Restoration of Shallow Lakes in Subtropical and Tropical China: Response of Nutrients and Water Clarity to Biomanipulation by Fish Removal and Submerged Plant Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Lakes
2.2. Monitoring of Physical and Chemical Parameters
2.3. Statistical Analyses
3. Results
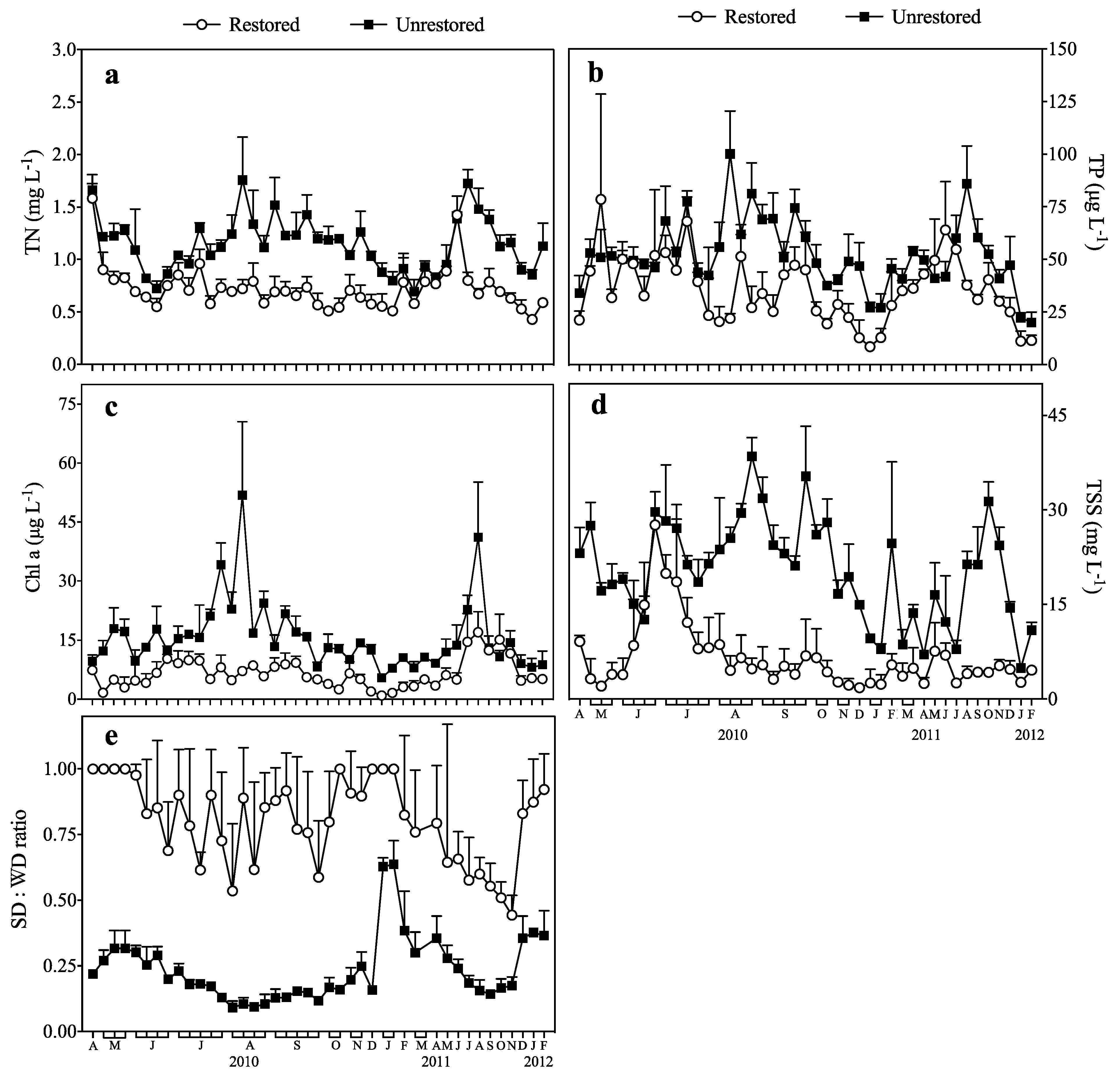
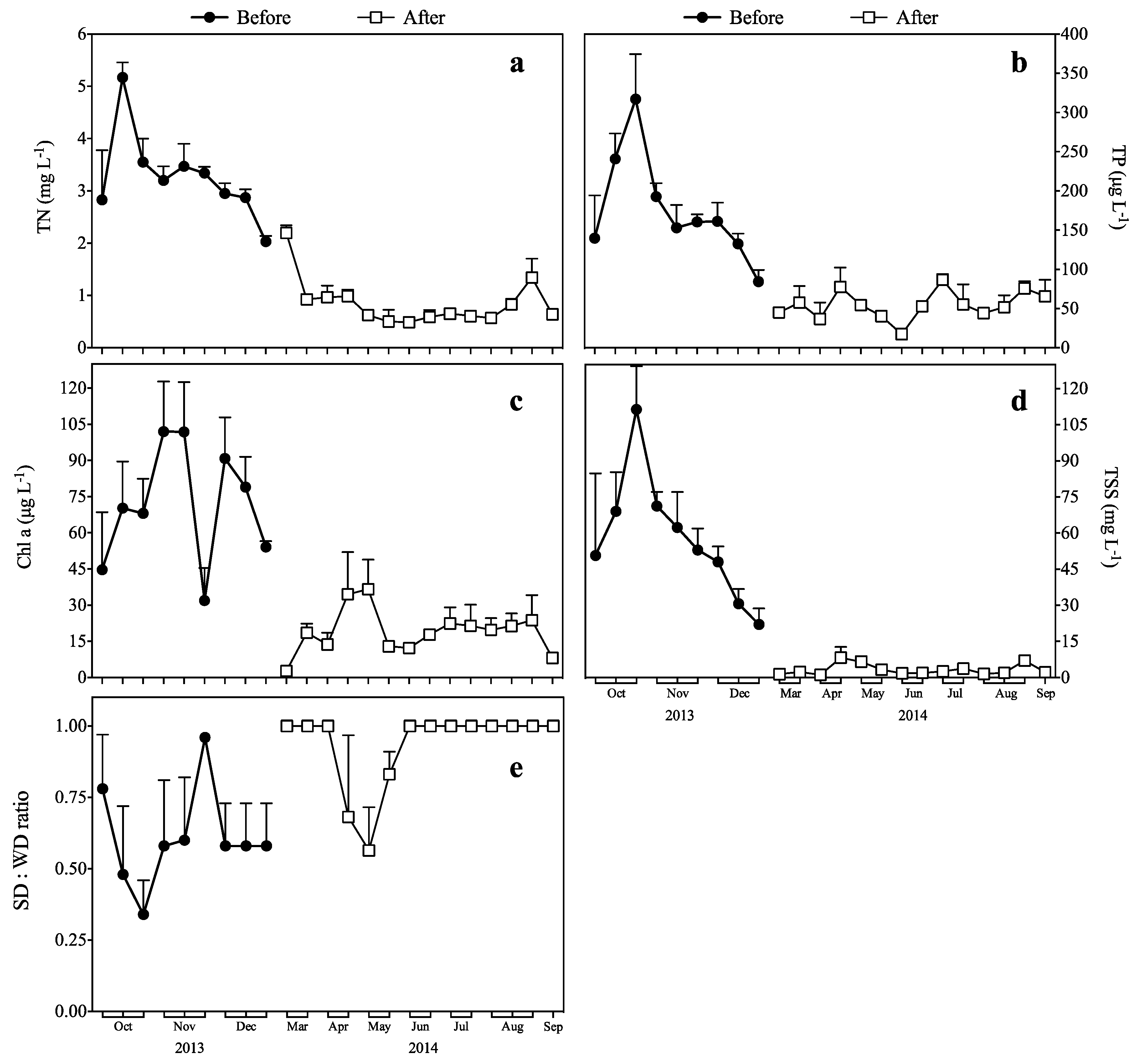
3.1. Response of TN and TP to Biomanipulation
3.2. Response of Chl a to Biomanipulation
3.3. Response of TSS to Biomanipulation
3.4. Response of the SD:WD Ratio to Biomanipulation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brooks, J.L.; Dodson, S.I. Predation, body size, and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–213. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; Özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a restoration tool to combat eutrophication: Recent advances and future challenges. Adv. Ecol. Res. 2012, 47, 411–488. [Google Scholar]
- Meijer, M.L.; Jeppesen, E.; Van Donk, E.; Moss, B.; Scheffer, M.; Lammens, E.; Van Nes, E.; Van Berkum, J.A.; de Jong, G.J.; Faafeng, B.A.; et al. Long-term responses to fish-stock reduction in small shallow lakes: Interpretation of five year results of four biomanipulation cases in the Netherlands and Denmark. Hydrobiologia 1994, 275, 457–466. [Google Scholar] [CrossRef]
- Scheffer, M.; Portielje, R.; Zambrano, L. Fish facilitate wave resuspension of sediment. Limnol. Oceanogr. 2003, 48, 1920–1926. [Google Scholar] [CrossRef]
- Volta, P.; Jeppesen, E.; Leoni, B.; Campi, B.; Sala, P.; Garibaldi, L.; Lauridsen, T.; Winfield, I.J. Recent invasion by a non-native cyprinid (common bream Abramis brama) is followed by major changes in the ecological quality of a shallow lake in southern Europe. Biol. Invasions 2013, 15, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Hansson, L.A.; Annadotter, H.; Bergman, E.; Hamrin, S.F.; Jeppesen, E.; Kairesalo, T.; Luokkanen, E.; Nilsson, P.A.; Søndergaard, M.; Strand, J. Biomanipulation as an application of food-chain theory: Constraints, synthesis, and recommendations for temperate lakes. Ecosystems 1998, 1, 558–574. [Google Scholar] [CrossRef]
