Desalination of Water Using ZVI (Fe0)
Abstract
:1. Introduction
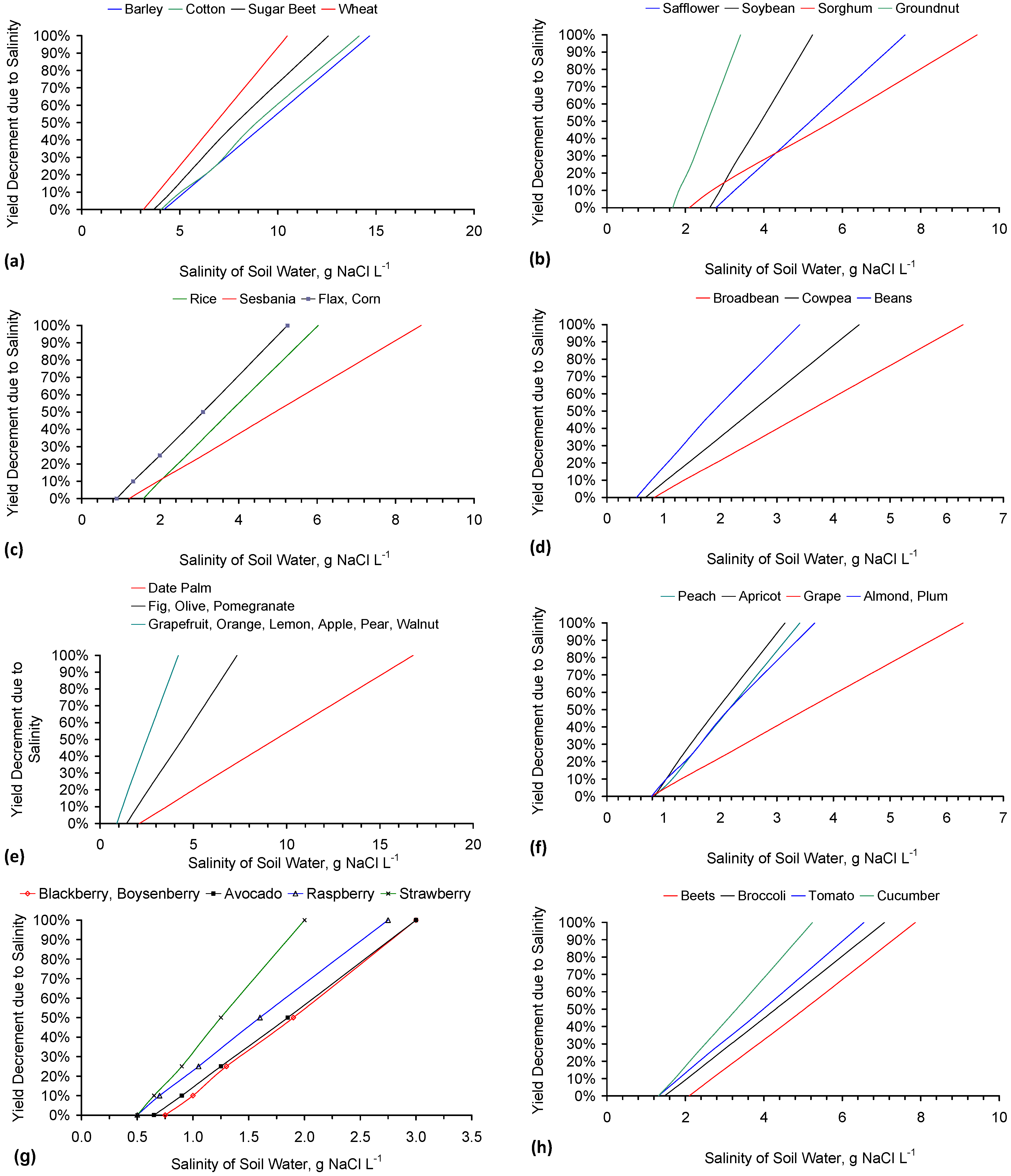
1.1. Potential Agricultural Application for Partial Desalination by ZVI
- (1)
- The impact of a decrease in water salinity on the crop yields;
- (2)
- The impact of a small decrease in water salinity on the number of crops which could be grown on an agricultural holding.
