Effects of Environmental Factors on the Temporal Stability of Phytoplankton Biomass in a Eutrophic Man-Made Lake
Abstract
:1. Introduction
2. Materials and Methods
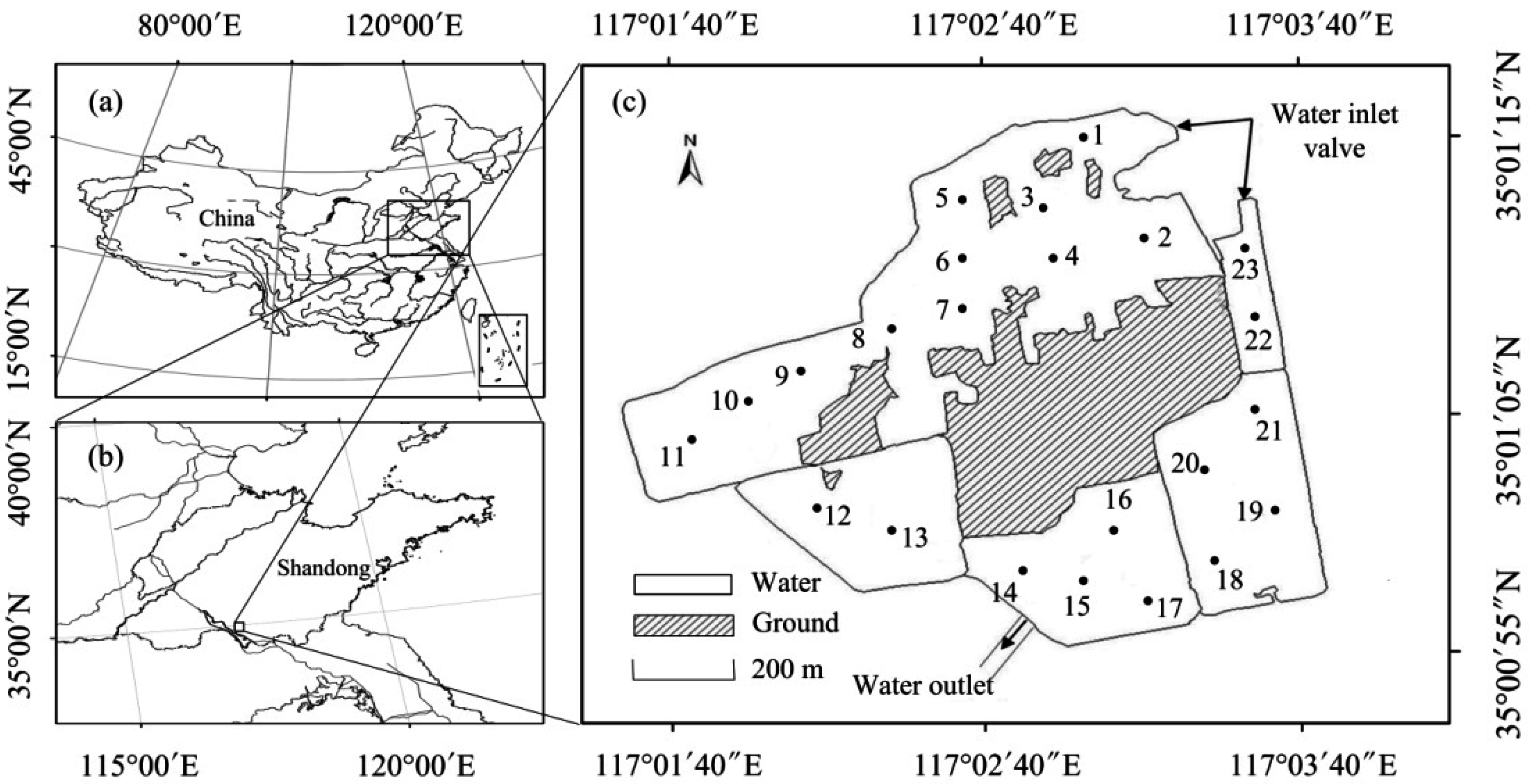
2.1. Study Area
2.2. Sampling and Measurements
2.3. Temporal Stability Index and Statistical Analyses
3. Results
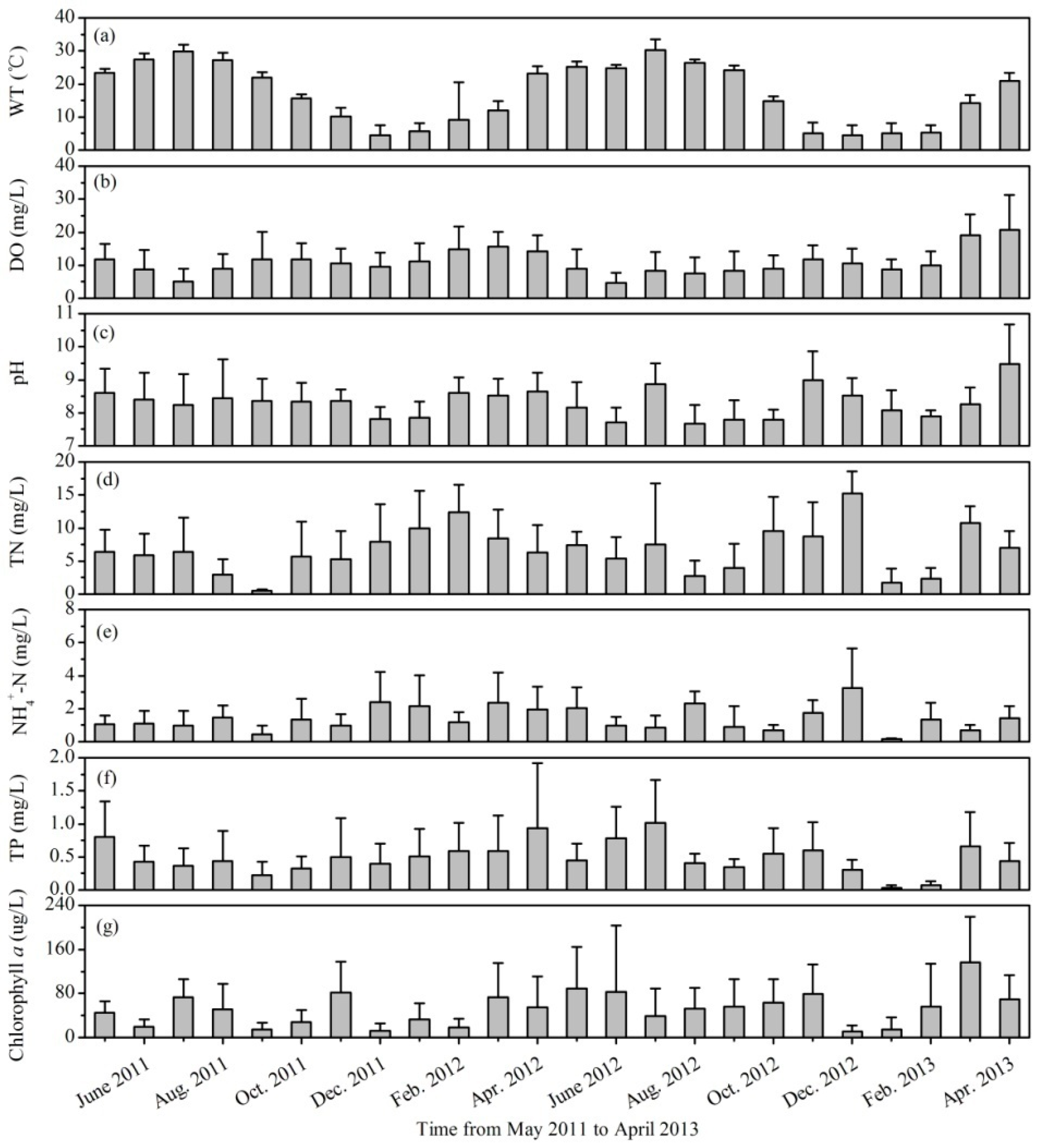
3.1. Variations of Environmental Factors and Phytoplankton Biomass
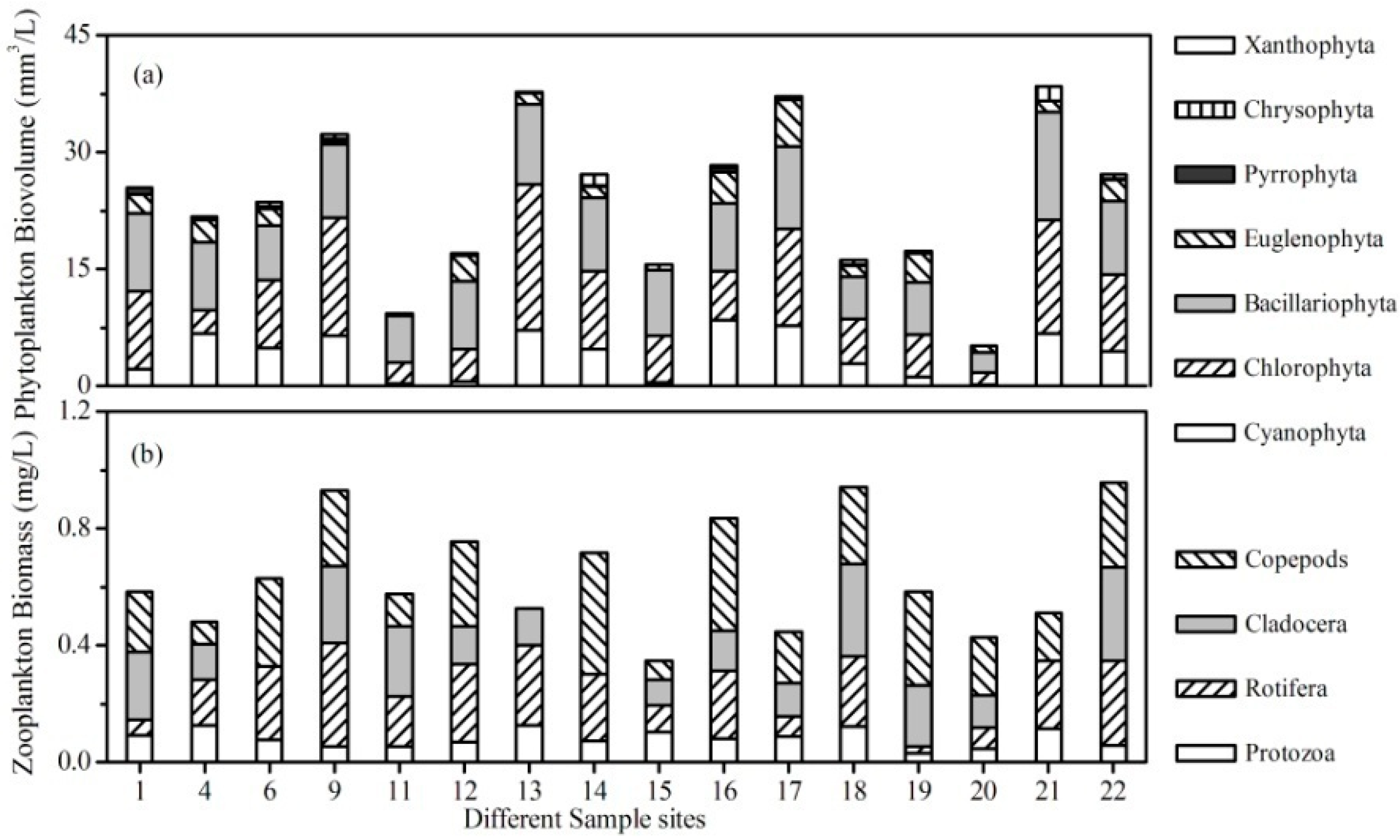
3.2. Plankton Community in the Lake
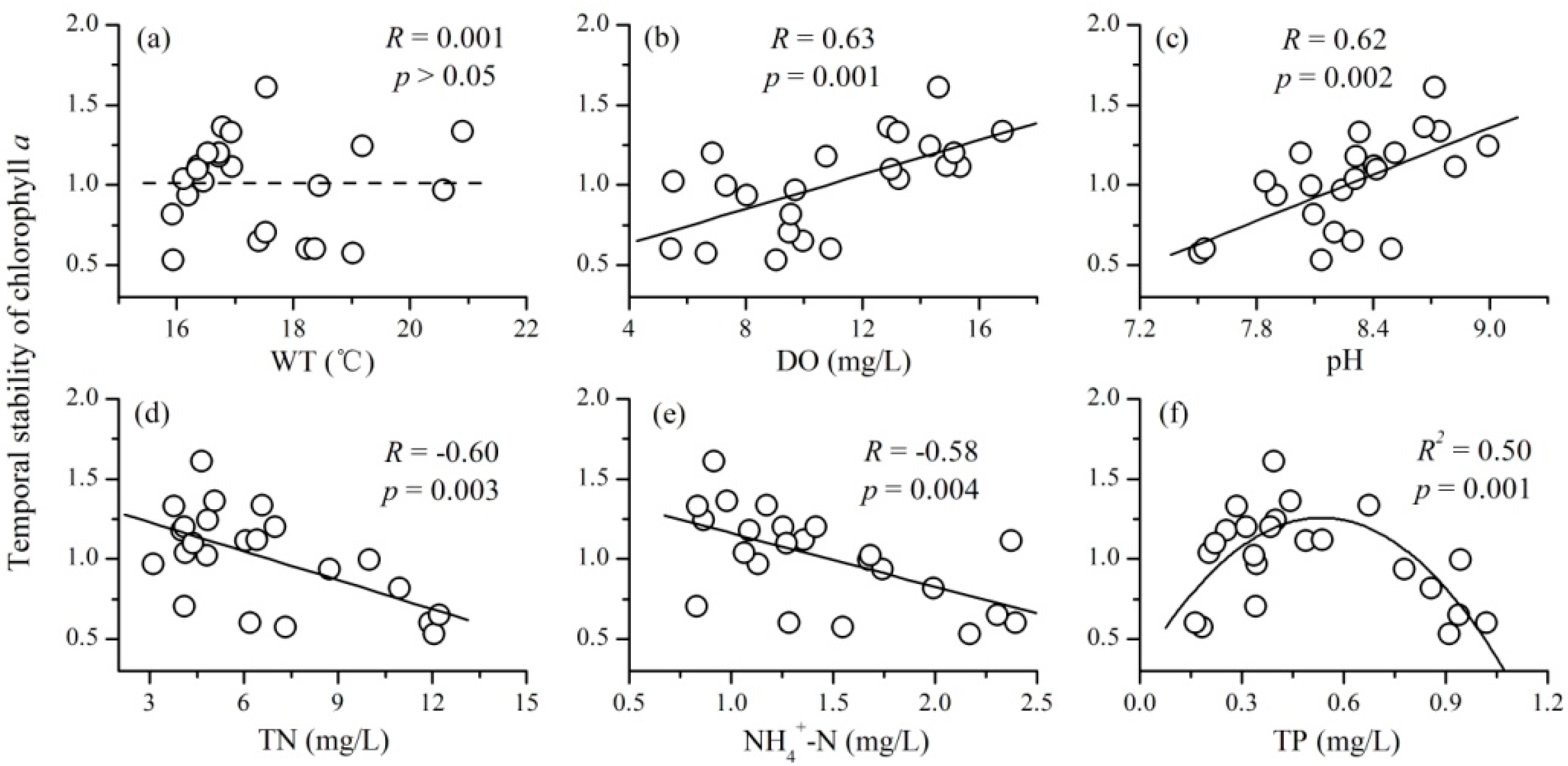
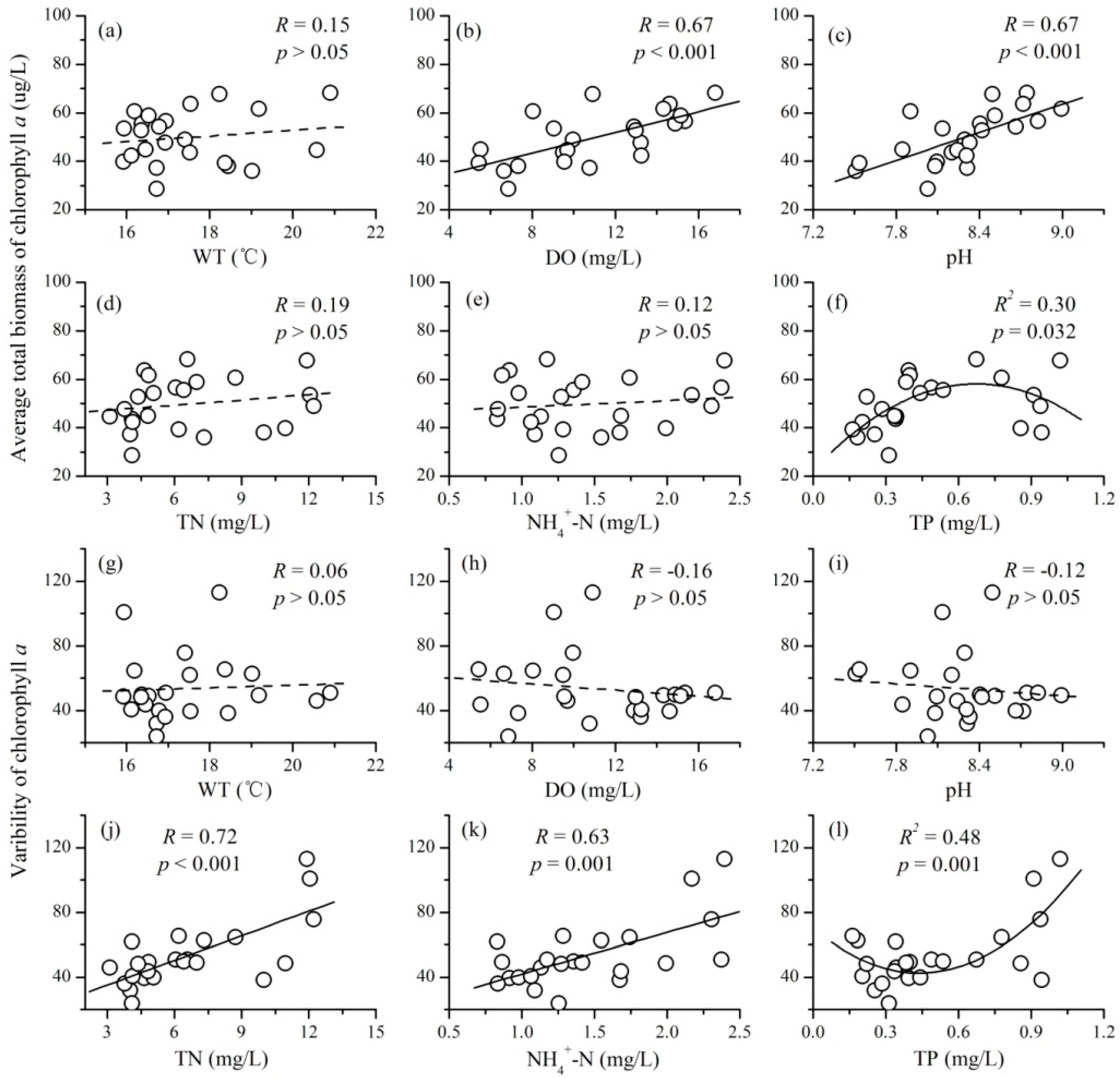
3.3. The Relationships between Mean Environmental Factors and Phytoplankton TSI
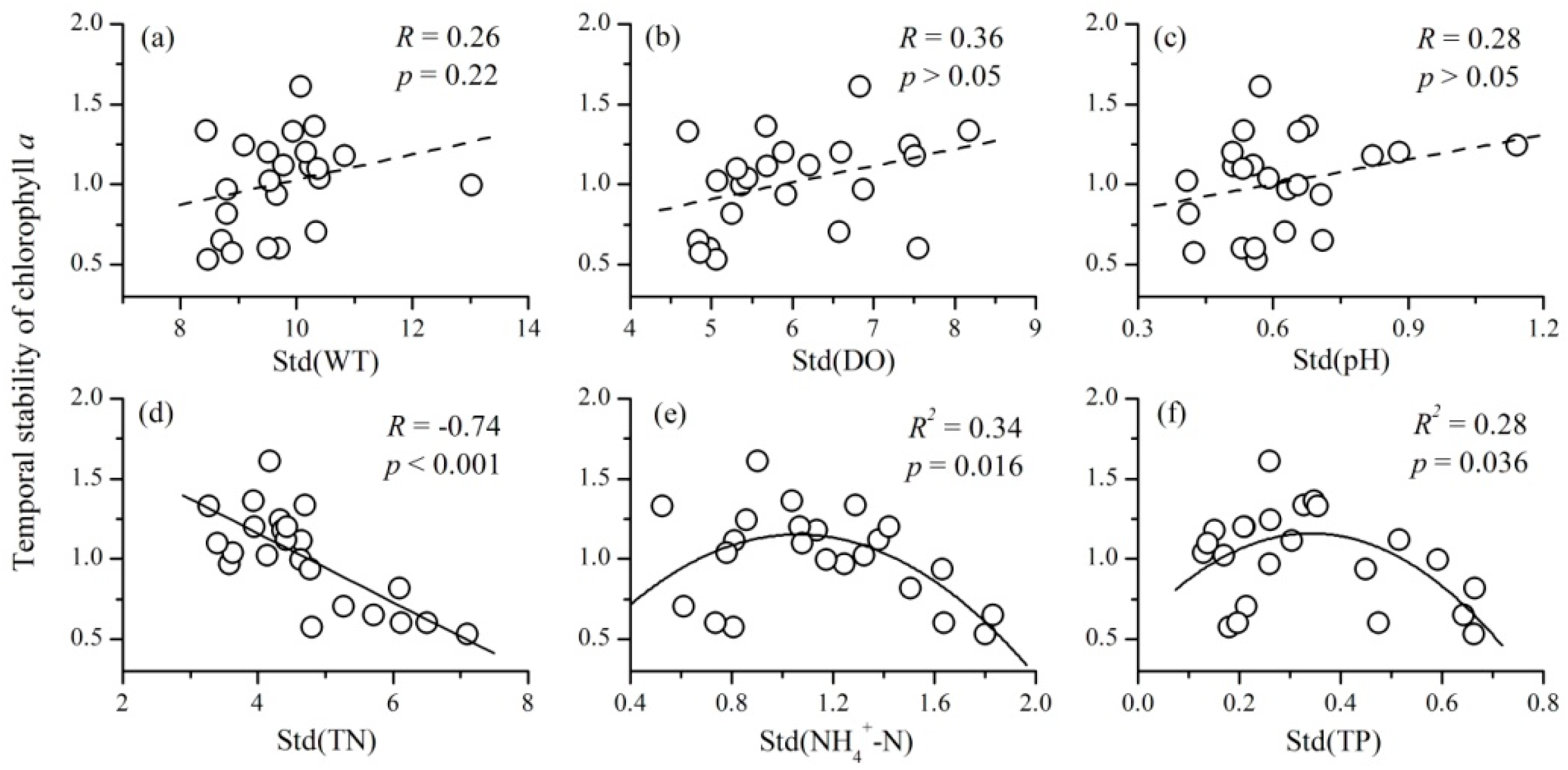
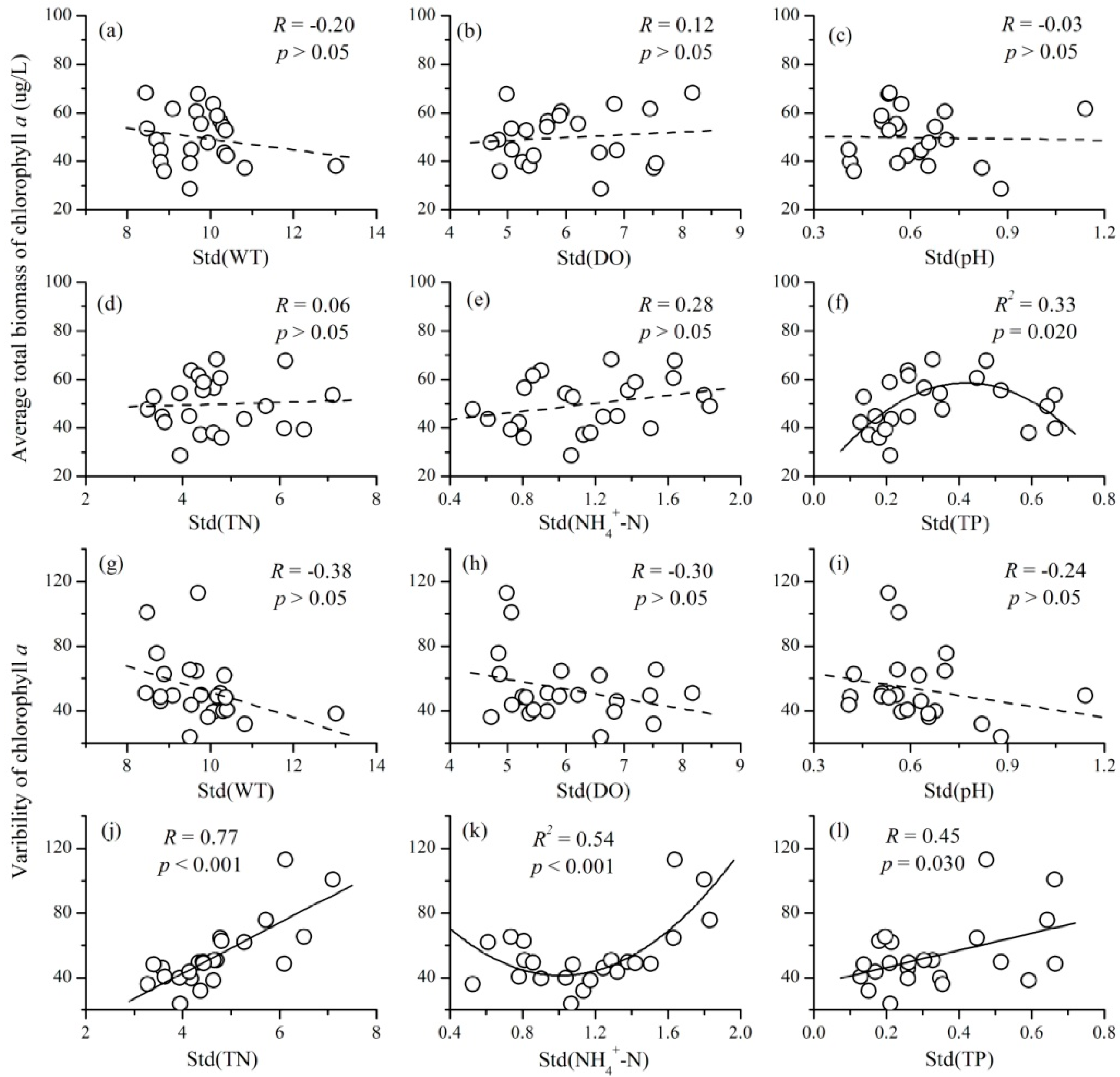
3.4. The Relationships between the Variability of Environmental Factors and Phytoplankton TSI
3.5. Combined Relationships between Environmental Factors and Phytoplankton TSI
4. Discussion
5. Conclusions
- (1)
- There were complex relationships between the mean environmental factors and phytoplankton TSI: a positive relationship for DO and pH, a negative relationship for TN and NH4+-N, a unimodal relationship for TP, and no relationship for WT. Mean values of DO and pH mainly increased the stability of phytoplankton through increasing the average total biomass; while that of TN and NH4+-N destabilized phytoplankton TSI primarily through increasing the variability of phytoplankton.
- (2)
