Monitoring of β-d-Galactosidase Activity as a Surrogate Parameter for Rapid Detection of Sewage Contamination in Urban Recreational Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Water and Fecal Samples
2.1.1. River Water Samples
2.1.2. Municipal Wastewater, Black Water and Human Feces
2.1.3. Bird Feces from Local Beaches
2.1.4. Feces from Farm Animals
2.1.5. Bathing Water Samples from Beaches
2.2. Determination of Precipitation and Water Flows in Rivers
2.3. Reference Methods for Detection of Fecal Indicator Bacteria
2.4. Field Kit for Measurement of GAL Activity
2.5. CALM Test for Automated Measurement of the GAL Activity in the Akerselva River
2.6. Effect of Salinity on the Measurement of GAL Activity
2.7. Statistical Analysis
3. Results
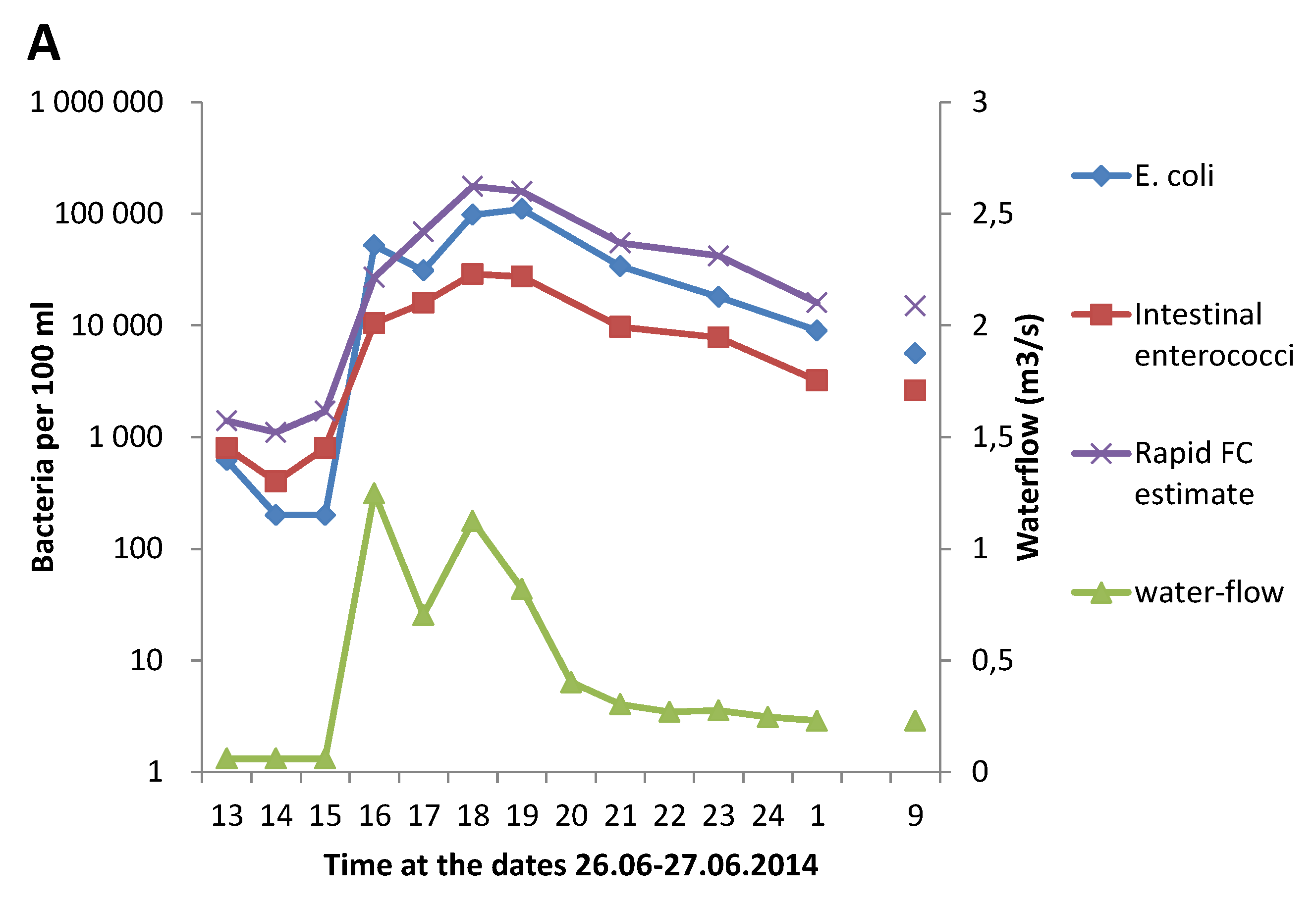
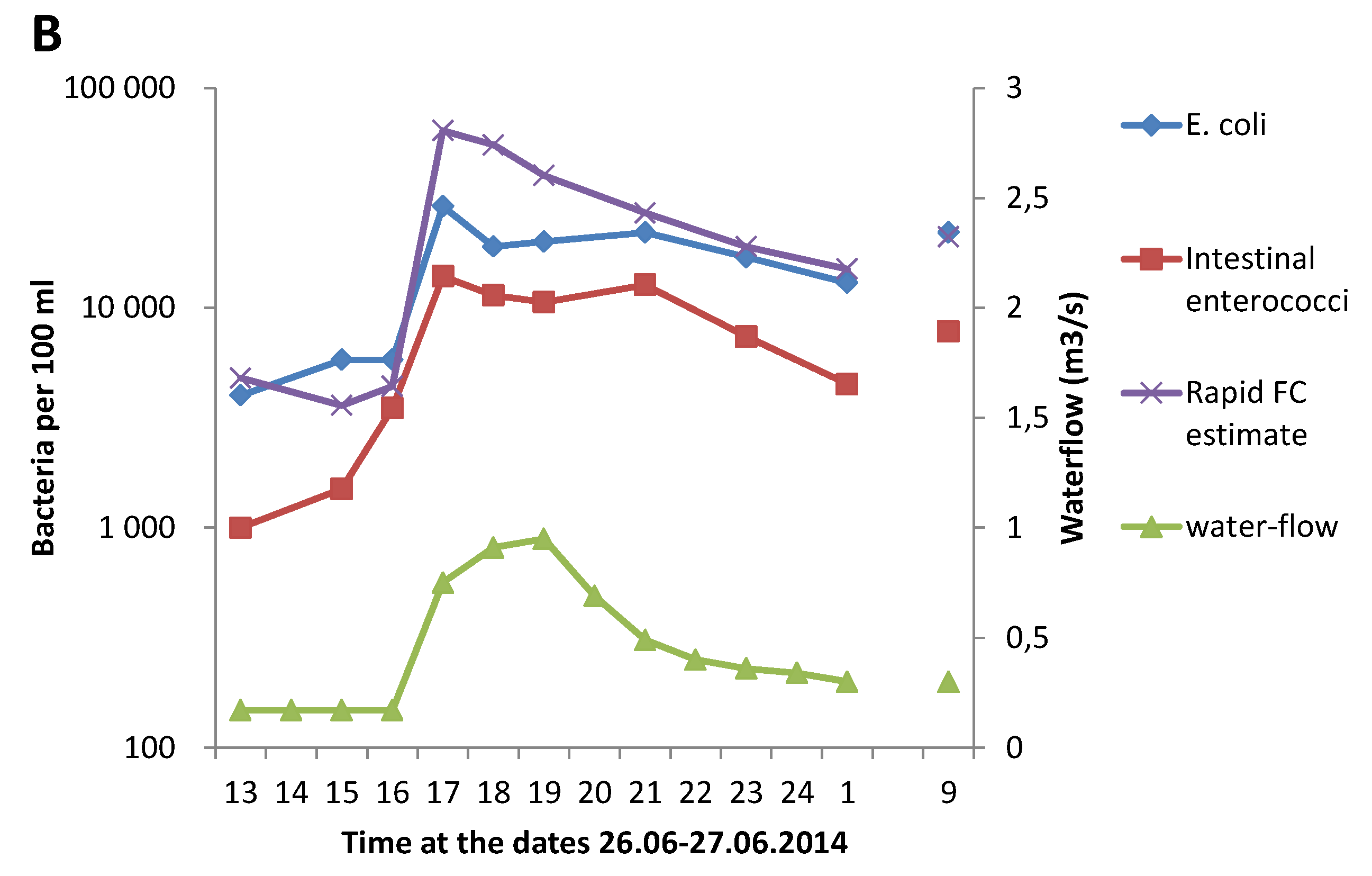
