemitz, D.; Pedersen, M.L.; Pletterbauer, F.; et al. From natural to degraded rivers and back again: A test of restoration ecology theory and practice. Adv. Ecol. Res. 2011, 44, 119–209. [Google Scholar]
- Morandi, B.; Piégay, H.; Lamouroux, N.; Vaudor, L. How is success or failure in river restoration projects evaluated? Feedback from French restoration projects. J. Environ. Manag. 2014, 137, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Aroviita, J.; Baattrup-Pedersen, A.; Brabec, K.; Buijse, T.; Ecke, F.; Friberg, N.; Gielczewski, M.; Januschke, K.; Köhler, J.; et al. Contrasting the roles of section length and instream habitat enhancement for river restoration success: A field study of 20 European restoration projects. J. Appl. Ecol. 2015, 52, 1518–1527. [Google Scholar] [CrossRef]
- Karr, J.R.; Fausch, K.D.; Angermeier, P.L.; Yant, P.R.; Schlosser, I.J. Assessing Biological Integrity in Running Waters: A Method and Its Rationale; Illinois Natural History Survey: Champaign, IL, USA, 1986. [Google Scholar]
- Lake, P.S.; Bond, N.; Reich, P. Linking ecological theory with stream restoration. Freshw. Biol. 2007, 52, 597–615. [Google Scholar] [CrossRef]
- Underwood, A.J. Beyond BACI: The detection of environmental impacts on populations in the real, but variable, world. J. Exp. Mar. Biol. Ecol. 1992, 161, 145–178. [Google Scholar] [CrossRef]
- Rosenfeld, J.S.; Hatfield, T. Information needs for assessing critical habitat of freshwater fish. Can. J. Fish. Aquat. Sci. 2006, 63, 683–698. [Google Scholar] [CrossRef]
- Jansson, R.; Nilsson, C.; Malmqvist, B. Restoring freshwater ecosystems in riverine landscapes: The roles of connectivity and recovery processes. Freshw. Biol. 2007, 52, 589–596. [Google Scholar] [CrossRef]
- Walters, C.J. Adaptive Management of Renewable Resources; MacMillan: New York, NY, USA, 1986. [Google Scholar]
- Haney, A.; Power, R.L. Adaptive management for sound ecosystem management. Environ. Manag. 1996, 20, 879–886. [Google Scholar] [CrossRef]
- Conroy, M.J.; Peterson, J.T. Decision Making in Natural Resource Management: A Structured, Adaptive Approach; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Woolsey, S.; Capelli, F.; Gonser, T.; Hoehn, E.; Hostmann, M.; Junker, B.; Paetzold, A.; Roulier, C.; Schweizer, S.; Tiegs, S.D.; et al. A strategy to assess river restoration success. Freshw. Biol. 2007, 52, 752–769. [Google Scholar] [CrossRef]
- Jansson, R.; Backx, H.; Boulton, A.J.; Dixon, M.; Dudgeon, D.; Hughes, F.M.R.; Nakamura, K.; Stanley, E.H.; Tockner, K. Stating mechanisms and refining criteria for ecologically successful river restoration: A comment on Palmer et al. (2005). J. Appl. Ecol. 2005, 42, 218–222. [Google Scholar] [CrossRef]
- Wohl, E.; Angermeier, P.L.; Bledsoe, B.; Kondolf, G.M.; MacDonnell, L.; Merritt, D.M.; Palmer, M.A.; Poff, N.L.; Tarboton, D. River restoration. Water Resour. Res. 2005, 41, 1–12. [Google Scholar] [CrossRef]
- Smallwood, K.S.; Beyea, J.; Morrison, M.L. Using the best scientific data for endangered species conservation. Environ. Manag. 1999, 24, 421–435. [Google Scholar] [CrossRef]
- Wilhere, G.F. Adaptive management in habitat conservation plans. Conserv. Biol. 2002, 16, 20–29. [Google Scholar] [CrossRef]
- Runge, M.C. An introduction to adaptive management for threatened and endangered species. J. Fish Wildl. Manag. 2011, 2, 220–233. [Google Scholar] [CrossRef]
- Tear, T.H.; Karieva, P.; Angermeier, P.L.; Comer, P.; Czech, B.; Kautz, R.; Landon, L.; Mehlman, D.; Murphy, K.; Ruckelshaus, M.; et al. How much is enough? The recurrent problem of setting measurable objectives in conservation. BioScience 2005, 55, 835–849. [Google Scholar] [CrossRef]
- Lobon-Cervia, J. Why, when and how do fish populations decline, collapse and recover? The example of brown trout (Salmo trutta) in Rio Chaballos (northwestern Spain). Freshw. Biol. 2009, 54, 1149–1162. [Google Scholar] [CrossRef]
- Ham, K.D.; Pearsons, T.N. Can reduced salmonid population abundance be detected in time to limit management impacts? Can. J. Fish. Aquat. Sci. 2000, 57, 17–24. [Google Scholar] [CrossRef]