- There were also complex relationships between the variability of environmental factors and phytoplankton TSI: a negative relationship for TN, a unimodal relationship for NH4+-N and TP, and no relationship for WT, DO, and pH. The variability of nutrient concentrations mainly affected phytoplankton TSI through influencing the variability of community biomass. Therefore, at a local scale, the fluctuations of nutrients will decrease the temporal stability of phytoplankton.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Schwaderer, A.S.; Yoshiyama, K.; de Tezanos Pinto, P.; Swenson, N.G.; Klausmeier, C.A.; Litchman, E. Eco-evolutionary differences in light utilization traits and distributions of freshwater phytoplankton. Limnol. Oceanogr. 2011, 56, 589–598. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Peter, H.; Willen, E. Shifts in phytoplankton species richness and biomass along a latitudinal gradient-consequences for relationships between biodiversity and ecosystem functioning. Freshw. Biol. 2013, 58, 612–623. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Y.; Liu, Z.; de Giesen, N.V.; Zhu, D. The influence of a eutrophic lake to the river downstream: Spatiotemporal algal composition changes and the driving factors. Water 2015, 7, 2184–2201. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- O’neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Smith, S.J.; Edmonds, J.; Hartin, C.A.; Mundra, A.; Calvin, K. Near-term acceleration in the rate of temperature change. Nat. Clim. Chang. 2015, 5, 333–336. [Google Scholar] [CrossRef]
- O’Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; Mclntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 10773–10781. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; O’Gorman, E.J.; Tian, W.; Ma, A.; Moore, J.C.; Borrett, S.R.; Woodward, G. Weighting and indirect effects identify keystone species in food webs. Ecol. Lett. 2016, 19, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.; Yacobi, Y.Z.; Pollingher, U. Lake Kinneret phytoplankton: Stability and variability during twenty years (1970–1989). Aquat. Sci. 1992, 54, 104–127. [Google Scholar] [CrossRef]
