This study further demonstrates the high level of predictability between regional reference stream channel dimensions and drainage area, thus validating its use as a planning and assessment tool for channel shape in natural channel design stream restoration efforts. Our results also confirm that, although considerably more variable than geomorphology, aquatic biota can be used as a reliable design tool and assessment guide to determine the ecological effectiveness of restoration efforts.
4.1. Geomorphology
The streams in our study area were selected based on being in apparent reference condition in terms of visible geomorphology, floodplain connectivity and vegetation structure, and instream habitat structure. Perhaps not surprisingly, identifying streams with all of these parameters proved to be one of the biggest challenges faced during the study. Based on observations from our field reconnaissance, the vast majority of streams we encountered in the Alabama Piedmont are either incised with bank height ratios much greater than 1.2, have channels lacking freely-formed meander patterns, are unstable or confined at bankfull width, and/or have an abundance of invasive species serving as the floodplain vegetation cover. This likely reflects the storied past of streams in this region of AL (and the southeastern Piedmont of the US in general), which experienced significant sediment inputs and subsequent erosion and stability declines due to an abundance of highly erosive land use during the early 1900s [
18]. Streams in the Piedmont also inherently may be more susceptible land use disturbance due to geology and local relief, thus increasing the persistence of legacy effects from past landscapes [
42,
43,
44].
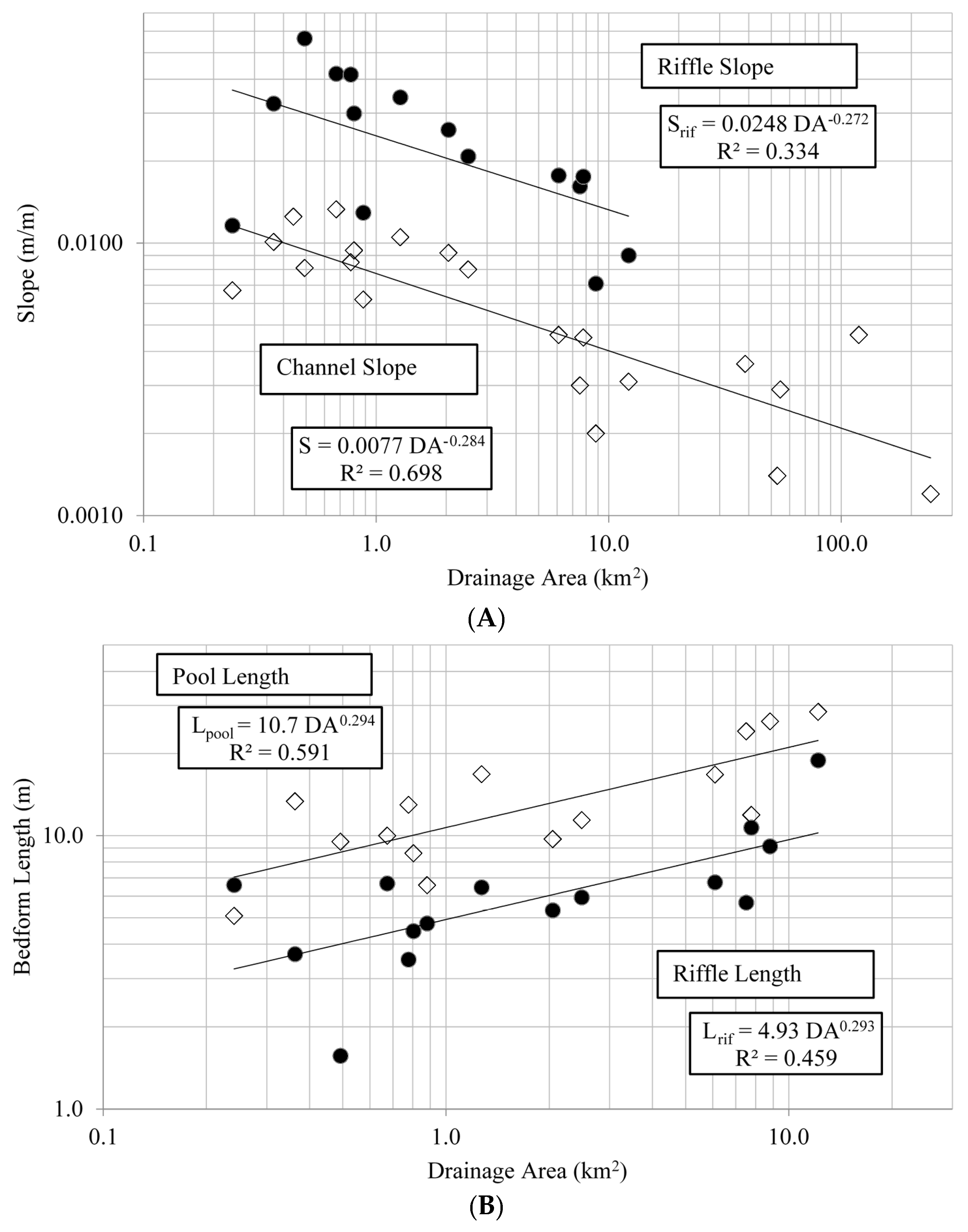
Most streams in the study area were C or E type channels based on Rosgen classification, indicated by a meandering pool-riffle morphology. Both of these classifications denote low-gradient, meandering channels with broad valleys and alluvial soils [
11]. Several of the streams had width/depth ratios of 10–14 and were borderline C or E channels. This type of variability suggests these streams lie in an area of geomorphic transition, a phenomenon observed by other researchers in the Piedmont, again likely a result of historical land use and subsequent floodplain fill [
25].