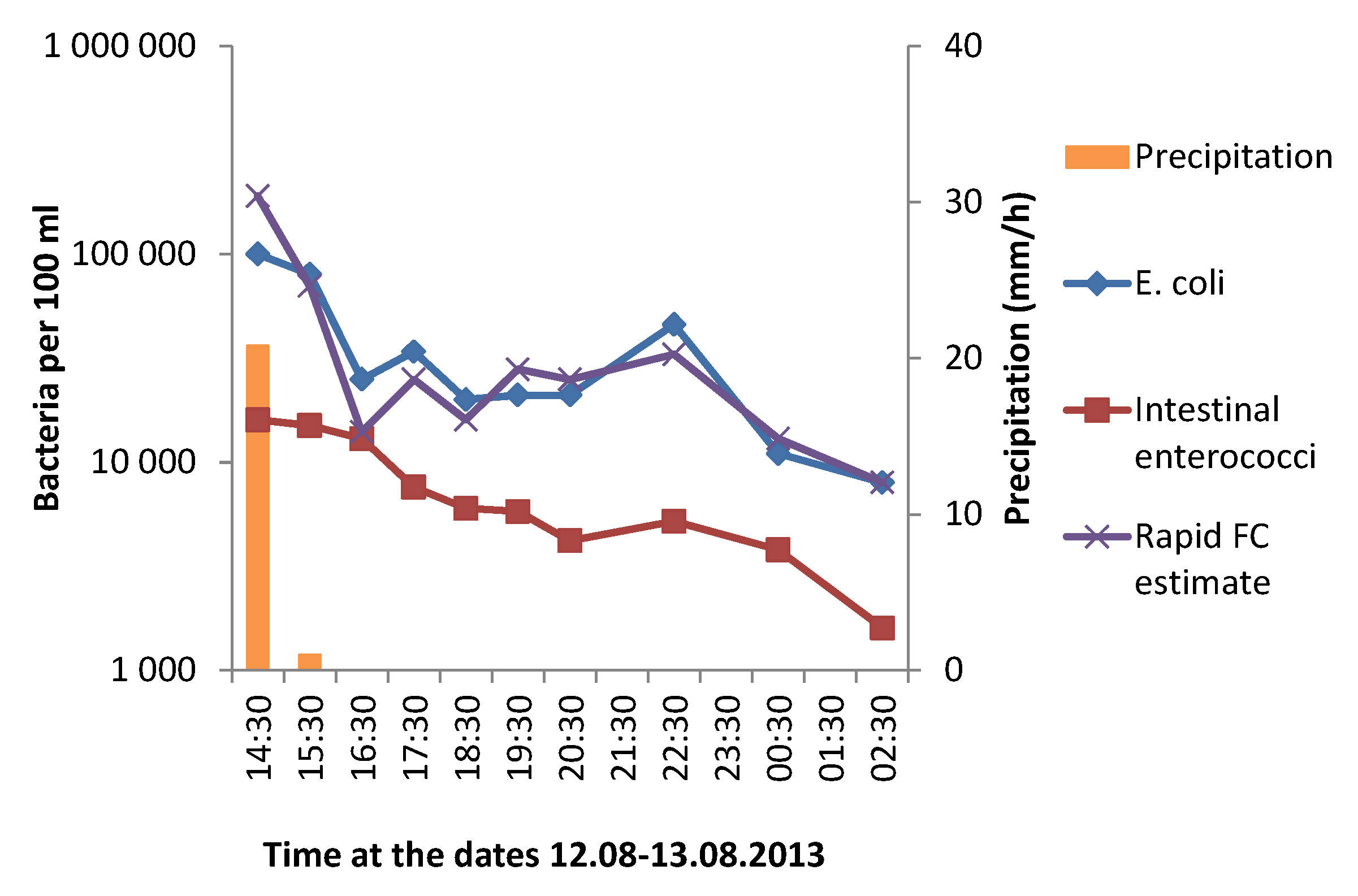
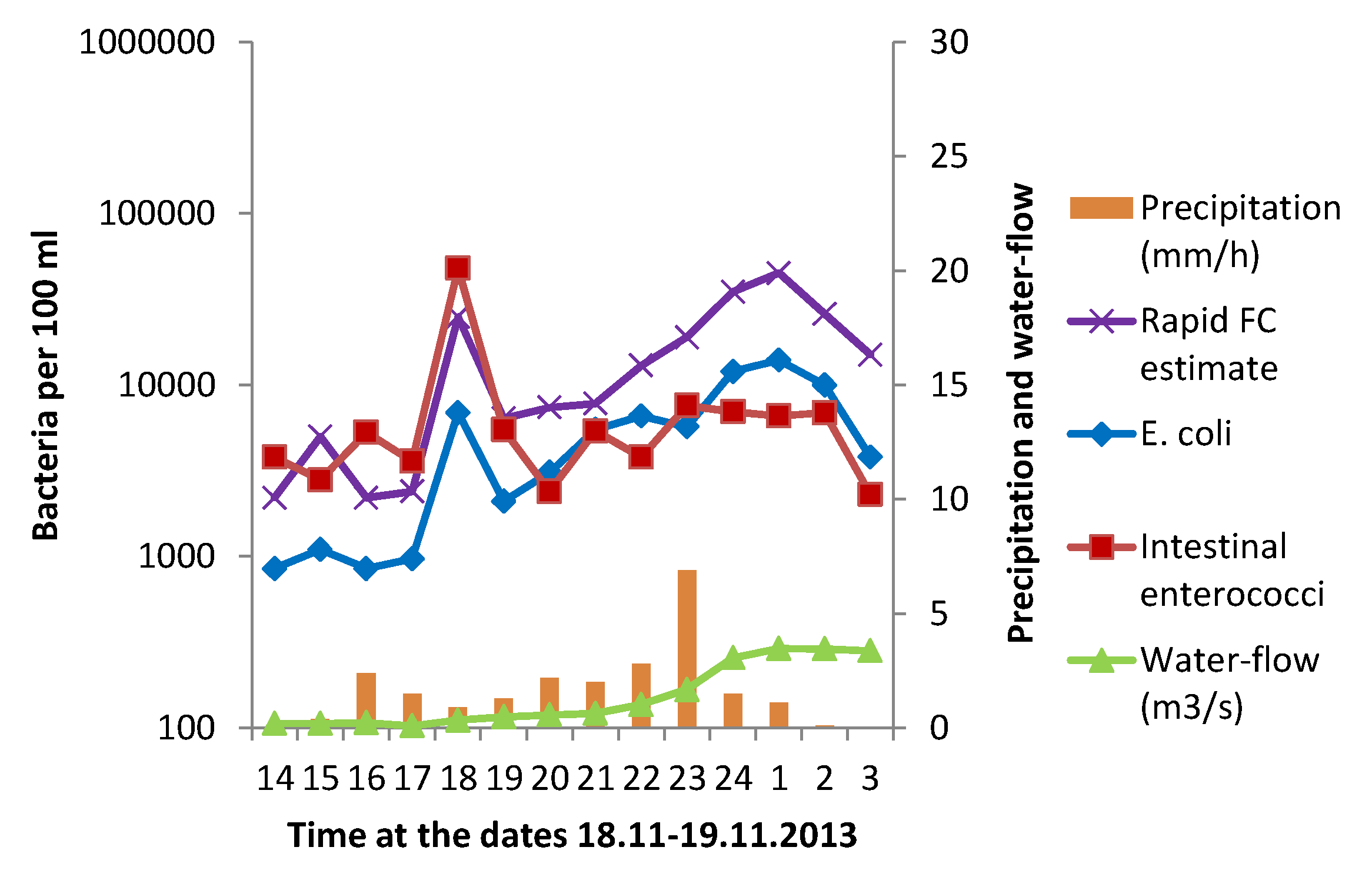
3.1. Effect of Heavy Rainfall on the Water Quality in Urban Rivers
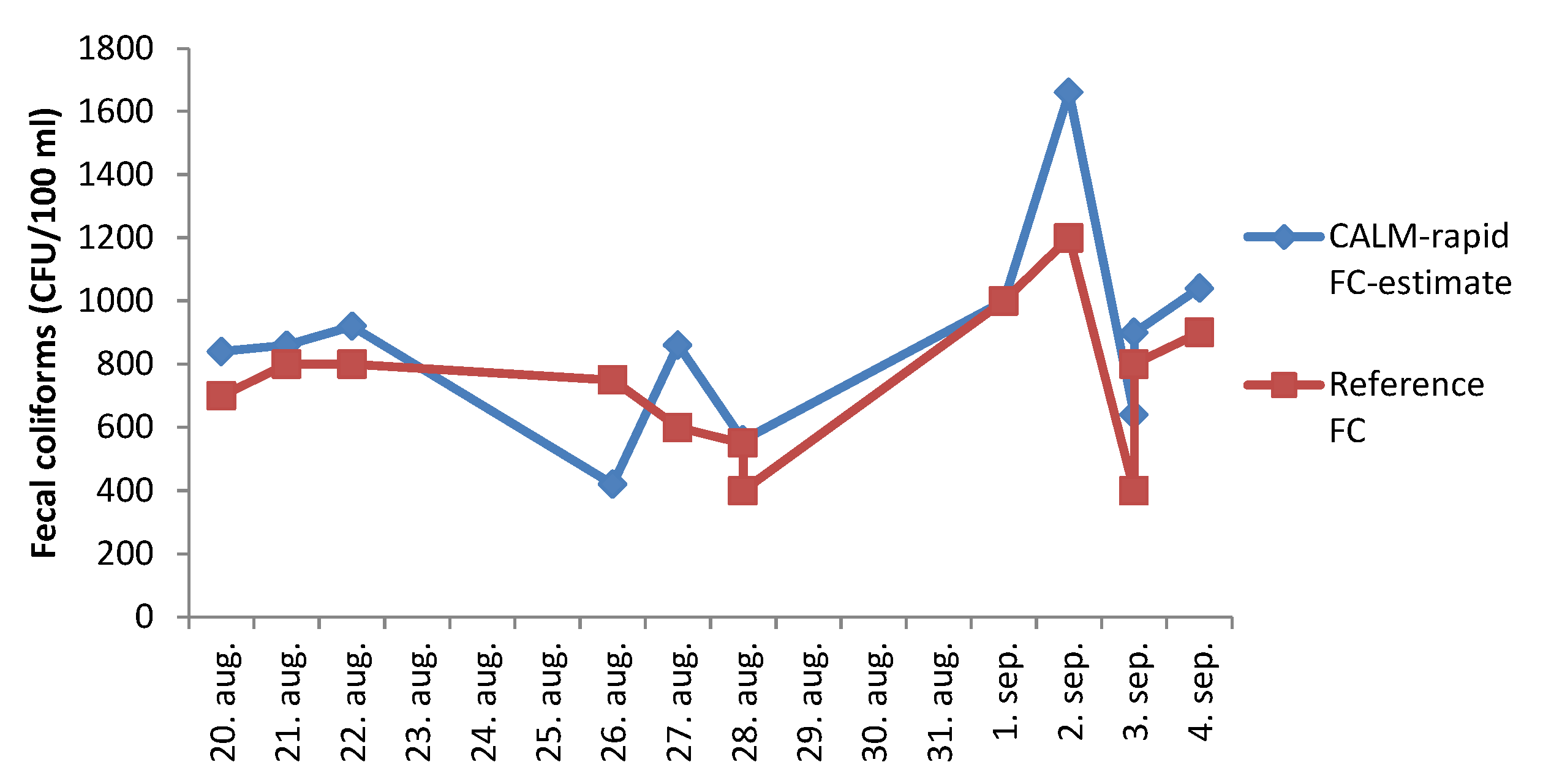
3.2. Test of a Fully Automated Instrument for Measurement of GAL Activity in River Water