- Søndergaard, M.; Liboriussen, L.; Pedersen, A.R.; Jeppesen, E. Lake restoration by fish removal: Short- and long-term effects in 36 Danish lakes. Ecosystems 2008, 11, 1291–1305. [Google Scholar] [CrossRef]
- Shapiro, J.; Wright, D.I. Lake restoration by biomanipulation: Round Lake, Minnesota, the first two years. Freshw. Biol. 1984, 14, 371–383. [Google Scholar]
- Meijer, M.L.; De Boois, I.; Scheffer, M.; Portielje, R.; Hosper, H. Biomanipulation in shallow lakes in the Netherlands and Denmark. Hydrobiologia 1999, 409, 13–30. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Lodge, D.M. Effects of submerged macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Jeppesen, E.; Lauridsen, T.L.; Kairesalo, T.; Perrow, M.R. Impact of submerged macrophytes on fish-zooplankton interactions in lakes. In The Structuring Role of Submerged Macrophytes in Lakes, 1st ed.; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Ecological Studies Series; Springer: New York, NY, USA, 1998; pp. 91–114. [Google Scholar]
- Moss, B. Engineering and biological approaches to the restoration from eutrophication of shallow lakes in which aquatic plant communities are important components. Hydrobiologia 1990, 200, 367–377. [Google Scholar] [CrossRef]
- Scheffer, M.; Jeppesen, E. Alternative stable states. In The Structuring Role of Submerged Macrophytes in Lakes, 1st ed.; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Ecological Studies Series; Springer: New York, NY, USA, 1998; pp. 397–406. [Google Scholar]
- Horppila, J.; Nurminen, L. Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi (southern Finland). Water Res. 2003, 37, 4468–4474. [Google Scholar] [CrossRef]
- Van Donk, E.; Gulati, R.D.; Iedema, A.; Meulemans, J.T. Macrophyte-related shifts in the nitrogen and phosphorus contents of the different trophic levels in a biomanipulated shallow lake. Hydrobiologia 1993, 251, 19–26. [Google Scholar] [CrossRef]
- Kosten, S.; Kamarainen, A.; Jeppesen, E.; van Nes, E.H.; Peeters, E.T.H.M.; Mazzeo, N.; Hauxwell, J.; Hansel-Welch, N.; Lauridsen, T.L.; Søndergaard, M.; et al. Likelihood of abundant submerged vegetation growth in shallow lakes differs across climate zones. Glob. Chang. Biol. 2009, 15, 2503–2517. [Google Scholar] [CrossRef]
- Gross, E.M. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 2003, 22, 313–339. [Google Scholar] [CrossRef]