- (1)
- 8 to 4 g·L−1 has the potential (Appendix B, Figure B1a) to increase the possible crop yield (t·ha−1) associated with wheat by 300%.
- (2)
- 4 to 2 g·L−1 has the potential (Appendix B, Figure B1i) to increase the possible crop yield (t·ha−1) associated with potato by >200%.
- (3)
- 3 to 1 g·L−1 has the potential (Appendix B, Figure B1g) to increase the possible crop yield (t·ha−1) associated with soft fruit such as blackberry, raspberry or strawberry by >800%.
- (i)
- Significantly reduce the proportion of global agricultural land which is adversely affected by salinity;
- (ii)
- Increase crop yields on individual agricultural holdings (Appendix B);
- (iii)
- Increase the range of crops that can be grown commercially on a specific agricultural holding (Appendix B).
1.2. Background
1.2.1. Historical ZVI Desalination Experiments
- (1)
- ZVM (Fe0 + Al0 + Cu0)-Ca-montmorillonite combination and ZVI (Fe0)-Ca-montmorillonite. This study [5] demonstrated (Temperature, T = 12–25 °C; Brunauer-Emmett-Teller (BET) surface area, as = 0.00289–0.01732 m2·g−1; ZVI (Fe0) concentration, Pw = 90 g·L−1) (in an open, unstirred, static flow, batch diffusion reactor operated at ambient temperatures) declines in salinity of 25%–50% over 60 days from an initial salinity of about 1 g·L−1.
- (2)
- ZVM (Fe0 and Fe0 + Al0 + Cu0). This study [6] demonstrated (T = 8–20 °C; as = 0.00289–0.01732 m2·g−1; Pw = 90 g·L−1) in an open, unstirred, static flow, batch diffusion reactor operated at ambient temperatures with an initial salinity of about 1 g·L−1, no effective decline in salinity over 60 days.
- (3)
- ZVI (Fe0). This study [20] established (T = 15.3 °C; as = 77.26 m2·g−1; Pw = 3.33 g·L−1) in an open, unstirred, static flow, batch diffusion reactor over 48 h a decline in Cl− concentration from 1.52913 to 1.19831 g·L−1. This was associated with an increase in electrical conductivity (EC) from 3.9 to 4.51 mS·cm−1, and an increase in pH from 6.47 to 9.59.
- (4)
- ZVI (Fe0). This study [21] established (T = 20–22 °C; as = 77.26 m2·g−1; Pw = 8 g·L−1) in an open, continuously stirred, batch diffusion reactor over a 24 h period a statistical relationship between Cl− concentration in the feed water [CF] and product water [CR] over a 24 h period, where CR, mg·L−1 = 1.1 CF0.98 [R2 = 0.99] [21]. The absorbed Cl−, mg·L−1 (CL) = CF − CR.
- (5)
- ZVI (n-Fe0). This study [25] established over a 3 h period using highly reduced n-Fe0, (structured with a Fe0 core and an outer carbon coating (Pw = 1.25 g·L−1; 0.1 gNO3−·L−1), in a fluidised, batch diffusion reactor saturated with argon), a linear relationship between Cl− removal [CL] (g Cl−·g−1 Fe0) and NaCl concentration g·L−1. CL = Adsorbed Cl−, gCl−·g−1 n-Fe0; after 3 h CL = 0.055 g Feed NaCl·L−1. At a salinity of 20 gNaCl·L−1, after 3 h [CL] = 1.1 gCl−·g−1 Fe0. This implies a NaCl removal of 1.77 gNaCl·g−1 Fe0.
- (1)
- (2)
- (3)
- (4)
- The rate of desalination can be significantly increased by combining ZVM with ion exchange material (e.g., aluminium silicates such as Ca-montmorillonite) [5].
- (5)
1.2.2. ZVI Composition
1.2.3. ZVI Cost
- (i)
- (ii)
- (iii)
1.2.4. Potential Cost of Partial Desalination Using ZVI
1.3. Study Structure
- (a)
- Appendix A: Microbiota, cations, and anions removed by ZVM.