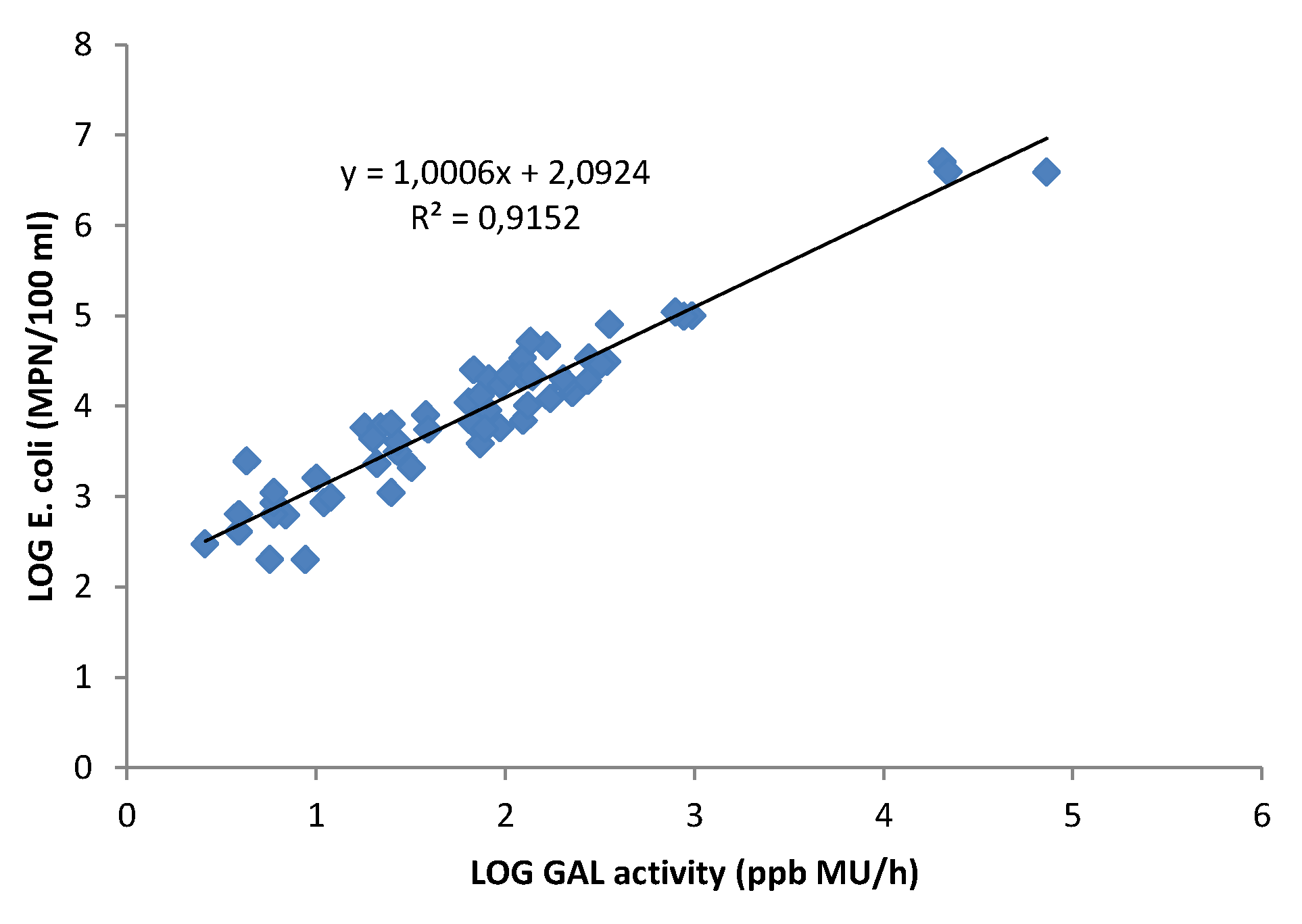
3.3. Correlation between GAL Activity and E. coli in Fresh Water Samples
3.4. Influence of Salinity on the Direct Measurement of GAL Activity
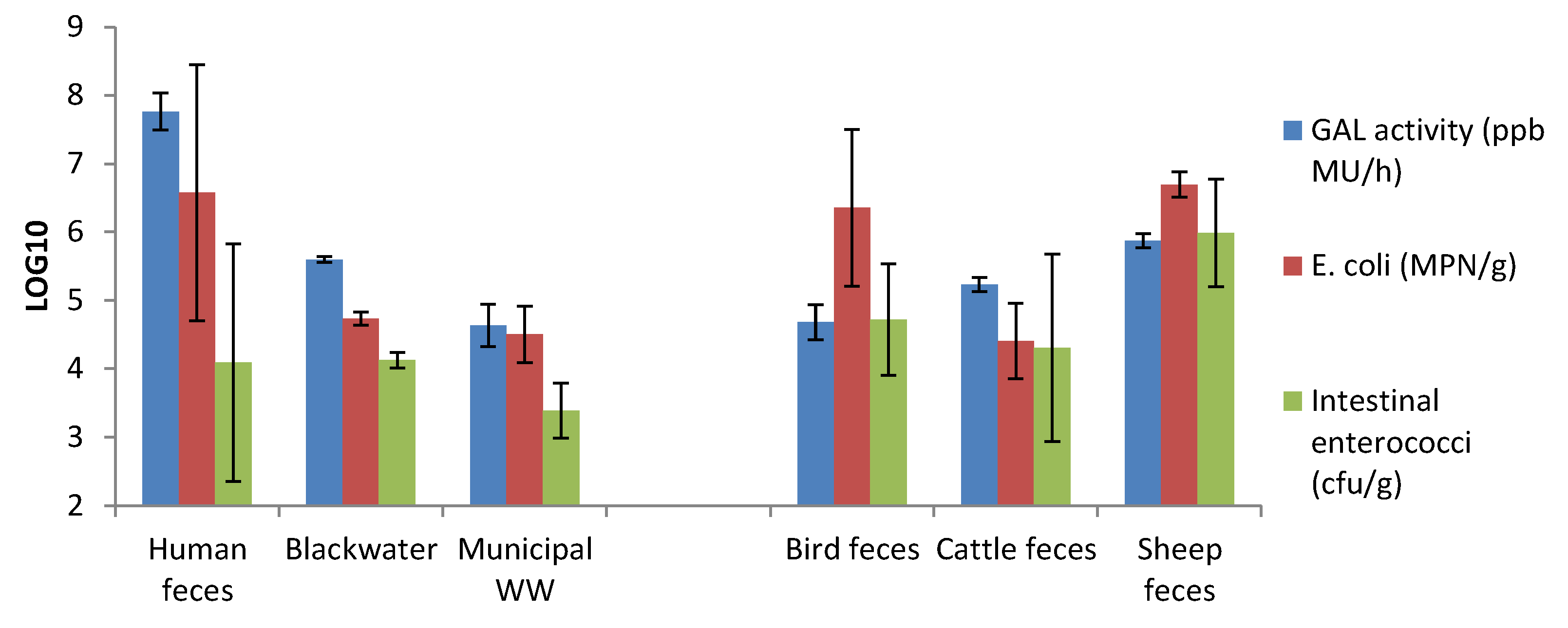
3.5. GAL Activity in Human and Animal Feces
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Union. Directive 2006/7/ec of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/eec. Available online: http://eur-lex.Europa.Eu/legal-content/en/txt/?Uri=celex:32006l0007 (assessed on 11 November 2015).