- Reed, J.M.; Blaustein, A.R. Biologically significant population declines and statistical power. Conserv. Biol. 1997, 11, 281–282. [Google Scholar] [CrossRef]
- Harding, E.K.; Crone, E.E.; Elderd, B.D.; Hoekstra, J.M.; McKerrow, A.J.; Perrine, J.D.; Regetz, J.; Rissler, L.J.; Stanley, A.G.; Walters, E.L.; et al. The scientific foundations of habitat conservation plans: A quantitative assessment. Conserv. Biol. 2001, 15, 488–500. [Google Scholar] [CrossRef]
- Orth, D.J. Ecological considerations in the development and application of instream flow-habitat models. Regul. Rivers Res. Manag. 1987, 1, 171–181. [Google Scholar] [CrossRef]
- Rosenfeld, J. Assessing the habitat requirements of stream fishes: An overview and evaluation of different approaches. Trans. Am. Fish. Soc. 2003, 132, 953–968. [Google Scholar] [CrossRef]
- Newcomb, T.J.; Orth, D.J.; Stauffer, D.F. Habitat Evaluation. In Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 843–886. [Google Scholar]
- Pretty, J.L.; Harrison, S.S.C.; Shepherd, D.J.; Smith, C.; Hildrew, A.G.; Hey, R.D. River rehabilitation and fish populations: Assessing the benefit of instream structures. J. Appl. Ecol. 2003, 40, 251–265. [Google Scholar] [CrossRef]
- Miller, S.W.; Budy, P.; Schmidt, J.C. Quantifying macroinvertebrate responses to instream habitat restoration: Applications of meta-analysis to river restoration. Restor. Ecol. 2010, 18, 8–19. [Google Scholar] [CrossRef]
- Lepori, F.; Palm, D.; Brännäs, E.; Malmquist, B. Does restoration of structural heterogeneity in streams enhance fish and macroinvertebrate diversity? Ecol. Appl. 2005, 15, 2060–2071. [Google Scholar] [CrossRef]
- Rios-Touma, B.; Prescott, C.; Axtell, S.; Kondolf, G.M. Habitat restoration in the context of watershed prioritization: The ecological performance of urban stream restoration projects in Portland, Oregon. River Res. Appl. 2015, 31, 755–766. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (USEPA). Wadeable Streams Assessment: A Collaborative Survey of the Nation’s Streams; EPA 841-B-06-002; Office of Research and Development and Office of Water, US Environmental Protection Agency: Columbia, WA, USA, 2006.
- Jelks, H.L.; Walsh, S.J.; Burkhead, N.M.; Contreras-Balderas, S.; Díaz-Pardo, E.; Hendrickson, D.A.; Lyons, J.; Mandrak, N.E.; McCormick, F.; Nelson, J.S.; et al. Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries 2008, 33, 372–407. [Google Scholar] [CrossRef]
- Waters, T.F. Sediment in Streams: Sources, Biological Effects, and Control; American Fisheries Society: Bethesda, MD, USA, 1995. [Google Scholar]
- Henley, W.; Patterson, M.; Neves, R.; Lemly, A.D. Effects of sedimentation and turbidity on lotic food webs: A concise review for natural resource managers. Rev. Fish. Sci. 2000, 8, 125–139. [Google Scholar] [CrossRef]
- Rabeni, C.F.; Smale, M.A. Effects of siltation on stream fishes and the potential mitigating role of the buffering riparian zone. Hydrobiologia 1995, 303, 211–219. [Google Scholar] [CrossRef]
- Lapointe, M.; Bergeron, N.; Berube, F.; Pouliot, M.; Johnston, P. Interactive effects of substrate sand and silt contents, redd-scale hydraulic gradients, and interstitial velocities on egg-to-emergence survival of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2004, 61, 2271–2277. [Google Scholar] [CrossRef]
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol. Process. 2011, 25, 1800–1821. [Google Scholar] [CrossRef]
- Arnold, J.G.; Moriasi, D.N.; Gassman, P.W.; Abbaspour, K.C.; White, M.J.; Srinivasan, R.; Santhi, C.; Harmel, R.D.; van Griensven, A.; van Liew, M.W.; et al. SWAT: Model use, calibration, and validation. Trans. Am. Soc. Agric. Biol. Eng. 2012, 55, 1491–1508. [Google Scholar]
- Hamilton, S.K. Biogeochemical time lags may delay responses of streams to ecological restoration. Freshw. Biol. 2012, 57 (Suppl. S1), 43–57. [Google Scholar] [CrossRef]
- Brown, L.R.; Cuffney, T.F.; Coles, J.F.; Fitzpatrick, F.; McMahon, G.; Steuer, J.; Bell, A.H.; May, J.T. Urban streams across the USA: Lessons learned from studies in 9 metropolitan areas. J. N. Am. Benthol. Soc. 2009, 28, 1051–1069. [Google Scholar] [CrossRef]