- Rojo, C.; Alvarez-Cobelas, M. Are there steady-state phytoplankton assemblages in the field? Hydrobiologia 2003, 502, 3–12. [Google Scholar] [CrossRef]
- Honti, M.; Istvánovics, V.; Osztoics, A. Stability and change of phytoplankton communities in a highly dynamic environment-the case of large, shallow Lake Balaton (Hungary). Hydrobiologia 2007, 581, 225–240. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Zhao, L.; Xu, X.; Huang, H. Plankton community stability and its relationship with phytoplankton species richness in Lake Nansihu, China. Water 2016, 8, 454. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Ives, A.R.; Carpenter, S.R. Stability and diversity of ecosystems. Science 2007, 317, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Donohue, I.; Petchey, O.L.; Montoya, J.M.; Jackson, A.L.; McNally, L.; Viana, M.; Healy, K.; Lurgi, M.; O’Connor, N.E.; Emmerson, M.C. On the dimensionality of ecological stability. Ecol. Lett. 2013, 16, 421–429. [Google Scholar] [CrossRef] [PubMed]
- McGrady-Steed, J.; Harris, P.M.; Morin, P.J. Biodiversity regulates ecosystem predictability. Nature 1997, 390, 162–165. [Google Scholar]
- Zhang, Q.G.; Zhang, D.Y. Species richness destabilizes ecosystem functioning in experimental aquatic microcosms. Oikos 2006, 112, 218–226. [Google Scholar] [CrossRef]
- Corcoran, A.A.; Boeing, W.J. Biodiversity increases the productivity and stability of phytoplankton communities. PLoS ONE 2012, 7, e49397. [Google Scholar] [CrossRef] [PubMed]