- Vanderstukken, M.; Mazzeo, N.; Van Colen, W.; Declerck, S.; Muylaert, K. Biological control of phytoplankton by the subtropical submerged macrophytes Egeria densa and Potamogeton illinoensis: A mesocosm study. Freshw. Biol. 2011, 56, 1837–1849. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Pedersen, L.J.; Jeppesen, E.; Søndergaard, M. The importance of macrophyte bed size for cladoceran composition and horizontal migration in a shallow lake. J. Plankton Res. 1996, 18, 2283–2294. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K.; Theil-Nielsen, J.; Jürgens, K. Cascading trophic interactions in the littoral zone: an enclosure experiment in shallow Lake Stigsholm, Denmark. Arch. Hydrobiol. 2002, 153, 533–555. [Google Scholar]
- Casselman, J.M.; Lewis, C.A. Habitat requirements of northern pike (Esox lucius). Can. J. Fish Aquat. Sci. 1996, 53, 161–174. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Engström-Öst, J.; Viitasalo, M. Turbidity decreases anti-predator behavior in pike larvae, Esox lucius. Environ. Biol. Fish 2005, 73, 1–8. [Google Scholar] [CrossRef]
- Salonen, M.; Engström-Öst, J. Prey capture of pike Esox Lucius larvae in turbid water. J. Fish Biol. 2010, 76, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, T.L.; Jeppesen, E.; Søndergaard, M. Colonization and succession of submerged macrophytes in shallow Lake Væng during the first five years following fish manipulation. Hydrobiologia 1994, 275, 233–242. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.; Meijer, M.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, J.; Peng, C.; Tang, Z.; Piao, S. Patterns of fish species richness in China’s lakes. Glob. Ecol. Biogeogr. 2006, 15, 386–394. [Google Scholar] [CrossRef]
- Teixeira-de Mello, F.; Meerhoff, M.; Pekcan-Hekim, Z.; Jeppesen, E. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshw. Biol. 2009, 54, 1202–1215. [Google Scholar] [CrossRef]
- Moss, B. Climate change, nutrient pollution and the bargain of Dr Faustus. Freshw. Biol. 2010, 55, 175–187. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Meerhoff, M.; Davidson, T.A.; Teixeira-de Mello, F.; Baattrup-Pedersen, A.; Jeppesen, E. Meta-analysis shows a consistent and strong latitude pattern in fish omnivory across ecosystems. Ecosystems 2012, 15, 492–503. [Google Scholar]