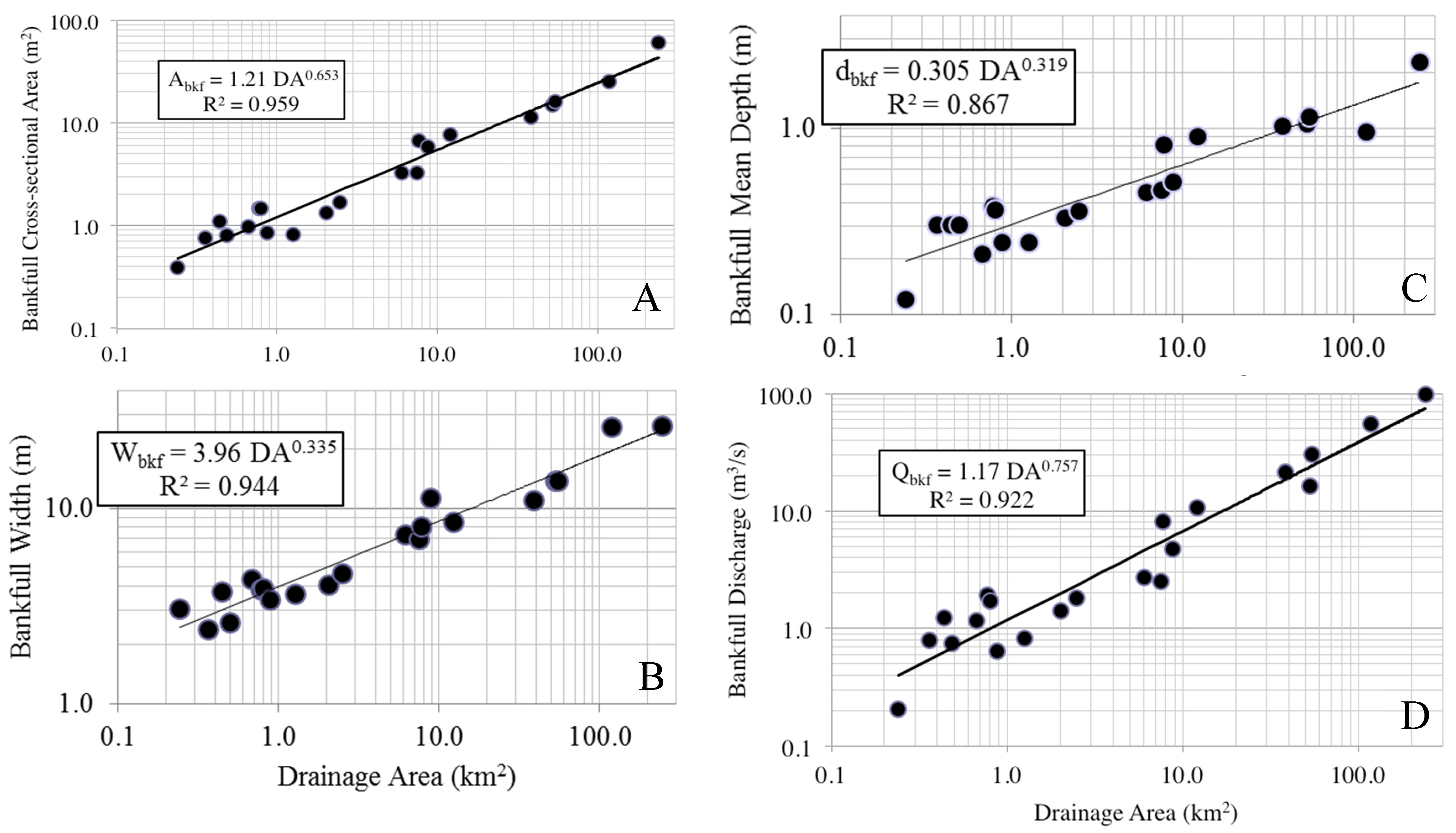
Regional bankfull curves were of high predictability, with A
bkf, W
bkf, d
bkf, and Q
bkf all largely explained by drainage area, a result similar to other studies in this region and beyond [
7,
29,
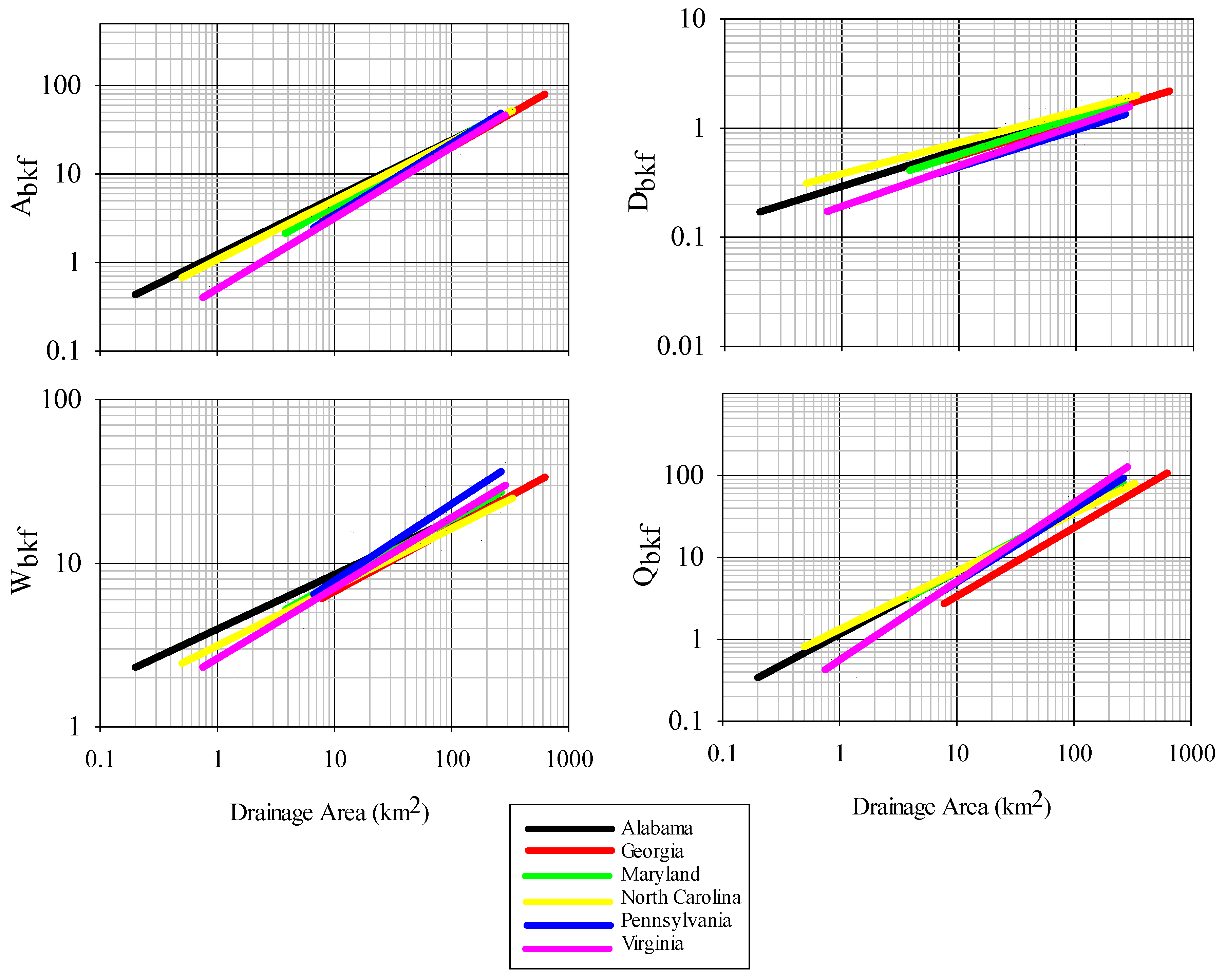
36]. Parameter estimates for all bankfull measures were within reported values from other studies in the Piedmont region for other states, however there were some differences, particularly for curves of bankfull area, width, and discharge between this study and those generated for Virginia and Pennsylvania. Variation in bankfull relationships have been reported across the Piedmont ecoregion, with higher bankfull discharge per drainage area in the northeast as compared to the southwest Piedmont, a phenomenon potentially resulting from higher runoff in the northeast Piedmont [
25]. We cannot confirm whether this also explains differences observed between Alabama and Virginia, although it is possible. Despite the causal factors, this regional variation in observed relationships is important to identify and leads to a more refined tool over broad-scale regional curves when considering restoration designs to match local reference conditions [
9]. The current set of regional curves for bankfull channel dimensions thus provides a reliable tool for verifying bankfull stage in field surveys and for estimating dimensions for stream restoration projects in the Piedmont of Alabama.