- Smol, J.P.; Wolfe, A.P.; Birks, H.J.B.; Douglas, M.S.; Jones, V.J.; Korhola, A.; Pienitz, R.; Rühland, K.; Sorvari, S.; Antoniades, D.; et al. Climate-driven regime shifts in the biological communities of arctic lakes. Proc. Natl. Acad. Sci. USA 2005, 102, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Giller, P.S.; Hillebrand, H.; Berninger, U.G.; Gessner, M.O.; Hawkins, S.; Inchausti, P.; Inglis, C.; Leslie, H.; Malmqvist, B.; Monaghan, M.T.; et al. Biodiversity effects on ecosystem functioning: Emerging issues and their experimental test in aquatic environments. Oikos 2004, 104, 423–436. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef] [PubMed]
- Filstrup, C.T.; Hillebrand, H.; Heathcote, A.J.; Harpole, W.S.; Downing, J.A. Cyanobacteria dominance influences resource use efficiency and community turnover in phytoplankton and zooplankton communities. Ecol. Lett. 2014, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Laamanen, M.; Huttunen, M. Long-term changes in summer phytoplankton communities of the open northern Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 71, 580–592. [Google Scholar] [CrossRef]
- Rothenberger, M.B.; Burkholder, J.A.M.; Wentworth, T.R. Use of long-term data and multivariate ordination techniques to identify environmental factors governing estuarine phytoplankton species dynamics. Limnol. Oceanogr. 2009, 54, 2107–2127. [Google Scholar] [CrossRef]
