Removal of Six Estrogenic Endocrine-Disrupting Compounds (EDCs) from Municipal Wastewater Using Aluminum Electrocoagulation
Abstract
:1. Introduction
2. Materials and Methods
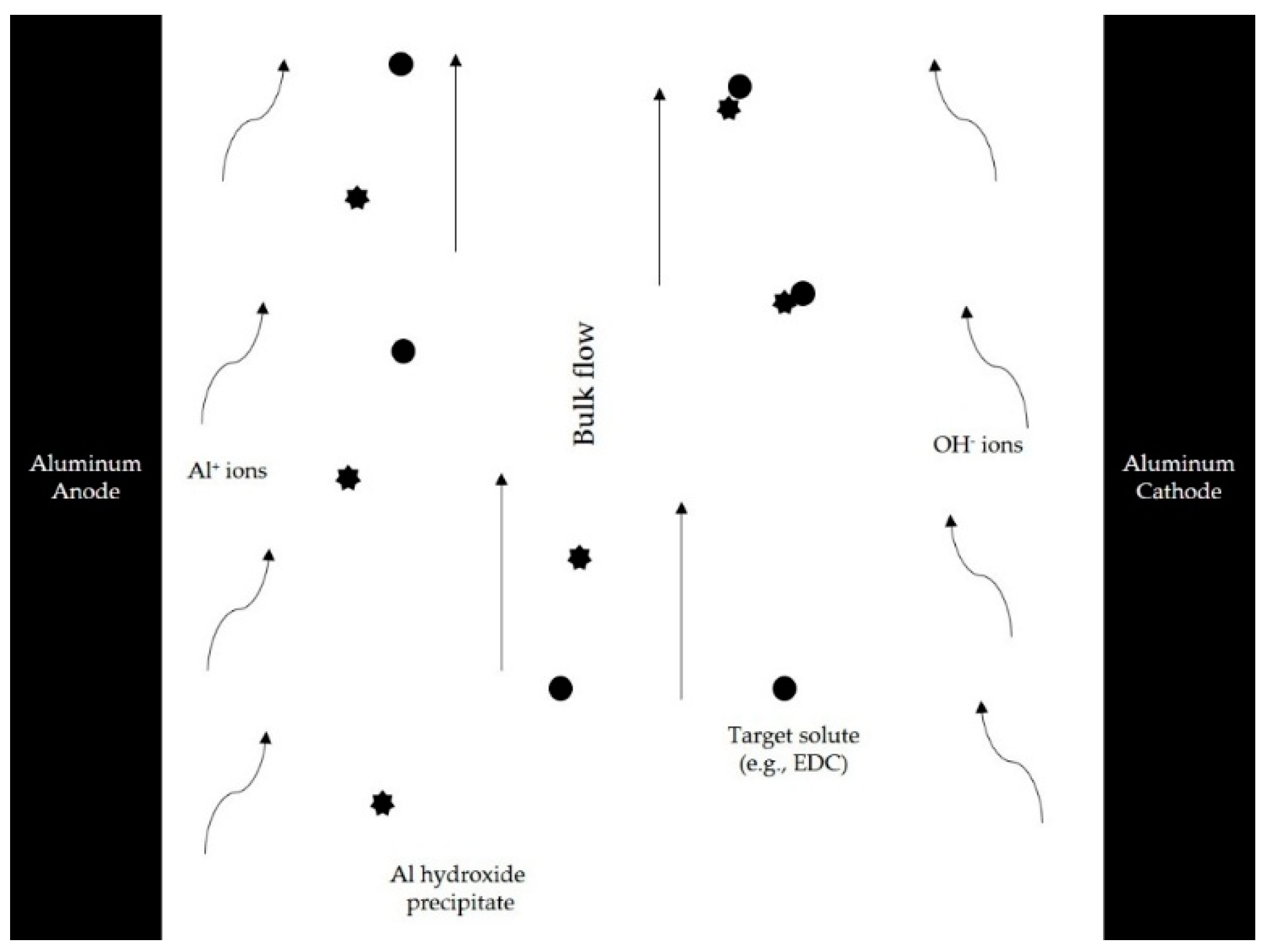
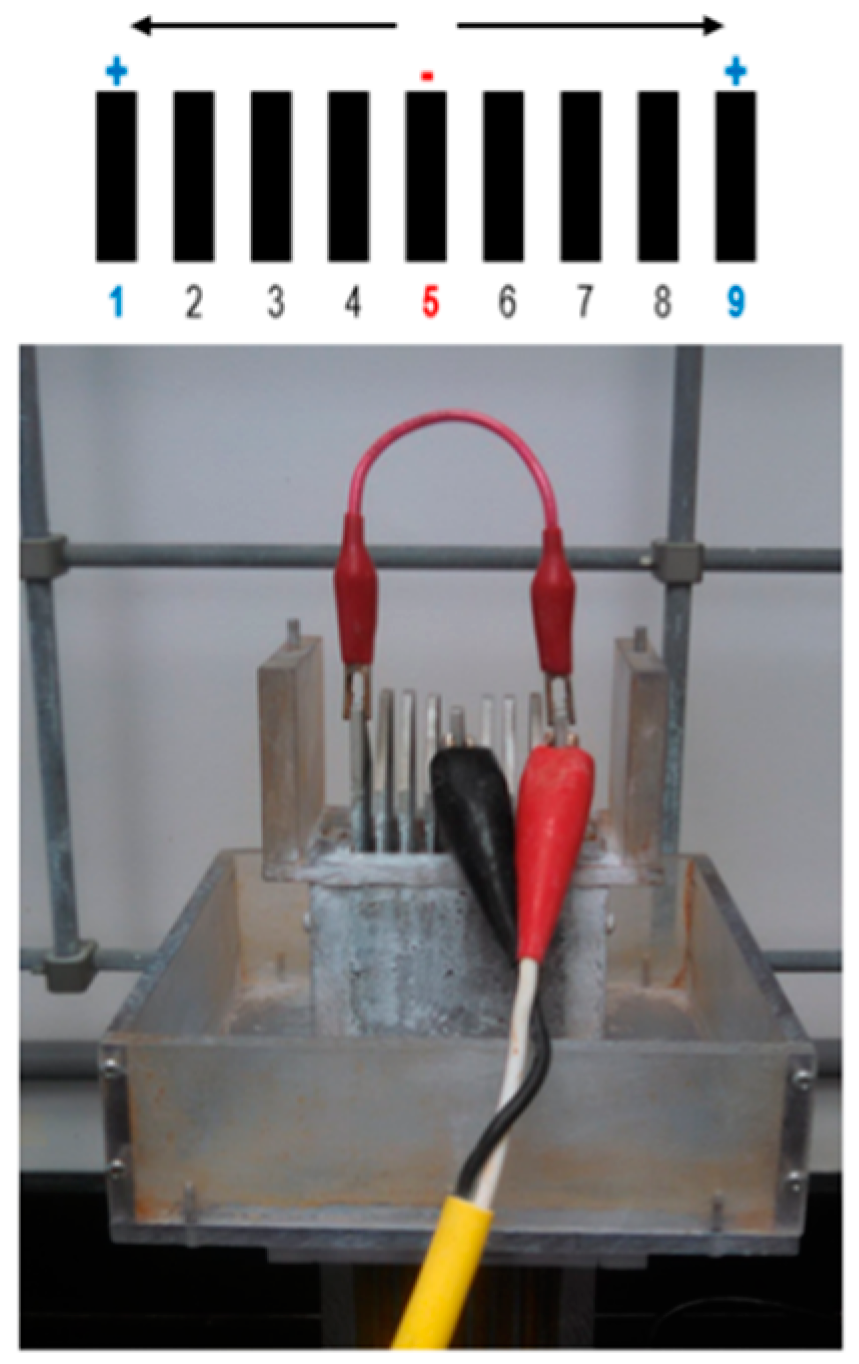
2.1. Electrocoagulation Unit
2.2. Preliminary Tests and Optimization of Parameters
2.3. Chemical Standards
2.4. Wastewater
2.5. Blanks