- Violin, C.R.; Cada, P.; Sudduth, E.B.; Hassett, B.A.; Penrose, D.L.; Bernhardt, E.S. Effects of urbanization and urban stream restoration on the physical and biological structure of stream ecosystems. Ecol. Appl. 2011, 21, 1932–1949. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lyons, J.; Kanehl, P.; Bannerman, R. Impacts of urbanization on stream habitat and fish across multiple spatial scales. Environ. Manag. 2001, 28, 255–266. [Google Scholar] [CrossRef]
- Morgan, R.P.; Cushman, S.E. Urbanization effects on stream fish assemblages in Maryland, USA. J. N. Am. Benthol. Soc. 2005, 24, 643–655. [Google Scholar] [CrossRef]
- Walsh, C.J.; Fletcher, T.D.; Ladson, A.R. Stream restoration in urban catchments through redesigning stormwater systems: Looking to the catchment to save the stream. J. N. Am. Benthol. Soc. 2005, 24, 690–705. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Angermeier, P.L.; Rosenberger, A.E. Impacts of new highways and subsequent landscape urbanization on stream habitat and biota. Rev. Fish. Sci. 2005, 13, 141–164. [Google Scholar] [CrossRef]
- Roberts, J.H.; Angermeier, P.L.; Hallerman, E.M. Distance, dams and drift: What structures populations of an endangered, benthic stream fish? Freshw. Biol. 2013, 58, 2050–2064. [Google Scholar] [CrossRef]
- Roberts, J.H.; Rosenberger, A.E.; Albanese, B.W.; Angermeier, P.L. Movement patterns of endangered Roanoke logperch (Percina rex). Ecol. Freshw. Fish 2008, 17, 374–381. [Google Scholar] [CrossRef]
- Rosenberger, A.E.; Angermeier, P.L. Ontogenetic shifts in habitat use by the endangered Roanoke logperch Percina rex. Freshw. Biol. 2003, 48, 1563–1577. [Google Scholar] [CrossRef]
- Burkhead, N.M. Ecological Studies of Two Potentially Threatened Fishes (the Orangefin madtom Noturus gilberti and Roanoke logperch Percina rex) Endemic to the Roanoke River Drainage; Final Report; U.S. Army Corps of Engineers: Wilmington, NC, USA, 1983. [Google Scholar]
- Roberts, J.H.; Angermeier, P.L. Assessing Impacts of the Roanoke River Flood Reduction Project on the Endangered Roanoke Logperch; Final Report to the Wilmington District; U.S. Army Corps of Engineers: Wilmington, NC, USA, 2006. [Google Scholar]
- Roberts, J.H.; Rosenberger, A.E. Threatened fishes of the world: Percina rex (Jordan and Evermann 1989) (Percidae). Environ. Biol. Fishes 2008, 83, 439–440. [Google Scholar] [CrossRef]
- Jenkins, R.E.; Burkhead, N.M. Freshwater Fishes of Virginia; American Fisheries Society: Bethesda, MD, USA, 1994. [Google Scholar]
- Rosenberger, A.E. An Update to the Roanoke Logperch Recovery Plan; Final Report; U.S. Fish and Wildlife Service: Gloucester, VA, USA, 2007.
- Roberts, J.H.; Angermeier, P.L.; Anderson, G.B. Population viability analysis for endangered Roanoke logperch. J. Fish Wildl. Manag. 2016. [Google Scholar] [CrossRef]
- Roberts, J.H.; Angermeier, P.L.; Hallerman, E.M. Extensive dispersal of Roanoke logperch (Percina rex) inferred from genetic marker data. Ecol. Freshw. Fish 2016, 25, 1–16. [Google Scholar] [CrossRef]
- Schlosser, I.J.; Angermeier, P.L. Spatial variation in demographic processes of lotic fishes: Conceptual models, empirical evidence, and implications for conservation. Am. Fish. Soc. Symp. 1995, 17, 392–401. [Google Scholar]
- Corrigan, P. The Floods of November 1985: Then and Now; National Oceanic and Atmospheric Administration, National Weather Service: Silver Spring, MD, USA, 2010. Available online: http://www.erh.noaa.gov/rnk/hydro/Flood%20of%201985_Then-Now.pdf (accessed on 31 January 2016).
- United States Army Corps of Engineers (USACE). Roanoke River Upper Basin, Virginia, Headwaters Area, Flood Damage Reduction—General Design Memorandum Volumes I and II; U.S. Army Corps of Engineers: Wilmington, NC, USA, 1989. [Google Scholar]
- Jenkins, R.E. Roanoke Logperch Percina rex (Jordan and Evermann 1889); Final Report; U.S. Fish and Wildlife Service: Gloucester, VA, USA, 1977.