- Salmaso, N. Long-term phytoplankton community changes in a deep subalpine lake: Responses to nutrient availability and climatic fluctuations. Freshw. Biol. 2010, 55, 825–846. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.M. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Thibaut, L.M.; Connolly, S.R. Understanding diversity-stability relationships: Towards a unified model of portfolio effects. Ecol. Lett. 2013, 16, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Fridley, J.D. Resource availability dominates and alters the relationship between species diversity and ecosystem productivity in experimental plant communities. Oecologia 2002, 132, 271–277. [Google Scholar] [CrossRef]
- Wardle, D.A.; Zackrisson, O. Effects of species and functional group loss on island ecosystem properties. Nature 2005, 435, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.B.; Anderson, M.T.; Smith, M.D.; Seabloom, E.; Andelman, S.J.; Meche, G.; Weiher, E.; Allain, L.K.; Jutila, H.; Sankaran, M.; et al. Does species diversity limit productivity in natural grassland communities? Ecol. Lett. 2007, 10, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.K.; Cardinale, B.J. Is the relationship between algal diversity and biomass in North American lakes consistent with biodiversity experiments? Oikos 2014, 123, 267–278. [Google Scholar] [CrossRef]
- Boyd, P.W.; Strzepek, R.; Fu, F.; Hutchins, D.A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 2010, 55, 1353–1376. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.H. Continuous monitoring of short term dissolved oxygen and algal dynamics. Water Res. 1995, 29, 2789–2796. [Google Scholar] [CrossRef]