2.6. Experimental Design/Electrocoagulation Processing
2.7. Solid Phase Extraction
2.8. Determination of EDC Concentrations
2.9. Statistical Analysis
3. Results and Discussion
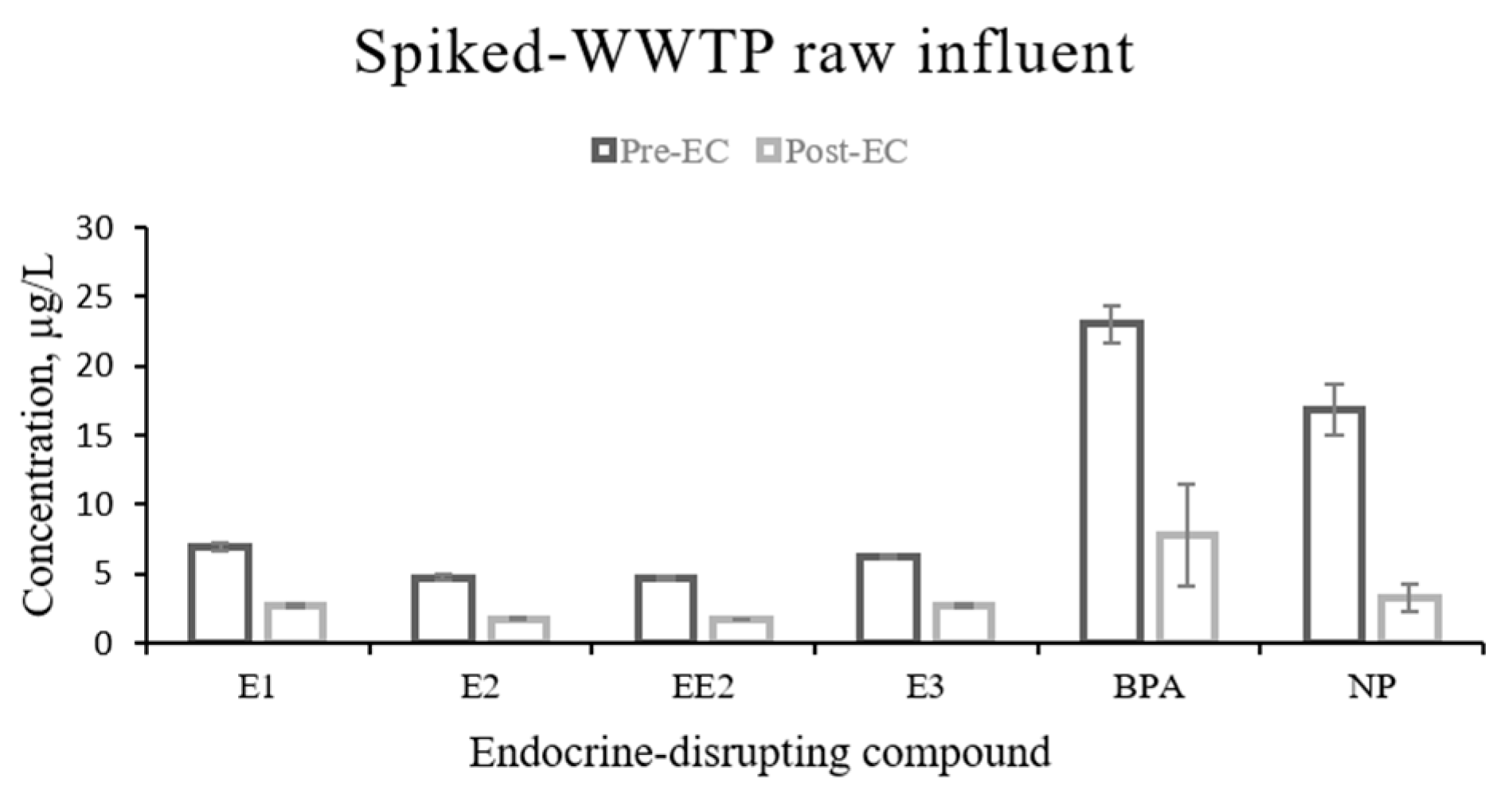
3.1. Removal of EDCs from Spiked-WWTP Influent by EC
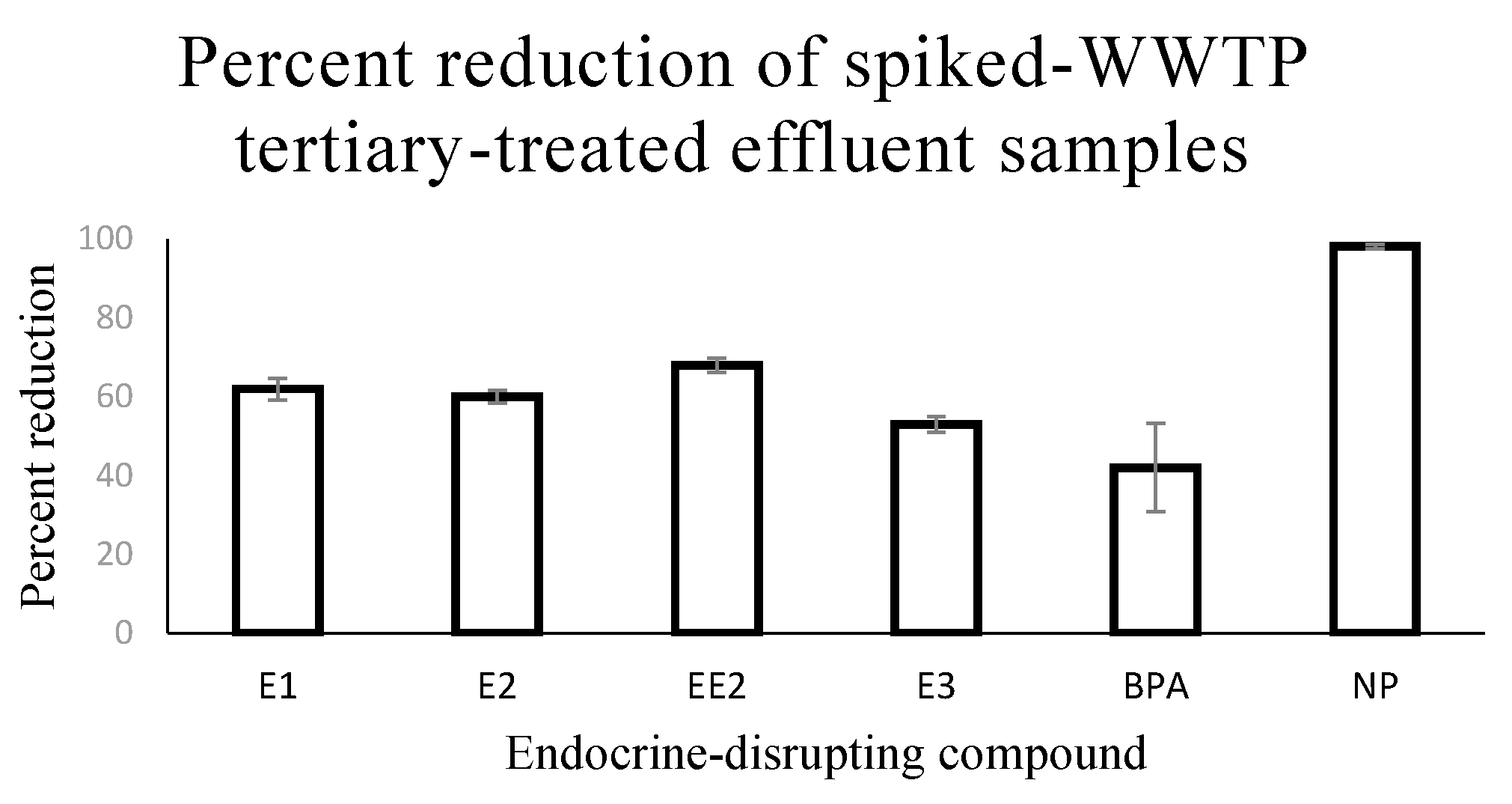
3.2. Removal of EDCs from Spiked-WWTP Tertiary-Treated Effluent by EC
3.3. Implementation Considerations and Concluding Remarks
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| WWTP | Wastewater treatment plant |
| EDC | Endocrine-disrupting compound |
| EC | Electrocoagulation |
| E1 | estrone |
| E2 | 17β-estradiol |
| E3 | estriol |
| EE2 | 17-ethinylestradiol |
| BPA | bisphenol-A |
| NP | nonylphenol |
| NPEO | nonylphenol ethoxylate |
| BSTFA | N, O-bis(trimethylsilyl)trifluoroacetamide |
| DI | deionized |
| GCMS | gas chromatography-mass spectrometry |
| MDL | method detection limit |
| TMCS | trimethylchlorosilane |
| EI | (electron impact) |
References
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Virkutyte, J.; Varma, R.S.; Jegatheesan, V. (Eds.) Treatment of Micropollutants in Water and Wastewater; IWA Publishing: London, UK, 2010; p. 483.