Riffles and pools can provide channel stability by minimizing energy loss, and their spacing and overall channel longitudinal profile can influence many physical and biotic processes within streams [
45,
46] For the Alabama Piedmont, riffle and pool lengths were positively related to drainage area, however displaying considerably more variation than what was observed with bankfull cross-section dimensions. This perhaps is not surprising as fine scale geomorphic features such as riffles and pools can be heavily influenced by flow obstacles induced by local geology, gradient, plant growth and animal activity, among other sources [
47,
48,
49]. This variation in riffle-pool morphology may be particularly true for the Piedmont ecoregion in general, given its geologic diversity, local topographic variation, and high biological diversity [
20,
50].
4.2. Biota
The influence of drainage area on the biotic composition of associated streams has been recognized for decades, with the river continuum concept being the most familiar framework for predictions of assemblage structure and ecosystem function considering placement within the watershed [
51]. More recently the complexities and true interactive nature between geomorphic processes and ecological components have been addressed, emphasizing the role of local scale phenomena [
15,
49,
52,
53]. Our results demonstrate that biotic assemblage structure is highly correlated with drainage area and many measures of bankfull dimensions, and as such are useful benchmarks for evaluating the need and/or success of restoration efforts in the Piedmont and beyond.
From our data, fish assemblages generally increase in richness and diversity and change rapidly in structure as stream systems initially increase in size, however these dynamics slow as system size continues to increase. There is a predictable, depauperate 2–3 species fish assemblage in small, 0.4–0.5 km
2 streams of this area, but assemblages increase to 10 species or more at 1 km
2 drainage area and to 20 species by 10 km
2 drainage area. Beyond 10 km
2 drainage area however, there is little change in species number, suggesting a potential cap in terms of fish diversity. Further, many measures of functional assemblage structure were strongly related to drainage area and measures of bankfull dimensions. Of particular note is the contribution of endemism to these patterns. The Mobile Basin has a high level of endemism in regards to fish, with the Tallapoosa claiming six species [
54,
55]. While these species are narrowly constrained geographically, they are often found in high abundance locally. In our sites, small- to mid-sized streams had high proportions of narrow-endemics (
i.e., Tallapoosa River basin endemics). This justifies the need for conserving and restoring small streams, as they often harbor many idiosyncratic taxa and contribute disproportionately to the overall biodiversity within a basin [
56]. Interestingly, broad endemics (
i.e., Mobile basin endemics) were absent from the smaller streams yet were found in increasing proportions in streams with drainage areas 8 km
2 and higher. This provides an identifiable biological ceiling, and if these assemblages are indeed indicative of reference condition, then this ceiling can provide a powerful biotic benchmark in the assessment of success and need of restoration from a biological perspective.
Invertebrate taxa also showed a predictable response patterns across the study streams. Our results suggest that average crayfish size increases as stream systems increase in size. Also, crayfish abundance (as interpreted from CPUE) significantly decreases with increasing channel size. Indeed juvenile crayfish and smaller sub-adults are often found in shallow habitats and vegetated refugia typical of small streams, where they presumably are partly released from predation by fish. Conversely, larger crayfish are susceptible to terrestrial predators and simultaneously less susceptible to fish predation, thus can often be found in deeper waters more common in high order streams [
57,
58]. Interestingly, M:F ratios increased with drainage area as well, possibly reflecting increased migration and dispersal of large males, a pattern that has been observed in other systems [
59]. However, sampling efficiency declines with increasing stream size due the inherent complexities of increased depth, flow, and habitat, thus to what degree observed crayfish patterns reflect a sampling bias or a true biological phenomena are unknown. Although crayfish assemblages are likely not as strong an ecological response signature as fish assemblages because of their inherently lower taxonomic diversity, these results do suggest that certain components of crayfish biology can be reasonably predicted with changing bankfull channel dimensions.