- Lopez-Archilla, A.I.; Moreira, D.; López-García, P.; Guerrero, C. Phytoplankton diversity and cyanobacterial dominance in a hypereutrophic shallow lake with biologically produced alkaline pH. Extremophiles 2004, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, J.E.; Montagnes, D.J.; Sharples, J.; Atkinson, D. Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnol. Oceanogr. 2008, 53, 487–493. [Google Scholar] [CrossRef]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Stomp, M.; Huisman, J.; Mittelbach, G.G.; Litchman, E.; Klausmeier, C.A. Large-scale biodiversity patterns in freshwater phytoplankton. Ecology 2011, 92, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, J.; Liu, L.; Yu, X.Q.; Zheng, Y.; Chiang, P.C. Temperature and nutrients are significant drivers of seasonal shift in phytoplankton community from a drinking water reservoir, subtropical China. Environ. Sci. Pollut. Res. Int. 2014, 21, 5917–5928. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Descamps-Julien, B. Population and community variability in randomly fluctuating environments. Oikos 2004, 106, 105–116. [Google Scholar] [CrossRef]
- Dokulil, M.; Chen, W.; Cai, Q. Anthropogenic impacts to large lakes in China: The Tai Hu example. Aquat. Ecosyst. Health 2000, 3, 81–94. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Ding, C.R.; Shi, H. Discussion on the problems of total phosphor determination by Mo-Sb anti-spectrophotometry method. Pollut. Control Technol. 2009, 22, 106–108. (In Chinese) [Google Scholar]
- Vörös, L.; Padisák, J. Phytoplankton biomass and chlorophyll-a in some shallow lakes in central Europe. Hydrobiologia 1991, 215, 111–119. [Google Scholar] [CrossRef]
- Felip, M.; Catalan, J. The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: Decoupling in their spatial and temporal maxima. J. Plankton Res. 2000, 22, 91–106. [Google Scholar] [CrossRef]