- United States Fish and Wildlife Service (USFWS). Biological Opinion on the Roanoke River Upper Basin, Headwaters Area, Flood Damage Reduction Project, in Roanoke, Virginia; U.S. Army Corps of Engineers: Wilmington, NC, USA, 1990. [Google Scholar]
- Stewart-Oaten, A.; Murdoch, W.W.; Parker, K.R. Environmental impact assessment: Pseudoreplication in time? Ecology 1986, 67, 929–940. [Google Scholar] [CrossRef]
- Anderson, G.B.; Angermeier, P.L. Assessing Impacts of the Roanoke River Flood Reduction Project on the Endangered Roanoke Logperch; Final Report; U.S. Army Corps of Engineers: Wilmington, NC, USA, 2015. [Google Scholar]
- Ensign, W.E.; Angermeier, P.L. Summary of Population Estimation and Habitat Mapping Procedures for the Roanoke River Flood Reduction Project; Final Report to the Wilmington District; U.S. Army Corps of Engineers: Wilmington, NC, USA, 1994. [Google Scholar]
- George, A.L.; Mayden, R.L. Conservation Genetics of Four Imperiled Fishes of the Southeast; Final Report; U.S. Forest Service: Washington, DC, USA, 2003. [Google Scholar]
- United States Fish and Wildlife Service (USFWS). Analysis of U.S. Army Corps of Engineers’ Roanoke River Flood Reduction Project with Respect to Natural Channel Morphology; U.S. Army Corps of Engineers: Wilmington, NC, USA, 2003. [Google Scholar]
- Roberts, J.H.; Angermeier, P.L. Monitoring Effects of the Roanoke River Flood Reduction Project on the Endangered Roanoke Logperch Percina rex; Final Report; U.S. Army Corps of Engineers: Wilmington, NC, USA, 2004. [Google Scholar]
- United States Fish and Wildlife Service (USFWS). Biological Opinion on the Roanoke River Upper Basin, Headwaters Area, Flood Damage Reduction Project, in Roanoke, Virginia; U.S. Army Corps of Engineers: Wilmington, NC, USA, 2005. [Google Scholar]
- Jastram, J.D.; Krstolic, J.L.; Moyer, D.L.; Hyer, K.E. Fluvial Geomorphology and Suspended-Sediment Transport during Construction of the Roanoke River Flood Reduction Project in Roanoke, Virginia, 2005–2012; Scientific Investigations Report 2015–5111; United States Geological Survey: Reston, VA, USA, 2015.
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.-L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Ensign, W.E.; Leftwich, K.N.; Angermeier, P.L.; Dolloff, C.A. Factors influencing stream fish recovery following a large-scale disturbance. Trans. Am. Fish. Soc. 1997, 126, 895–907. [Google Scholar] [CrossRef]
- Argentina, J.E.; Roberts, J.H. Habitat Associations for Young-of-Year Roanoke Logperch in Roanoke River; Final Report; Virginia Department of Game and Inland Fisheries: Richmond, VA, USA, 2014. [Google Scholar]
- Dutton, D.J.; Roberts, J.H.; Angermeier, P.L.; Hallerman, E.M. Microsatellite markers for the endangered Roanoke logperch Percina rex (Percidae) and their potential utility for other darter species. Mol. Ecol. Resour. 2008, 8, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Ensign, W.E.; Angermeier, P.L.; Dolloff, C.A. Use of line transect methods to estimate abundance of benthic stream fishes. Can. J. Fish. Aquat. Sci. 1995, 52, 213–222. [Google Scholar] [CrossRef]
- Walters, C.J.; Holling, C.S. Large-scale management experiments and learning by doing. Ecology 1990, 71, 2060–2068. [Google Scholar] [CrossRef]
- Vaudor, L.; Lamouroux, N.; Olivier, J.; Forcellini, M. How sampling influences the statistical power to detect changes in abundance: An application to river restoration. Freshw. Biol. 2015, 60, 1192–1207. [Google Scholar] [CrossRef]
- Korman, J.; Higgins, P.S. Utility of escapement time series data for monitoring the response of salmon populations to habitat alteration. Can. J. Fish. Aquat. Sci. 1997, 54, 2058–2067. [Google Scholar] [CrossRef]
- Kimmerer, W.J.; Murphy, D.D.; Angermeier, P.L. A Landscape-level model for ecosystem restoration in the San Francisco Estuary and its watershed. San Franc. Estuary Watershed Sci. 2005, 2. Available online: http://repositories.cdlib.org/jmie/sfews/vol3/iss1/art2 (accessed on 29 April 2016). [Google Scholar]
- Skalski, J.R. Statistical considerations in the design and analysis of environmental damage assessment studies. J. Environ. Manag. 1995, 43, 67–85. [Google Scholar] [CrossRef]