- Auriol, M.; Filali-Meknassi, Y.; Tyagi, R.D.; Adams, C.D.; Surampalli, R.Y. Endocrine disrupting compounds removal from wastewater, a new challenge. Process Biochem. 2006, 41, 525–539. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Hester, R.E.; Harrison, R.M. (Eds.) Marine Pollution and Human Health; Royal Society of Chemistry: Cambridge, UK, 2011; p. 182.
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from the endocrine society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Chapman, H.F.; Moore, M.R.; Ranmuthugala, G. Endocrine-disrupting compounds: A review of their challenge to sustainable and safe water supply and water reuse. Environ. Toxicol. 2006, 21, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 46, 1–59. [Google Scholar] [CrossRef]
- Jobling, S.; Casey, D.; Rogers-Gray, T.; Oehlmann, J.; Schulte-Oehlmann, U.; Pawlowski, S.; Baunbeck, T.; Turner, A.P.; Tyler, C.R. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat. Toxicol. 2004, 66, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Janex-Habibi, M.-L.; Huyard, A.; Esperanza, M.; Bruchet, A. Reduction of endocrine disruptor emissions in the environment: The benefit of wastewater treatment. Water Res. 2009, 43, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Nadzialek, S.; Vanparys, C.; Van der Heiden, E.; Michaux, C.; Brose, F.; Scippo, M.-L.; De Coen, W.; Kestemont, P. Understanding the gap between the estrogenicity of an effluent and its real impact into the wild. Sci. Total Environ. 2010, 408, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.L.; Bonnema, R.; McNamara, M.C. Helping women choose appropriate hormonal contraception: Update on risks, benefits, and indications. Am. J. Med. 2009, 122, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Cicek, N.; Londry, K.; Oleszkiewicz, J.A.; Wong, D.; Lee, Y. Removal of selected natural and synthetic estrogenic compounds in a canadian full-scale municipal wastewater treatment plant. Water Environ. Res. 2007, 79, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Danzl, E.; Sei, K.; Soda, S.; Ike, M.; Fujita, M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int. J. Env. Res. Public Health 2009, 6, 1472–1484. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, I.; Ince, N.H. Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes. J. Environ. Manag. 2007, 85, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K.; Xie, B.; Thompson, M.L.; Sung, S.; Ong, S.K.; Van Leeuwent, J. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ. Sci. Technol. 2006, 40, 6537–6546. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.P.; Noguerol, T.-N.; Lacorte, S.; Buchanan, I.; Piña, B. Toxicity identification fractionation of environmental estrogens in waste water and sludge using gas and liquid chromatography coupled to mass spectrometry and recombinant yeast assay. Anal. Bioanal. Chem. 2009, 393, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.; Kumar, A.; Shareef, A.; Doan, H.; Stuetz, R.; Kookana, R. Fate of indicator endocrine disrupting chemicals in sewage during treatment and polishing for non-potable reuse. Water Sci. Technol. 