- Panasiuk, O.; Hedstrom, A.; Marsalek, J.; Ashley, R.M.; Viklander, M. Contamination of stormwater by wastewater: A review of detection methods. J. Environ. Manag. 2015, 152, 241–250. [Google Scholar] [CrossRef] [PubMed]
- McLellan, S.L.; Eren, A.M. Discovering new indicators of fecal pollution. Trends Microbiol. 2014, 22, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Colford, J.M.; Schiff, K.C.; Griffith, J.F.; Yau, V.; Arnold, B.F.; Wright, C.C.; Gruber, J.S.; Wade, T.J.; Burns, S.; Hayes, J.; et al. Using rapid indicators for enterococcus to assess the risk of illness after exposure to urban runoff contaminated marine water. Water Res. 2012, 46, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.E.; Ashbolt, N.J. Assessing pathogen risk to swimmers at non-sewage impacted recreational beaches. Environ. Sci. Technol. 2010, 44, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Harwood, V.J.; Gyawali, P.; Sidhu, J.P.S.; Toze, S. Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl. Environ. Microbiol. 2015, 81, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, U.; Hart, I.; Armon, R. Field evaluation of colilert 3000 for ground, raw and treated surface water and comparison with standard membrane filtration method. Water Air Soil Pollut. 2008, 188, 3–8. [Google Scholar] [CrossRef]
- Ryzinska-Paier, G.; Lendenfeld, T.; Correa, K.; Stadler, P.; Blaschke, A.P.; Mach, R.L.; Stadler, H.; Kirschner, A.K.T.; Farnleitner, A.H. A sensitive and robust method for automated on-line monitoring of enzymatic activities in water and water resources. Water Sci. Technol. 2014, 69, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Tryland, I.; Eregno, F.E.; Braathen, H.; Khalaf, G.; Sjolander, I.; Fossum, M. On-line monitoring of Escherichia coli in raw water at oset drinking water treatment plant, Oslo (Norway). Int. J. Environ. Res. Public Health 2015, 12, 1788–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, J.D.; Fiksdal, L. Rapid detection of total and fecal coliforms in water by enzymatic-hydrolysis of 4-methylumbelliferone-beta-d-galactoside. Appl. Environ. Microbiol. 1988, 54, 2118–2122. [Google Scholar] [PubMed]
- Davies, C.M.; Apte, S.C. An evaluation of potential interferences in a fluorimetric assay for the rapid detection of thermotolerant coliforms in sewage. Lett. Appl. Microbiol. 2000, 30, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fiksdal, L.; Tryland, I. Application of rapid enzyme assay techniques for monitoring of microbial water quality. Curr. Opin. Biotechnol. 2008, 19, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Sicard, C.; Shek, N.; White, D.; Bowers, R.J.; Brown, R.S.; Brennan, J.D. A rapid and sensitive fluorimetric beta-galactosidase assay for coliform detection using chlorophenol red-β-D-galactopyranoside. Anal. Bioanal. Chem. 2014, 406, 5395–5403. [Google Scholar] [CrossRef] [PubMed]
- Edberg, S.C.; Allen, M.J.; Smith, D.B. National field-evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and escherichia-coli from drinking-water—comparison with the standard multiple tube fermentation method. Appl. Environ. Microbiol. 1988, 54, 1595–1601. [Google Scholar] [PubMed]
- Tryland, I.; Samset, I.D.; Hermansen, L.; Berg, J.D.; Rydberg, H. Early warning of faecal contamination of water: A dual mode, automated system for high- (<1 h) and low-levels (6–11 h). Water Sci. Technol. 2001, 43, 217–220. [Google Scholar] [PubMed]
- VanPoucke, S.O.; Nelis, H.J. Limitations of highly sensitive enzymatic presence-absence tests for detection of waterborne coliforms and Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 771–774. [Google Scholar]
- Wallenfels, K.; Weil, R. B-galactosidase. In The Enzymes; Academic Press: New York, NY, USA, 1972; Volume VIII, pp. 617–663. [Google Scholar]
- Tryland, I.; Fiksdal, L. Enzyme characteristics of β-D-galactosidase- and β-D-glucuronidase-positive bacteria and their interference in rapid methods for detection of waterborne coliforms and Escherichia coli. Appl. Environ. Microbiol. 1998, 64, 1018–1023. [Google Scholar] [PubMed]
- Arukha, A.P.; Mukhopadhyay, B.C.; Mitra, S.; Biswas, S.R. A constitutive unregulated expression of β-galactosidase in lactobacillus fermentum M1. Curr. Microbiol. 2015, 70, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tryland, I. Rapid Enzymatic Detection of Microbial Water Quality. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 1999. [Google Scholar]
- The Norwegian Meteorological Institute. Available online: http://sharki.oslo.dnmi.no/portal/page?_pageid=73,39035,73_39049&_dad=portal&_schema=PORTAL (accessed on 16 February).