- Lorenz, A.W.; Feld, C.K. Upstream river morphology and riparian land use overrule local restoration effects on ecological status assessment. Hydrobiologia 2013, 704, 489–501. [Google Scholar] [CrossRef]
- Fausch, K.D.; Torgersen, C.E.; Baxter, C.V.; Li, H.W. Landscapes to riverscapes: Bridging the gap between research and conservation of stream fishes. BioScience 2002, 52, 483–498. [Google Scholar] [CrossRef]
- Cormack, R. Estimates of survival from the sighting of marked animals. Biometrika 1964, 51, 429–438. [Google Scholar] [CrossRef]
- Jolly, G.M. Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika 1965, 52, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Seber, G.A.F. A note on the multiple-recapture census. Biometrika 1965, 52, 249–259. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Academic Press/Elsvier: Burlington, MA, USA, 2006. [Google Scholar]
- Kéry, M.; Schmidt, B.R. Imperfect detection and its consequences for monitoring for conservation. Community Ecol. 2008, 9, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Lele, S.R.; Moreno, M.; Bayne, E. Dealing with detection error in site occupancy surveys: What can we do with a single survey? J. Plant Ecol. 2012, 5, 22–31. [Google Scholar] [CrossRef]
- Sólymos, P.; Lele, S.; Bayne, E. Conditional likelihood approach for analyzing single visit abundance survey data in the presence of zero inflation and detection error. Environmetrics 2012, 23, 197–205. [Google Scholar] [CrossRef]
- Stewart-Oaten, A.; Bence, J.R.; Osenberg, C.W. Assessing effects of unreplicated perturbations: No simple solutions. Ecology 1992, 73, 1396–1404. [Google Scholar] [CrossRef]
- Baldigo, B.P.; Warren, D.R.; Ernst, A.G.; Mulvihill, C.I. Response of fish populations to natural channel design restoration in streams of the Catskill Mountains, New York. N. Am. J. Fish. Manag. 2008, 28, 954–969. [Google Scholar] [CrossRef]
- Smith, E.P. BACI designs. In Encyclopedia of Environmetrics; El-shaarawi, A.H., Piegorsch, W.W., Eds.; Wiley: Chickester, UK, 2002; pp. 141–148. [Google Scholar]
- Baldigo, B.P.; Warren, D.R. Detecting the response of fish assemblages to stream restoration: Effects of different sampling designs. N. Am. J. Fish. Manag. 2008, 28, 919–934. [Google Scholar] [CrossRef]
- Peterman, R.M. Statistical power analysis can improve fisheries research and management. Can. J. Fish. Aquat. Sci. 1990, 47, 2–15. [Google Scholar] [CrossRef]
- Ludwig, D.; Hilborn, R.; Walters, C. Uncertainty, resource exploitation, and conservation: Lessons from history. Ecol. Appl. 1993, 3, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Che-Castaldo, J.P.; Crouse, D.; Davis, F.W.; Epanchin-Niell, R.; Flather, C.H.; Frohlich, R.K.; Goble, D.D.; Li, Y.W.; Male, T.D.; et al. Species recovery in the United States: Increasing the effectiveness of the Endangered Species Act. Issues Ecol. 2016, 20, 1–28. [Google Scholar]





| Phases and Steps | Key Considerations | Potential Pitfalls | RRFRP Examples |
|---|---|---|---|
| Planning Phase | |||
| Step 1. Communicate with stakeholders to identify valued biological and environmental attributes and desired or acceptable endpoints. | Identify (a) attributes expected to respond to proposed alteration, (b) realistic endpoints, and (c) endpoints based on non-arbitrary, ecologically meaningful criteria. | Selection of attributes that (a) cannot be indexed with measurable indicators or (b) are unresponsive to proposed alteration, or (c) are arbitrary or unrealistic, so results have unclear interpretations. | 25%–75% annual reduction in adult logperch abundance had no clear implication for population viability. |
| Step 2. Define spatiotemporal scope and objectives of proposed alteration. | Social, economic, and environmental factors may limit potential alterations. | Unanticipated factors may impinge on the project’s scope. | Construction was delayed for several years. |
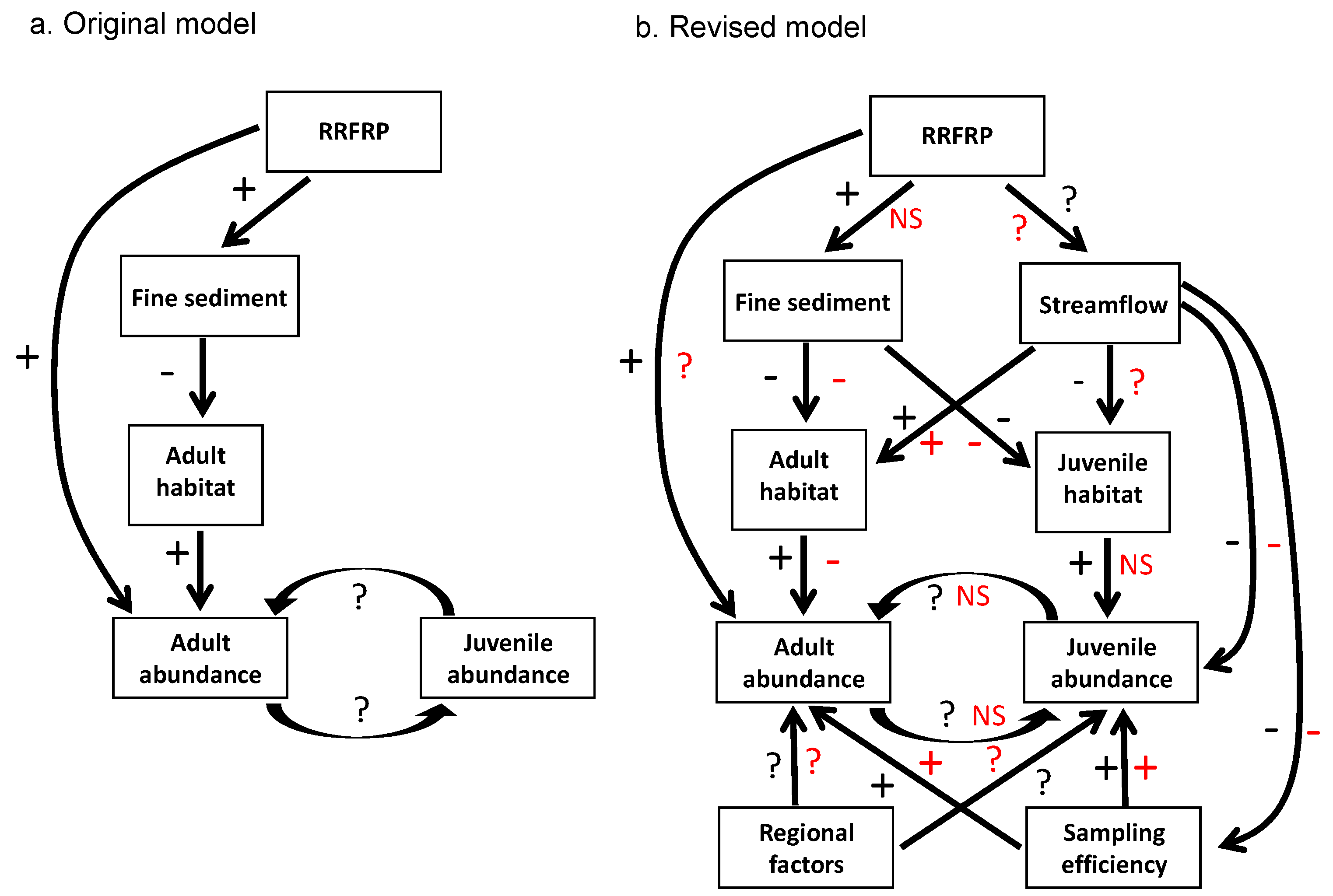
| Step 3. Develop initial conceptual model relating major factors and processes to each other, based on best available knowledge (see Figure 1a). | Account for all important direct and indirect linkages among factors, processes, and proposed alteration. | Knowledge gaps may result in conceptual model failing to account for (a) non-linear or indirect relations between known factors or (b) unknown factors. | Lack of basic dispersal information on logperch. Lagged effects of construction on logperch abundance. Logperch recruitment and detection driven by river flow. |