- Kasprzak, P.; Padisak, J.; Koschel, R.; Krienitz, L.; Gervais, F. Chlorophyll a concentration across a trophic gradient of lakes: An estimator of phytoplankton biomass? Limnol.-Ecol. Manag. Inland Waters 2008, 38, 327–338. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D.Y.; Qian, S.B. Study on phytoplankton biomass I. Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanol. Sin. 1999, 21, 75–85. (In Chinese) [Google Scholar]
- Zhang, H.; Cui, B.; Zhang, Z.; Fan, X. Species diversity and distribution for zooplankton in the inter-tidal wetlands of the Pearl River estuary, China. Procedia Environ. Sci. 2012, 13, 2383–2393. [Google Scholar]
- Akaike, H. Information theory as an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akaemiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Symonds, M.R.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Lauster, G.H.; Hanson, P.C.; Kratz, T.K. Gross primary production and respiration differences among littoral and pelagic habitats in northern Wisconsin lakes. Can. J. Fish. Aquat. Sci. 2006, 63, 1130–1141. [Google Scholar] [CrossRef]
- Staehr, P.A.; Kaj, S.J. Temporal dynamics and regulation of lake metabolism. Limnol. Oceanogr. 2007, 52, 108–120. [Google Scholar] [CrossRef]
- Tilman, D.; Kilham, S.S.; Kilham, P. Phytoplankton community ecology: The role of limiting nutrients. Annu. Rev. Ecol. Syst. 1982, 13, 349–372. [Google Scholar] [CrossRef]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol.-Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Hansen, P.J. Effect of high pH on the growth and survival of marine phytoplankton: Implications for species succession. Aquat. Microb. Ecol. 2002, 28, 279–288. [Google Scholar] [CrossRef]