2010, 62, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Basile, T.; Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, V.; Colasuonno, S.; Petruzzelli, D. Review of endocrine-disrupting-compound removal technologies in water and wastewater treatment plants: An EU perspective. Ind. Eng. Chem. Res. 2011, 50, 8389–8401. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water Air Soil Pollut. 2013, 224, 1–29. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Rodriguez, J.; Stopic, S.; Krause, G.; Friedrich, B. Feasibility assessment of electrocoagulation towards a new sustainable wastewater treatment. Environ. Sci. Poll. Res. Int. 2007, 14, 477–482. [Google Scholar] [CrossRef]
- Canizares, P.; Jimenez, C.; Martinez, F.; Saez, C.; Rodrigo, M.A. Study of the electrocoagulation process using aluminum and iron electrodes. Ind. Eng. Chem. Res. 2007, 46, 6189–6195. [Google Scholar] [CrossRef]
- Gadd, A.; Ryan, D.; Kavanagh, J.; Beaurain, A.-L.; Luxem, S.; Barton, G. Electrocoagulation of fermentation wastewater by low carbon steel (Fe) and 5005 aluminium (Al) electrodes. J. Appl. Electrochem. 2010, 40, 1511–1517. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.; Cook, M.; McQuaig, S.; Ulrich, R.; Schenck, R.; Lukasik, J.; Van Vleet, E.; Breitbart, M. Reduction of nutrients, microbes, and personal care products in domestic wastewater by a benchtop electrocoagulation unit. Sci. Rep. 2015, 5, 9380. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, G.A.; Radovan, C.; Vlaicu, I.; Masu, S. Removal of nonylphenol ethoxylates by electrochemically-generated coagulants. J. Appl. Electrochem. 2002, 32, 561–567. [Google Scholar] [CrossRef]
- Martins, A.F.; Wilde, M.L.; Vasconcelos, T.G.; Henriques, D.M. Nonylphenol polyethoxylate degradation by means of electrocoagulation and electrochemical fenton. Sep. Purif. Technol. 2006, 50, 249–255. [Google Scholar] [CrossRef]
- Ivashechkin, P.; Corvini, P.F.; Dohmann, M. Behaviour of endocrine disrupting chemicals during the treatment of municipal sewage sludge. Water Sci. Technol. 2004, 50, 133–140. [Google Scholar] [PubMed]
- Chang, S.; Waite, T.D.; Schäfer, A.I.; Fane, A.G. Adsorption of trace steroid estrogens to hydrophobic hollow fibre membranes. Desalination 2002, 146, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Govindaraj, M.; Sudhir, A.; Sukumar, C.; Hariprakash, B.; Pattabhi, S. Treatment of endocrine disrupting chemical from aqueous solution by electrocoagulation. Sep. Sci. Technol. 2012, 48, 295–302. [Google Scholar] [CrossRef]
- Cook, M. Endocrine-Disrupting Compounds: Measurement in Tampa Bay, Removal From Sewage and Development of an Estrogen Receptor Model. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2015. [Google Scholar]
- Akbal, F.; Camcı, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 2011, 269, 214–222. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Parmar, S. Removal of strontium by electrocoagulation using stainless steel and aluminum electrodes. Desalination 2011, 282, 63–67. [Google Scholar] [CrossRef]