- (b)
- Appendix B: Impact of changing water salinity of arable crop yields and changing feed water salinity on livestock yields;
- (c)
- Appendix C: Trial results: feed and product water cations and anion analyses, gas flow rates, Eh, pH, EC, salinity vs. time, UV-visible spectroscopy analyses of entrained particles.
- (d)
- Appendix D: ZVM compositional and pre-treatment details; Control Reactor Trials;
- (e)
- Appendix E: Interpretation of salinity from electrical conductivity and UV-visible absorbance data.
- (f)
- Appendix F: Fe, Al, Cu corrosion in saline water; Relationship between EC (salinity) and reaction kinetics;
- (g)
- Appendix G: Corrosion species involved in desalination;
- (h)
- Appendix H: Identification of radicals removed during desalination;
1.3.1. ZVM TP and ZVM TPA
- Treat the iron powders (and ZVM combinations) prior to use to form air stable, attrition resistant porous pellets which can partially desalinate water over a 30–250 day trial period. This treated ZVM is termed ZVM TP in this study; TP = treatment product;
- Use a combination of untreated Fe0 and potassium aluminium silicate powder (K-feldspar) to force partial desalination to occur with a 1–24 h period. This combination of untreated Fe0 + K-feldspar is termed ZVM TPA in this study; TPA = treatment product, Type A.
- The ZVM TP are provided in Section 4 and Section 6, Appendix C (Table C1, Table C2, Table C3 and Table C4; Figure C1, Figure C2, Figure C3, Figure C4, Figure C5, Figure C6, Figure C7, Figure C8, Figure C9, Figure C10, Figure C11, Figure C12, Figure C13, Figure C14, Figure C15, Figure C16, Figure C17 and Figure C18), Appendix D (Figure D1, Figure D2, Figure D3, Figure D4, Figure D5 and Figure D6), Appendix F.
- The ZVM TPA are provided in Section 5, Appendix C (Table C5, Table C6, Table C7, Table C8, Table C9, Table C10, Table C11, Table C12 and Table C13; Figure C19, Figure C20, Figure C21, Figure C22, Figure C23, Figure C24, Figure C25, Figure C26, Figure C27, Figure C28, Figure C29, Figure C30, Figure C31, Figure C32, Figure C33, Figure C34, Figure C35, Figure C36 and Figure C37), Appendix H.
1.3.2. Partial Desalination Information Provided in This Study
- Control Trials and Reference Data (Section 2, Table C1, Table C2, Table C3 and Table C4, Figure D1)
- Natural spring water used to construct the synthetic saline water used in the trials;
- Initial Control Data Set: Variation in Eh, pH, EC of untreated ZVM (Fe0, Al0, Fe0 + Al0, Fe0 + Cu0, Fe0 + Al0 + Cu0) in fresh water and saline water;
- Trial Control Data Set: Variation in Eh, pH, EC of treated particulate ZVM TP (P1) in fresh water (P1c) and saline water (P1);
- Saline feed water used in the trials, at the trial onset and trail conclusion;
- ZVM TP (Section 4 and Section 6, Table C1, Table C2, Table C3 and Table C4; Figure C1, Figure C2, Figure C3, Figure C4, Figure C5, Figure C6, Figure C7, Figure C8, Figure C9, Figure C10, Figure C11, Figure C12, Figure C13, Figure C14, Figure C15, Figure C16, Figure C17 and Figure C18, Table D1 and Table D2, Figure D1, Figure D2, Figure D3, Figure D4, Figure D5 and Figure D6)
- Impact of ZVM pre-treatment on desalination rates;
- Variation in EC (salinity) with time;
- Variation in Eh and pH with time;
- Relationship between desalination and ZVM TP loading (g·L−1);
- Relationship between desalination, particle size and capacitance;
- Economics of partial desalination using ZVM TP.
- ZVM TPA (Section 5, Table C5, Table C6, Table C7, Table C8, Table C9, Table C10, Table C11, Table C12 and Table C13, Figure C19, Figure C20, Figure C21, Figure C22, Figure C23, Figure C24, Figure C25, Figure C26, Figure C27, Figure C28, Figure C29, Figure C30, Figure C31, Figure C32, Figure C33, Figure C34, Figure C35, Figure C36 and Figure C37, Table D3)
- Relationship between desalination rate, ZVM TPA reuse, and time;
- Economics of partial desalination.