| Step 4. Choose specific biological and environmental indicators to represent factors and processes of interest, including any thresholds for acceptability or success. | Identify measurable indicators that (a) can be measured with sufficient precision and accuracy and (b) index the status of valued attributes. | Selection of indicators that (a) cannot be measured with sufficient accuracy or precision or (b) do not covary with valued attributes. | Logperch abundance was difficult to measure. Relation between habitat availability and logperch abundance obscured by river flow. |
| Step 5. Develop comprehensive set of testable alternative hypotheses regarding effects of proposed alteration on indicators. | Develop hypotheses that account for all reasonable types and routes of influence of the proposed alteration. Ensure that all potential results can be interpreted via alternative hypotheses (avoid surprises). | Overly simplistic hypotheses may fail to account for important types and routes of influences. Failure to consider a priori all potential results may necessitate post hoc arm-waving and weaken inferences. | Original BACI test assumed sudden, constant effects on indicators; revised tests included lagged and gradual effects. |
| Step 6. Design monitoring studies (including spatiotemporal attributes and analytical tools) capable of testing hypotheses. | Available resources (i.e., time and money) may be severely limiting. Allocate enough spatial and temporal replication to characterize mean and variance of indicators in each treatment group and provide adequate statistical power to detect changes of interest, including lagged effects. For samples of animal abundance, account for imperfect detection. | Monitoring studies that cannot provide valid statistical tests of effects of proposed alteration. Inadequate spatial or temporal replication results in poor estimates of mean and variance and/or low statistical power to detect effects of proposed alteration. | Initial plan for pre-construction phase included a single year of monitoring. We did not use power analysis to inform the spatiotemporal design of the monitoring program. Power analysis showed we had low statistical power to detect lagged post-treatment effects. We conducted capture-recapture studies to estimate logperch detection probability. |
| Implementation phase | |||
| Step 7. Conduct pre-treatment monitoring. | Measure all selected indicators and their drivers. Collect all samples as consistently as possible. | Important indicators and drivers of indicators are not measured and cannot be accounted for when testing hypotheses. | Confounding effects of non-RRFRP actions within the catchment. We accounted for variation in logperch detectability in statistical models. |
| Environmental and human variability between replicate samples obscures relations among indicators. | |||
| Step 8. Re-evaluate conceptual model (see Figure 1b), hypotheses, and monitoring design; revise as appropriate. | Use data from pre-treatment monitoring and/or concurrent data collected by others to update knowledge and enhance monitoring effectiveness. | Best available knowledge is not applied to monitoring effort; monitoring effectiveness is compromised. | Knowledge gained during pre-treatment monitoring was used to a) add young-of-year monitoring and b) revise the logperch-abundance “trigger” that indicated excessive take. |
| Step 9. Implement proposed alteration and conduct post-treatment monitoring. | Same as Step 7. | Same as Step 7. | Same as Step 7. |
| Step 10. Test hypotheses using appropriate analytical tools, then draw inferences and conclusions. | Examine and account for covariates, indirect relations, and autocorrelation when testing hypotheses. Use statistical effect sizes to evaluate change in indicators vis a vis desired or acceptable endpoints. | Failure to account for covariates, indirect relations, and autocorrelation obscures relations between alteration and indicators. Use of simple statistical significance thresholds (e.g., p < 0.05) provides hypothesis-test results with no clear biological interpretation. | Statistical models accounted for effects of logperch detectability and spatiotemporal autocorrelation but did not account for other potential influences on adult abundance. We based conclusions about project impacts on the magnitude and direction of effect sizes for various hypothesized RRFRP-related effects. |