- Gamage, N.P.; Chellam, S. Aluminum electrocoagulation pretreatment reduces fouling during surface water microfiltration. J. Membr. Sci. 2011, 379, 97–105. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, D.; Du, P.; Li, H.; Liu, C.; Li, Y.; Cao, H.; Crittenden, J.C.; Huang, Q. A combination of electro-enzymatic catalysis and electrocoagulation for the removal of endocrine disrupting chemicals from water. J. Hazard. Mater. 2015, 297, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Attour, A.; Touati, M.; Tlili, M.; Ben Amor, M.; Lapicque, F.; Leclerc, J.P. Influence of operating parameters on phosphate removal from water by electrocoagulation using aluminum electrodes. Sep. Purif. Technol. 2014, 123, 124–129. [Google Scholar] [CrossRef]




| Compound | Type | Retention Time (min) | Quantitative Ion | Confirmatory Ion(s) | Method Detection Limit (ng/L) | Structure |
|---|---|---|---|---|---|---|
| Estrone (E1) | Natural estrogen | 21.9 | 342 | 218, 257 | 2 |  |
| 17β-Estradiol (E2) | Principal natural estrogen | 22.4 | 416 | 129, 285 | 1 |  |
| 17α-Ethinylestradiol (EE2) | Synthetic estrogen | 23.8 | 425 | 440 | 1 |  |
| Estriol (E3) | Natural estrogen | 24.8 | 504 | 386 | 3 |  |
| Bisphenol-A (BPA) | Industrial estrogen mimic | 16.2 | 357 | 358, 372 | 1 |  |
| Nonylphenol (NP) | Industrial estrogen mimic | 11.8 | 179 | 180, 292 | 2 |  |
| 5α-androstanol | Internal standard | 18.0 | 333 | 258 | N/A |  |
| EDC | Mean Pre-EC Conc ± SD (µg/L) | Mean Post-EC Conc ± SD (µg/L) | Test Statistic | p Value | % Removal |
|---|---|---|---|---|---|
| E1 | 7 ± 0.3 | 3 ± 0.1 | F = 1194.45 | <0.0001 | 61 |
| E2 | 5 ± 0.2 | 2 ± 0.1 | F = 954.56 | <0.0001 | 63 |
| EE2 | 5 ± 0.1 | 2 ± 0.1 | F = 2079.79 | <0.0001 | 64 |
| E3 | 6 ± 0.2 | 3 ± 0.1 | F = 1021.31 | <0.0001 | 56 |
| BPA | 23 ± 1 | 8 ± 4 | F = 85.15 | <0.0001 | 66 |
| NP | 17 ± 2 | 3 ± 1 | F = 133.28 | <0.0001 | 81 |
| EDC | Mean Pre-EC Conc ± SD (µg/L) | Mean Post-EC Conc ± SD (µg/L) | Test Statistic | p Value | % Removal |
|---|---|---|---|---|---|
| E1 | 7 ± 0.1 | 3 ± 0.2 | F = 1125.53 | <0.0001 | 62 |
| E2 | 5 ± 0.1 | 2 ± 0.1 | F = 803.89 | <0.0001 | 60 |
| EE2 | 5 ± 0.1 | 2 ± 0.1 | F = 2304.72 | <0.0001 | 68 |
| E3 | 7 ± 0.2 | 3 ± 0.1 | F = 984.47 | <0.0001 | 53 |
| BPA | 26 ± 0.2 | 15 ± 3 | F = 36.01 | <0.0001 | 42 |
| NP | 21 ± 2 | 0.4 ± 0.1 | F = 250.89 | <0.0001 | 98 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, M.M.; Symonds, E.M.; Gerber, B.; Hoare, A.; Van Vleet, E.S.; Breitbart, M. Removal of Six Estrogenic Endocrine-Disrupting Compounds (EDCs) from Municipal Wastewater Using Aluminum Electrocoagulation. Water 2016, 8, 128. https://doi.org/10.3390/w8040128
Cook MM, Symonds EM, Gerber B, Hoare A, Van Vleet ES, Breitbart M. Removal of Six Estrogenic Endocrine-Disrupting Compounds (EDCs) from Municipal Wastewater Using Aluminum Electrocoagulation. Water. 2016; 8(4):128. https://doi.org/10.3390/w8040128
Chicago/Turabian StyleCook, Monica M., Erin M. Symonds, Bert Gerber, Armando Hoare, Edward S. Van Vleet, and Mya Breitbart. 2016. "Removal of Six Estrogenic Endocrine-Disrupting Compounds (EDCs) from Municipal Wastewater Using Aluminum Electrocoagulation" Water 8, no. 4: 128. https://doi.org/10.3390/w8040128