- Chen, K.; Bao, C.; Zhou, W. Ecological restoration in eutrophic Lake Wuli: A large enclosure experiment. Ecol. Eng. 2009, 35, 1646–1655. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Jeppesen, E. Fish community assemblages changed but biomass remained similar after lake restoration by biomanipulation in a Chinese tropical eutrophic lake. Hydrobiologia 2014, 724, 127–140. [Google Scholar] [CrossRef]
- Meerhoff, M.; Mazzeo, N.; Moss, B.; Rodríguez-Gallego, L. The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat. Ecol. 2003, 37, 377–391. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Jakobsen, B.A.; Hansen, R.S.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Mazzeo, N.; Branco, C. Restoration of shallow lakes by nutrient control and biomanipulation—The successful strategy depends on lake size and climate. Hydrobiologia 2007, 581, 269–288. [Google Scholar] [CrossRef]
- Meerhoff, M.; Clemente, J.M.; Teixeira-de Mello, F.; Iglesias, C.; Pedersen, A.R.; Jeppesen, E. Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Glob. Chang. Biol. 2007, 13, 1888–1897. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhong, P.; Zhang, X.; Ning, J.; Larsen, S.E.; Jeppesen, E. Successful restoration of a tropical shallow eutrophic lake: Strong bottom-up but weak top-down effects recorded. Australia–China wetland network research partnership. In Proceedings of the Australia–China Wetland Network Research Partnership Symposium, Nanjing, China, 23–28 March 2014; Kattel, G., Ed.; Federation University Australia: Mt. Helen, Australia, 2014; pp. 78–86. [Google Scholar]
- Rao, W.; Ning, J.; Zhong, P.; Jeppesen, E.; Liu, Z. Size-dependent feeding of omnivorous Nile tilapia in a macrophyte-dominated lake: Implications for lake management. Hydrobiologia 2015, 749, 125–134. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Z.; He, H.; Zhen, W.; Guan, B.; Chen, F.; Li, K.; Zhong, P.; Teixeira-de Mello, F.; Jeppesen, E. Submerged macrophytes facilitate dominance of omnivorous fish in a subtropical shallow lake: Implications for lake restoration. Hydrobiologia 2016, 775, 97–107. [Google Scholar] [CrossRef]