| Step 11. Revise conceptual model (see Figure 1b) and evaluate monitoring methods based on results. | Build an adaptive management approach into the project. | Administrative, regulatory, and/or funding limitations may preclude changes to the monitoring program after it begins. | Some flexibility to alter monitoring and analyses emerged but was not planned at the outset; the basic spatiotemporal scope and main methods used were not adaptive. |
| Communication phase | |||
| Step 12. Communicate results to stakeholders and articulate applications for future projects. | Communication with stakeholders and application of new knowledge more effective if communication occurs continually rather than sporadically or simply when monitoring is completed. | Stakeholders who have use or need of new knowledge do not have timely access to it. | As findings emerged, our communication with state and federal managers of logperch led to changes in their management tactics and priorities for research to inform logperch conservation. |
| BACI Assumption | Potential Reasons for Violation | Potential Consequences of Violation | Potential Solutions |
|---|---|---|---|
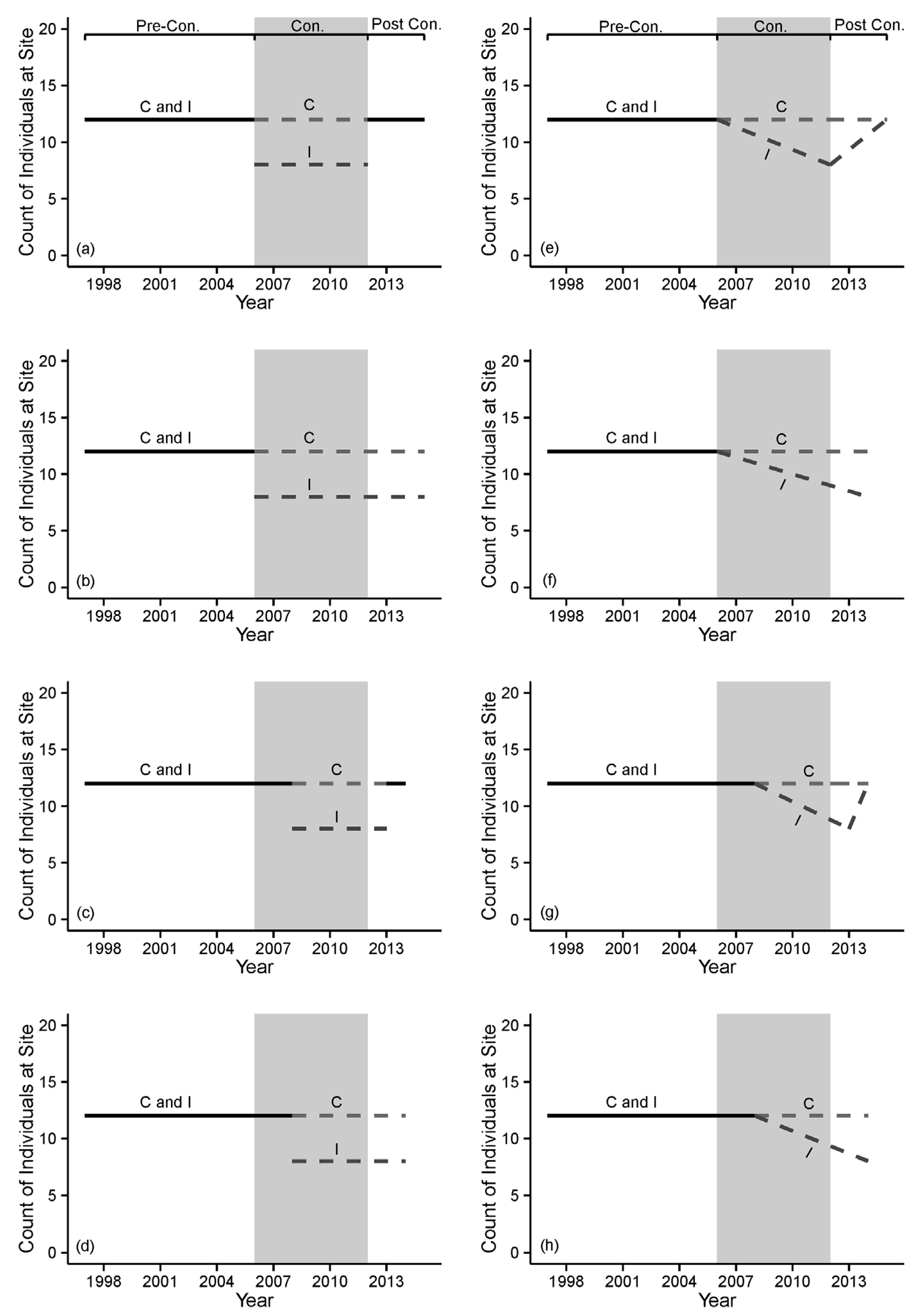
| Treatment occurs instantly at the B-A transition and then is applied uniformly to I sites, throughout the A period. | Proposed alteration does not occur all at once, resulting in spatiotemporal variation in project effects. | Increased spatial and temporal variance in A-I replicate samples; Some A-I replicates do not accurately characterize treatment effects; Cumulative effects of project are underestimated. | Only assign those sites receiving the treatment to the I group; Use regression-based models that allow treatment effect size to vary. |
| Indicator variables respond instantly at the B-A transition, via a change in their means. | Biological/ecological responses are immediate, but responses are not observable in indicator variables until well into the A period. | Early A-I replicate samples do not accurately characterize treatment effects; Statistical power to detect change is reduced. | Measure indicator variables most likely to detect change (e.g., juvenile logperch abundance); Develop a set of competing a priori hypotheses representing different functional forms of response in indicator variables; In this case, anticipate a lagged observed response and allocate replicate A-I samples to appropriate treatment groups. |
| Indicator variables exhibit a lagged response, once a critical threshold of cumulative environmental change is reached | Early A-I replicate samples do not accurately characterize treatment effects; Statistical power to detect change is reduced | Measure indicator variables most likely to detect change (e.g., habitat); Develop a set of competing a priori hypotheses representing different functional forms of response in indicator variables; In this case, anticipate a lagged observed response and allocate replicate A-I samples to appropriate treatment groups. | |
| Indicator variables respond via a gradual change, due to chronic effects (i.e., “press” disturbance). | ANOVA-type tests for change in mean values (intercepts) are inappropriate and underestimate ultimate changes. | Develop a set of competing a priori hypotheses representing different functional forms of response in indicator variables; In this case, anticipate a change in the temporal slope (not intercept) of the indicator variable; Utilize regression-based models. | |
| All measured differences between B-A and C-I are due to the project. | Other environmental variables change over space and time. | Depending on the spatial and temporal distribution of extrinsic influences, these may bias statistical tests toward or away from detecting effects; They likely will reduce precision as well, reducing the probability of detecting real effects. | To the extent possible, either control or block for extrinsic sources of variability; For other sources, incorporate them into models as random effects or covariates. |