- Engel, A. Direct relationship between CO2 uptake and transparent exopolymer particles production in natural phytoplankton. J. Plankton Res. 2002, 24, 49–53. [Google Scholar] [CrossRef]
- Olli, K. Diel vertical migration of phytoplankton and heterotrophic flagellates in the Gulf of Riga. J. Mar. Syst. 1999, 23, 145–163. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.J.; Xu, W.J.; Wang, G.S.; Chen, Z.M.; Sun, C.B. Diurnal vertical migration laws of plankton in late breeding period of marsupenaeus japonicus in intensive pond culture. J. Anhui Agric. Sci. 2015, 43, 72–79. (In Chinese) [Google Scholar]
- McQueen, D.J.; Johannes, M.R.; Post, J.R.; Stewart, T.J.; Lean, D.R. Bottom-up and top-down impacts on freshwater pelagic community structure. Ecol. Monogr. 1989, 59, 289–309. [Google Scholar] [CrossRef]
- Elser, J.J.; Goldman, C.R. Zooplankton effects on phytoplankton in lakes of contrasting trophic status. Limnol. Oceanogr. 1971, 36, 64–90. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Zhang, H.; Zhao, L.; Xiong, Y.; Huang, H. Effects of Environmental Factors on the Temporal Stability of Phytoplankton Biomass in a Eutrophic Man-Made Lake. Water 2016, 8, 582. https://doi.org/10.3390/w8120582
Tian W, Zhang H, Zhao L, Xiong Y, Huang H. Effects of Environmental Factors on the Temporal Stability of Phytoplankton Biomass in a Eutrophic Man-Made Lake. Water. 2016; 8(12):582. https://doi.org/10.3390/w8120582
Chicago/Turabian StyleTian, Wang, Huayong Zhang, Lei Zhao, Yuanwu Xiong, and Hai Huang. 2016. "Effects of Environmental Factors on the Temporal Stability of Phytoplankton Biomass in a Eutrophic Man-Made Lake" Water 8, no. 12: 582. https://doi.org/10.3390/w8120582