- Zhu, S.P. Variety of water quality in whole year in the northern of Taihu Lake. Oceanol. Limnol. Sin. 1959, 2, 146–162. (In Chinese) [Google Scholar]
- Wu, X.W. Investigation of limnology in Lake Wuli in 1951. Acta Hydrobiol. Sin. 1962, 1, 63–113. (In Chinese) [Google Scholar]
- Chen, F.; Shu, T.; Jeppesen, E.; Liu, Z.; Chen, Y. Restoration of a subtropical eutrophic shallow lake in China: effects on nutrient concentrations and biological communities. Hydrobiologia 2013, 718, 59–71. [Google Scholar] [CrossRef]
- Zeng, H.; Zhong, P.; Zhao, X.; Li, C.; He, X.; Liu, Z. Response of metazoan zooplankton communities to ecological restoration in a tropical shallow lake. J. Lake Sci. 2016, 28, 170–177. (In Chinese) [Google Scholar]
- Jin, X.; Tu, Q. The Standard Methods for Observation and Analysis in Lake Eutrophication, 2nd ed.; Chinese Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- SEPA. Analytical Methods for Water and Wasterwater Monitor, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Ibelings, B.W.; Portielje, R.; Lammens, E.H.R.R.; Noordhuis, R.; Van den Berg, M.S.; Joosse, W.; Meijer, M.L. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 2007, 10, 4–16. [Google Scholar] [CrossRef]
- Meerhoff, M.; Teixeira-de Mello, F.; Kruk, C.; Alonso, C.; González-Bergonzoni, I.; Pacheco, J.P.; Lacerot, G.; Arim, M.; Beklioğlu, M.; Brucet, S.; et al. Environmental warming in shallow lakes: A review of potential changes in community structure as evidenced from space-for-time substitution approaches. Adv. Ecol. Res. 2012, 46, 259–349. [Google Scholar]
- Nagdali, S.S.; Gupta, P.K. Impact of mass mortality of a mosquito fish, Gambusia affinis on the ecology of a fresh water eutrophic lake (Lake Naini Tal, India). Hydrobiologia 2002, 468, 45–52. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Meerhoff, M.; Lacerot, G.; Clemente, J.; Scasso, F.; Kruk, C.; Goyenola, G.; Garcia, J.; Amsinck, S.L.; et al. High predation is the key factor for dominance of small-bodied zooplankton in warm lakes—Evidence from lakes, fish exclosures and surface sediment. Hydrobiologia 2011, 667, 133–147. [Google Scholar] [CrossRef]
- Liu, W.; Hu, W.; Chen, Y.; Gu, X.; Hu, Z.; Chen, Y.; Ji, L. Temporal and spatial variation of aquatic macrophytes in West Taihu Lake. Acta Ecol. Sin. 2007, 27, 159–170. (In Chinese) [Google Scholar]