- Mechanism of desalination (Section 4 and Section 5, Appendix F, Appendix G, Appendix H)
- Removal of NaCl by incorporation into Fe corrosion products;
- Removal of NaCl by inclusion in a hydration shell;
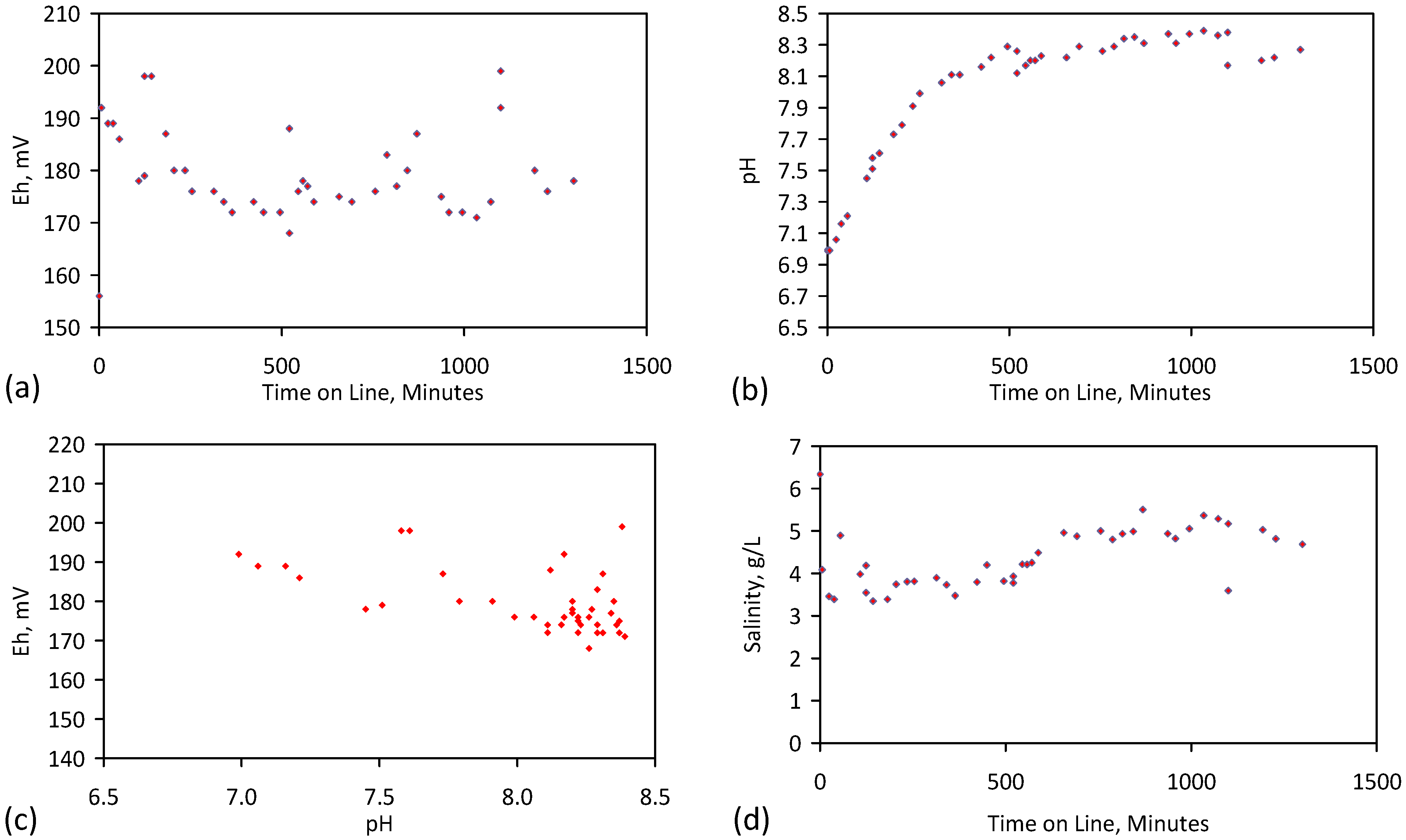
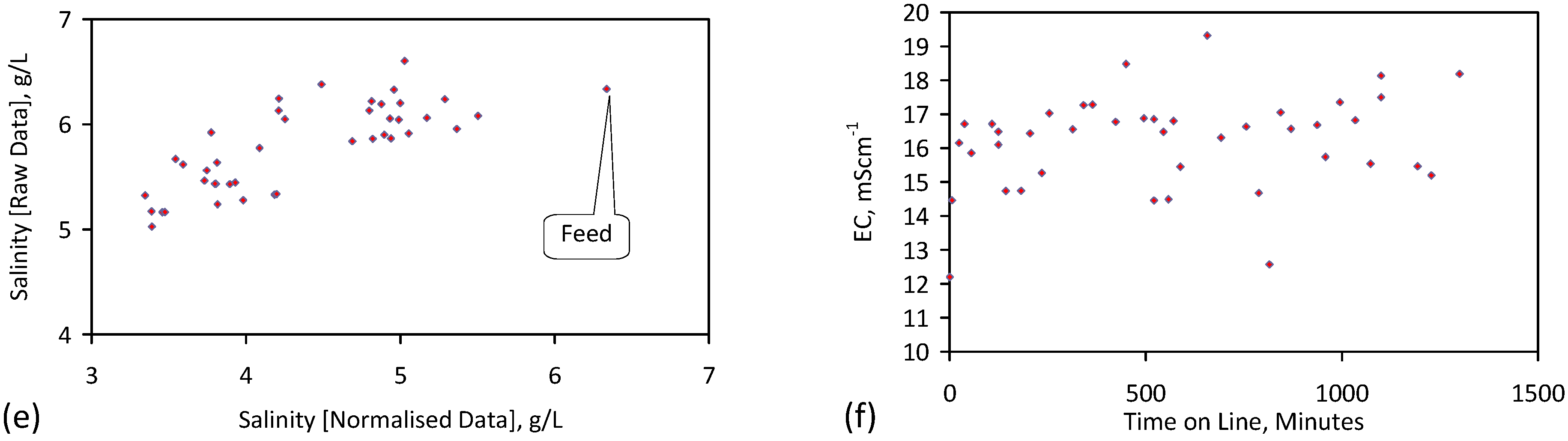
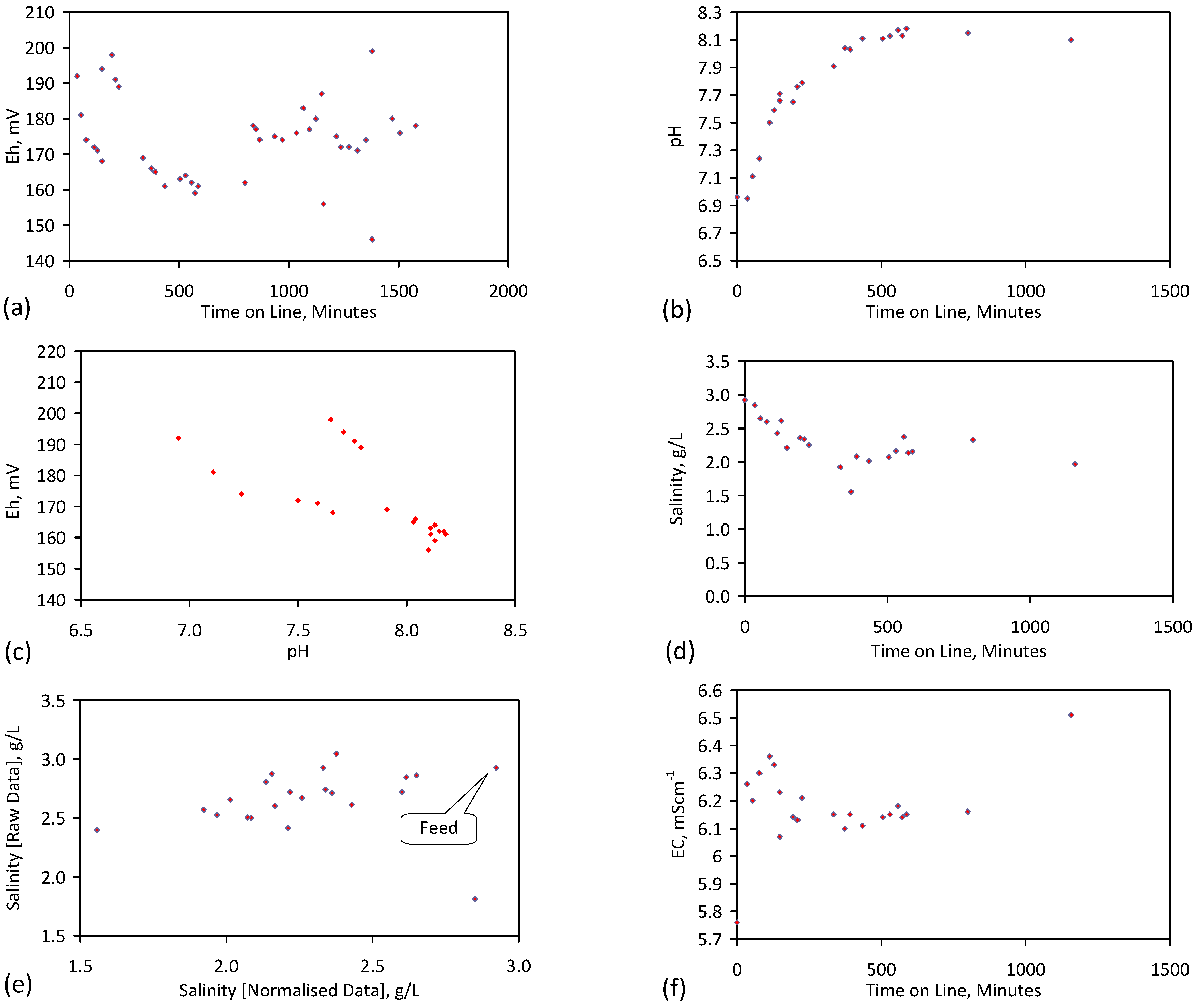
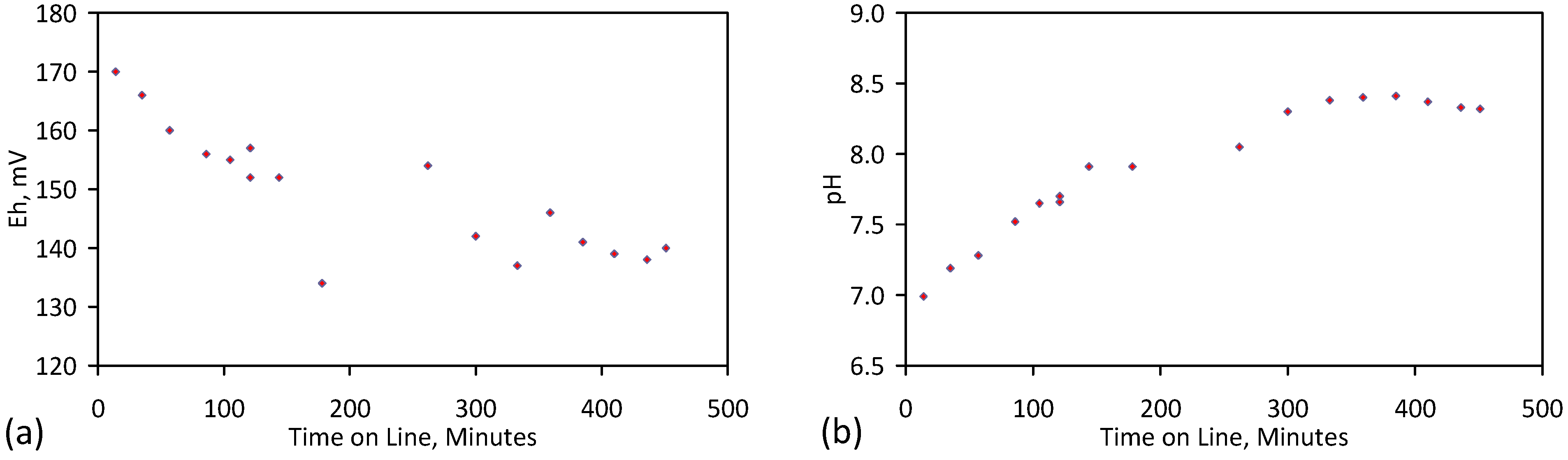