| Indicator variables are measured without error. | Sampling sites do not represent area-wide conditions. | Measured variation in indicators within sample sites does not reflect biological/ecological trends across the study area. | Randomize site selection; Use GLMMs to account for random effects of sites when partitioning sources of variation. |
| Environmental and investigator variability over space and time introduces measurement error. | Depending on the spatial and temporal distribution of these influences, they may bias statistical tests toward or away from detecting effects; They likely will reduce precision as well, reducing the probability of detecting real effects. | Use repeated-sampling methods (e.g., occupancy or mark-recapture models) to reduce influences of observation/measurement error on estimates of indicators. | |
| Treatment has no effect on C sites. | Environmental effects of treatment are transmitted beyond the I area. | A-C sites do not accurately represent the reference condition; Statistical power to detect change is reduced; Ultimate effects of the project are underestimated. | Select C and I sites that are as environmentally correlated as possible, but where C sites are not affected by the treatment being applied to I. |
| C and I sites may be demographically interdependent. | A-C sites do not accurately characterize reference conditions; Statistical power to detect change is reduced; Ultimate effects of the project are underestimated. | Select C and I sites that are as environmentally correlated as possible, but where C and I sites are not demographically interdependent on each other. | |
| B and A periods are long enough to accurately represent background (interannual) mean and variance of indicator variables and statistically detect the desired effect size with the desired level of power. | Project timeline is short, monitoring resources are limited, temporal variance is higher than expected, or no power analysis has been conducted. | Tests have insufficient power to detect the desired effect size with the desired level of power; Ultimate effects of the project are underestimated; Biological/ecological meaning of monitoring results are unclear. | Conduct preliminary sampling to estimate mean and variance of indicators, then use power analyses to determine necessary spatial and temporal replication; Allow sufficient pre- and post- time to collect data necessary to assess change. |
| C and I sites are numerous enough to accurately represent mean and variance of these groups and statistically detect the desired effect size with the desired level of power. | Monitoring resources are limited, spatial variance is higher than expected, few replicate habitats are available, or no power analysis has been conducted. | Tests have insufficient power to detect the desired effect size with the desired level of power; Ultimate effects of the project are underestimated; Biological/ecological meaning of the monitoring results are unclear. | Conduct preliminary sampling to estimate the mean and variance of indicator, then use power analyses to determine necessary spatial and temporal replication; Allow sufficient pre- and post- time to collect data necessary to assess change. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, J.H.; Anderson, G.B.; Angermeier, P.L. A Long-Term Study of Ecological Impacts of River Channelization on the Population of an Endangered Fish: Lessons Learned for Assessment and Restoration. Water 2016, 8, 240. https://doi.org/10.3390/w8060240
Roberts JH, Anderson GB, Angermeier PL. A Long-Term Study of Ecological Impacts of River Channelization on the Population of an Endangered Fish: Lessons Learned for Assessment and Restoration. Water. 2016; 8(6):240. https://doi.org/10.3390/w8060240
Chicago/Turabian StyleRoberts, James H., Gregory B. Anderson, and Paul L. Angermeier. 2016. "A Long-Term Study of Ecological Impacts of River Channelization on the Population of an Endangered Fish: Lessons Learned for Assessment and Restoration" Water 8, no. 6: 240. https://doi.org/10.3390/w8060240