- Søndergaard, M.; Lauridsen, T.L.; Johansson, L.S.; Jeppesen, E. Repeated fish removal to restore lakes: case study Lake Væng, Denmark-two biomanipulations during 30 years of monitoring. Water 2016, in press. [Google Scholar]

| Parameters | Lake Wuli | Lake Qinhu | South Lake* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unrestored | Restored | F value | P value | Unrestored | Restored | F value | P value | Pre-restoration | Post-restoration | |
| TN (mg L−1) | 1.2 ± 0.3 | 0.7 ± 0.2 | 155.5 | <0.0001 | 1.3 ± 0.2 | 0.8 ± 0.2 | 143.8 | <0.0001 | 3.3 ± 0.9 | 0.9 ± 0.5 |
| TP (μg L−1) | 51.9 ± 16.4 | 35.6 ± 16.0 | 84.7 | 0.001 | 66.3 ± 25.9 | 48.4 ± 17.1 | 2.2 | 0.212 | 175.7 ± 68.0 | 54.3 ± 18.1 |
| Chl a (μg L−1) | 15.6 ± 8.9 | 6.6 ± 3.7 | 545.0 | <0.0001 | 17.0 ± 8.4 | 9.1 ± 4.8 | 23.3 | 0.009 | 71.4 ± 24.7 | 19.0 ± 9.2 |
| TSS (mg L−1) | 20.3 ± 8.0 | 6.4 ± 5.2 | 89.1 | 0.001 | 34.1 ± 14.5 | 5.2 ± 2.1 | 746.7 | <0.0001 | 57.6 ± 25.9 | 3.3 ± 2.3 |
| SD:WD ratio | 0.2 ± 0.1 | 0.8 ± 0.2 | 184.1 | <0.0001 | 0.2 ± 0.1 | 0.7 ± 0.2 | 192.5 | <0.0001 | 0.6 ± 0.2 | 0.9 ± 0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Liu, Z.; Li, K.; Chen, F.; Guan, B.; Hu, Y.; Zhong, P.; Tang, Y.; Zhao, X.; He, H.; et al. Restoration of Shallow Lakes in Subtropical and Tropical China: Response of Nutrients and Water Clarity to Biomanipulation by Fish Removal and Submerged Plant Transplantation. Water 2016, 8, 438. https://doi.org/10.3390/w8100438
Yu J, Liu Z, Li K, Chen F, Guan B, Hu Y, Zhong P, Tang Y, Zhao X, He H, et al. Restoration of Shallow Lakes in Subtropical and Tropical China: Response of Nutrients and Water Clarity to Biomanipulation by Fish Removal and Submerged Plant Transplantation. Water. 2016; 8(10):438. https://doi.org/10.3390/w8100438
Chicago/Turabian StyleYu, Jinlei, Zhengwen Liu, Kuanyi Li, Feizhou Chen, Baohua Guan, Yaohui Hu, Ping Zhong, Yali Tang, Xuefeng Zhao, Hu He, and et al. 2016. "Restoration of Shallow Lakes in Subtropical and Tropical China: Response of Nutrients and Water Clarity to Biomanipulation by Fish Removal and Submerged Plant Transplantation" Water 8, no. 10: 438. https://doi.org/10.3390/w8100438