2. Materials, Methods and Equipment
2.1. Feed Water
2.2. Equipment
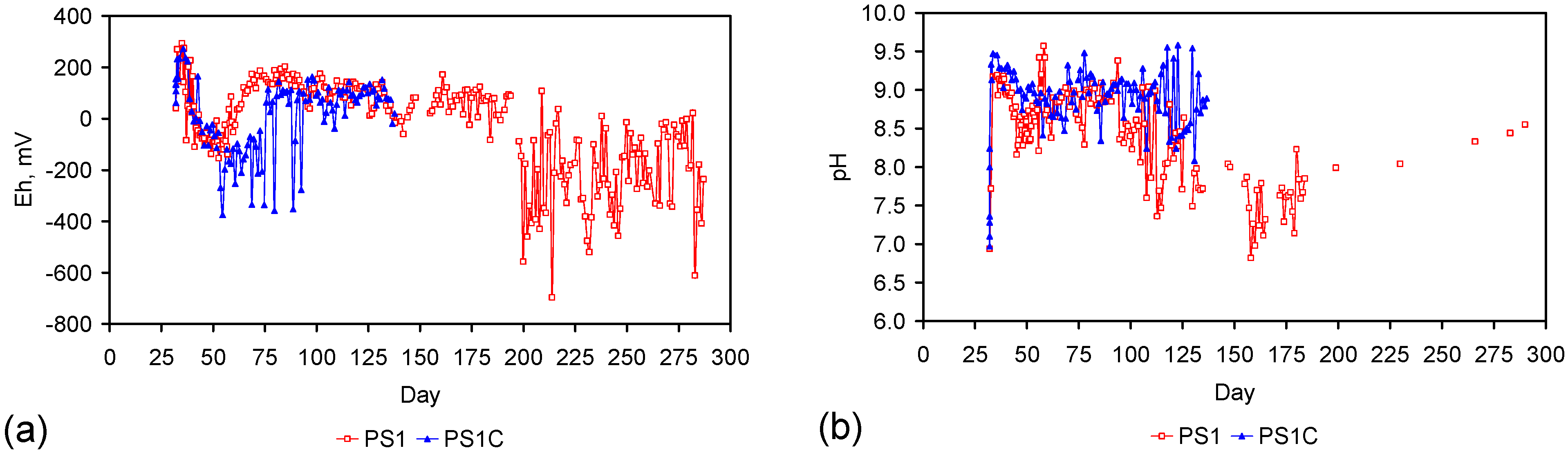
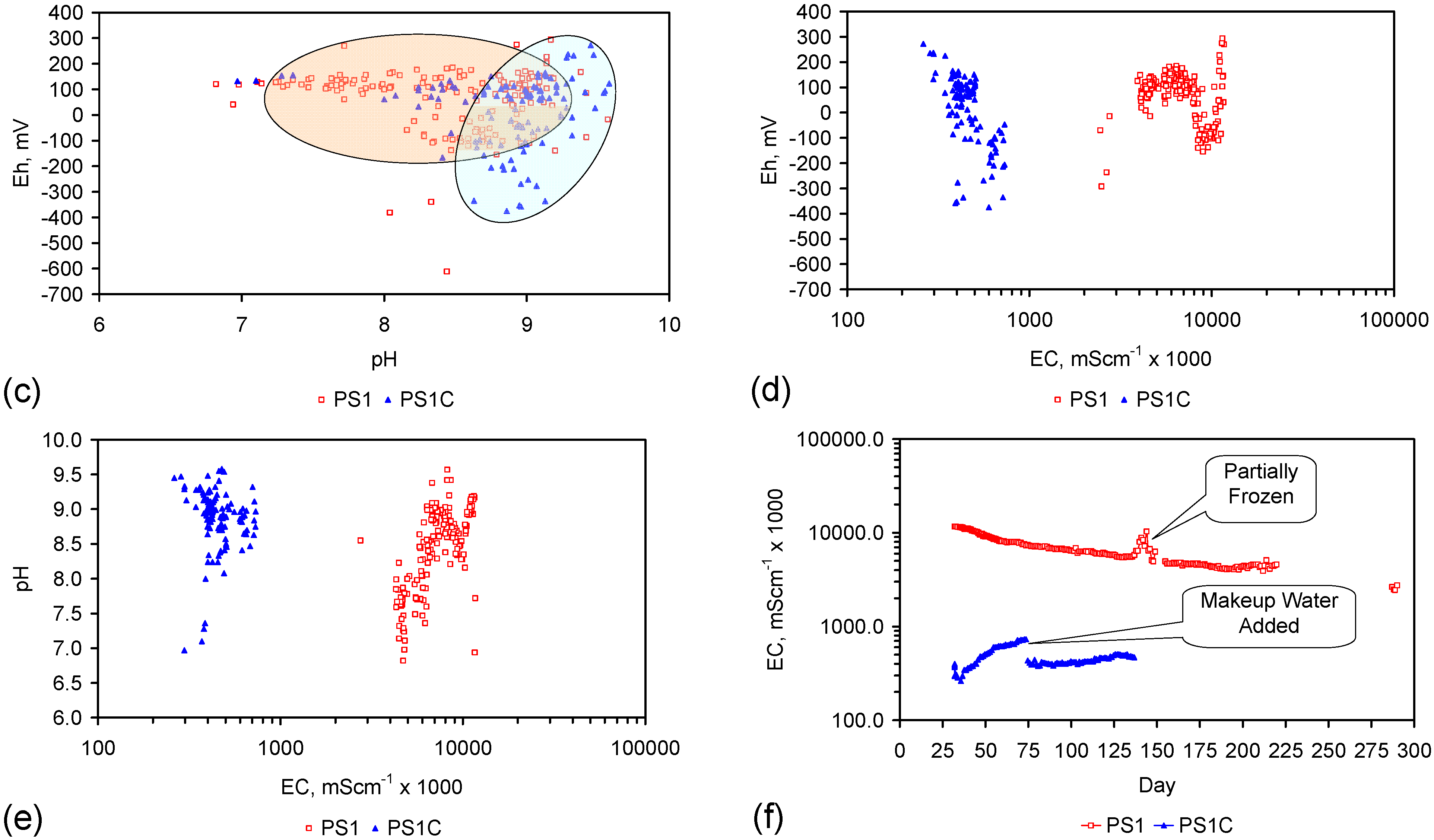
2.3. Control and Reference Trials
2.3.1. Reference Trials: Feedwater Analysed at the Start and End of Each Trial
2.3.2. Initial Control Trials Using Untreated ZVM
2.3.3. Control Trial Using ZVM TP
2.4. ZVM TP Trials