Somewhat surprisingly, our results suggest aquatic insect taxa richness and diversity do not predictably change in terms of channel dimensions over the study sites. This is surprising as macroinvertebrate taxonomic diversity and richness are typical biological response indicators of various environmental conditions [
60]. As such, their use as a design and assessment tool may be limited in this area, at least compared to fish. It also should be noted that all of the streams were considered reference condition, and that the major axis of change was system size. Thus taxonomic richness/diversity may be too coarse of a measure for these purposes, although the measures do provide a biological ceiling that appears to be consistent irrespective of drainage area or geomorphology in reference condition. Trends found with compositional measures (e.g., scraper richness, % collector/filterers,
etc.) however are likely more nuanced and more informative as they incorporate the functional response to physical change associated with increasing system size. Indeed collector/filterer and scraper richness significantly increased with measures of increasing system size whereas shredder richness decreased. This is in concordance with general theory regarding functional shifts in macroinvertebrate assemblages as stream systems become larger [
50]. Based on observations, low-order streams in this area appear detritus-driven and tightly coupled with organic inputs from the surrounding watershed, whereas higher-order streams contain higher abundances of fine-particulate organic matter and periphyton growth. These changing environmental conditions influence assemblage structure in a predictable way and this structure can be used as a benchmark for design and assessment tools that may be used to estimate the range of aquatic insect assemblage composition in restoration projects. Although designers should consider natural variability in these data, particular attention should be paid to the lower bounds of positive relationships as they represent the critical biological ceiling across these systems.
Overall, fishes appear to have more promise than crayfishes or aquatic insects at this stage as an ecological endpoint for restoration design and assessment tools, at least in the context of this effort. However, all three groups have value in determining the ‘biological ceiling’ of reference condition in this area, and can be useful as such. The lack of strong relationships with aquatic insects may be a result of a disparity in scales, as macroinvertebrates are small-bodied organisms responding to fine-scale environmental phenomena (<10 m), and our measures of geomorphology were at the reach scale (100 m). Such disparities in scale among various taxonomic groups and environmental predictors have been implicated in other studies [
61]. Scale mismatch issues may explain why we observed stronger relationships with fish than insects, as fish are considerably more mobile in water and likely respond to broader-scale phenomena, on par with geomorphology measurements.
4.3. Determination of Reference Conditions
Identifying reference condition, whether for restoration efforts, developing indices of biotic or ecological integrity, or other endeavors needing a point of reference, is a challenging yet integral issue for the effective management of ecological systems [
19,
62,
63]. Reference conditions are used to determine restoration goals, assess the relative success of restoration efforts, and provide general context of the current ecological or physical state to that of a relatively pristine, undisturbed state. Several perspectives have been used to identify reference condition, including historic condition, best attainable condition, and least disturbed condition approach [
63]. Additionally, there are multiple methods of estimating this condition, including reference site approach, best professional judgement, comparison with historical conditions, among others [
63]. In this study, the streams evaluated would best be described as “least disturbed” as there are few (if any) entirely reference condition stream remaining in the southeastern Piedmont due to the current and historic land use of the area [
19]. Further, identification of biological reference conditions generally proved to be highly variable as compared to reference geomorphic condition. However this is not surprising given the stochastic nature of aquatic assemblages. Of particular interest however is the strong relationships observed with taxonomic and functional measures of stream fish assemblages and drainage area. These measures have high potential to be useful as biological benchmarks, and in conjunction with predictable geomorphological benchmarks, can be effective ecological benchmarks for restoration efforts in this region.
The tools derived from this study will be useful in site assessment, project selection, restoration design and implementation, and follow-up monitoring for evaluating the success of ecosystem restoration projects in the Appalachian Plateau. They can also serve as context and point of reference for similar efforts elsewhere, perhaps ultimately leading to the development of regional ecological endpoint curves. Development of such tools that integrate ecological conditions will result in improved stream evaluations and designs increasing the effectiveness of stream restoration projects and thus improved watershed functions.