- Tryland, I.; Braathen, H.; Beschorner, A.-L.; Muthanna, T. Monitoring of hygienic bathing water quality. Vann 2012, 2, 194–206. (in Norwegian). [Google Scholar]
- Wutor, V.C.; Togo, C.A.; Pletschke, B.I. Comparison of the direct enzyme assay method with the membrane filtration technique in the quantification and monitoring of microbial indicator organisms—Seasonal variations in the activities of coliforms and E-coli, temperature and ph. Water SA 2007, 33, 107–110. [Google Scholar] [CrossRef]
- Baudart, J.; Servais, P.; De Paoli, H.; Henry, A.; Lebaron, P. Rapid enumeration of Escherichia coli in marine bathing waters: Potential interference of nontarget bacteria. J. Appl. Microbiol. 2009, 107, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.M.; Apte, S.C.; Peterson, S.M. Possible interference of lactose-fermenting marine vibrios in coliform β-D-galactosidase assays. J. Appl. Bacteriol. 1995, 78, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.M.; Apte, S.C.; Peterson, S.M.; Stauber, J.L. Plant and algal interference in bacterial β-D-galactosidase and β-D-glucuronidase assays. Appl. Environ. Microbiol. 1994, 60, 3959–3964. [Google Scholar] [PubMed]
- Davies, C.M.; Apte, S.C.; Peterson, S.M. β-D-galactosidase activity of viable, non-culturable coliform bacteria in marine waters. Lett. Appl. Microbiol. 1995, 21, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Fiksdal, L.; Tryland, I. Effect of UV Light irradiation, starvation and heat on Escherichia coli β-D-galactosidase activity and other potential viability parameters. J. Appl. Microbiol. 1999, 87, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pommepuy, M.; Fiksdal, L.; Gourmelon, M.; Melikechi, H.; Caprais, M.P.; Cormier, M.; Colwell, R.R. Effect of seawater on Escherichia coli β-galactosidase activity. J. Appl. Bacteriol. 1996, 81, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tryland, I.; Pommepuy, M.; Fiksdal, L. Effect of chlorination on β-D-galactosidase activity of sewage bacteria and Escherichia coli. J. Appl. Microbiol. 1998, 85, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Molina-Munoz, M.; Poyatos, J.M.; Vilchez, R.; Hontoria, E.; Rodelas, B.; Gonzalez-Lopez, J. Effect of the concentration of suspended solids on the enzymatic activities and biodiversity of a submerged membrane bioreactor for aerobic treatment of domestic wastewater. Appl. Microbiol. Biotechnol. 2007, 73, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Wutor, V.C.; Togo, C.A.; Pletschke, B.I. The effect of physico-chemical parameters and chemical compounds on the activity of β-D-galactosidase (B-GAL), a marker enzyme for indicator microorganisms in water. Chemosphere 2007, 68, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Rada, V.; Vlkova, E.; Nevoral, J.; Trojanova, I. Comparison of bacterial flora and enzymatic activity in faeces of infants and calves. FEMS Microbiol. Lett. 2006, 258, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Shrier, I.; Heilpern, D.; Je, J.S.; Park, S.; Chong, G.; Lalonde, C.; Cote, L.F.; Lee, B. Differential impact of lactose/lactase phenotype on colonic microflora. Can. J. Gastroenterol. 2010, 24, 373–379. [Google Scholar] [PubMed]
| GAL Activity <6.7 ppb MU/h | GAL Activity >6.7 ppb MU/h | |
|---|---|---|
| E. coli concentration <1000 E. coli/100 mL | 33 | 0 |
| E. coli concentration >1000 E. coli/100 mL | 3 | 2 |
| Seawater | NaCl | |
|---|---|---|
| Low salinity (3.4 PSU) | 100% | 100% |
| Medium salinity (17 PSU) | 90% | 93% |
| High salinity (34 PSU) | 65% | 70% |
| Arithmetic Mean | Geometric Mean | Minimum | Maximum 1 | LOG Variation 2 | |
|---|---|---|---|---|---|
| GAL activity (ppb MU/h) | 6.9 × 107 | 5.8 × 107 | 1.7 × 107 | 1.8 × 108 | 1.0 |
| E. coli (MPN/g) 3 | 3.4 × 108 | 3.7 × 106 | <1 × 103 | 3.1 × 109 | >6.5 |
| Intestinal enterococci (cfu/g) | 4.7 × 106 | 1.8 × 104 | <1 × 102 | 6.0 × 107 | >5.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tryland, I.; Braathen, H.; Wennberg, A.C.; Eregno, F.; Beschorner, A.-L. Monitoring of β-d-Galactosidase Activity as a Surrogate Parameter for Rapid Detection of Sewage Contamination in Urban Recreational Water. Water 2016, 8, 65. https://doi.org/10.3390/w8020065
Tryland I, Braathen H, Wennberg AC, Eregno F, Beschorner A-L. Monitoring of β-d-Galactosidase Activity as a Surrogate Parameter for Rapid Detection of Sewage Contamination in Urban Recreational Water. Water. 2016; 8(2):65. https://doi.org/10.3390/w8020065
Chicago/Turabian StyleTryland, Ingun, Henrik Braathen, Aina C. Wennberg, Fasil Eregno, and Anna-Lena Beschorner. 2016. "Monitoring of β-d-Galactosidase Activity as a Surrogate Parameter for Rapid Detection of Sewage Contamination in Urban Recreational Water" Water 8, no. 2: 65. https://doi.org/10.3390/w8020065