2.4.1. Temperatures and Pressures Used in the ZVM TP Trials and ZVM TP Control Trial
2.4.2. Manufacture of ZVM TP
- Type A: [16.79% CH4 + 16.88% H2 + 11.97% CO + 8.33% CO2 + 46.03% N2], Trials: AS1–AS3, PS5; Manufacturing time = 17 days; Manufacturing time for Trial PS15 = 35 Days;
- Type B: [16.79% CH4 + 16.88% H2 + 11.97% CO + 8.33% CO2 + 46.03% N2 for 110 days] followed by [80% N2 + 20% CO2 for 135 days] followed by [100% N2 for 46 days]; Trials: ST1–ST7, AS4–AS6, PS1–PS4, PS1C; Manufacturing time = 291 days; Manufacturing time for Trial PS16 = 65 Days;
- Type C: [N2] Trials: PS8–PS10, ST8, MT1, MT2; Manufacturing time = 34 days;
- Type D: [air] Trials: PS11, PS12, PS13, MT3, MT4; Manufacturing time = 42 days.
2.4.3. ZVM Composition Used to Manufacture ZVM TP (Table D1 and Table D2)
- (i)
- Copper sheathed pellets (Figure 2),
- (ii)
- MDPE sheathed pellets,
- (iii)
- A cartridge.

2.4.4. Reactors: ZVM TP Tests at Ambient Temperatures
2.4.5. Reactors: ZVM TP Tests Using Pressured Reactors where a Gas Is Used to Maintain the Pressure
2.5. ZVM TPA Trials
2.5.1. Cartridge and Particle Manufacture: ZVM TPA (Table D3)
2.5.2. Reactors: Used for Testing ZVM TPA
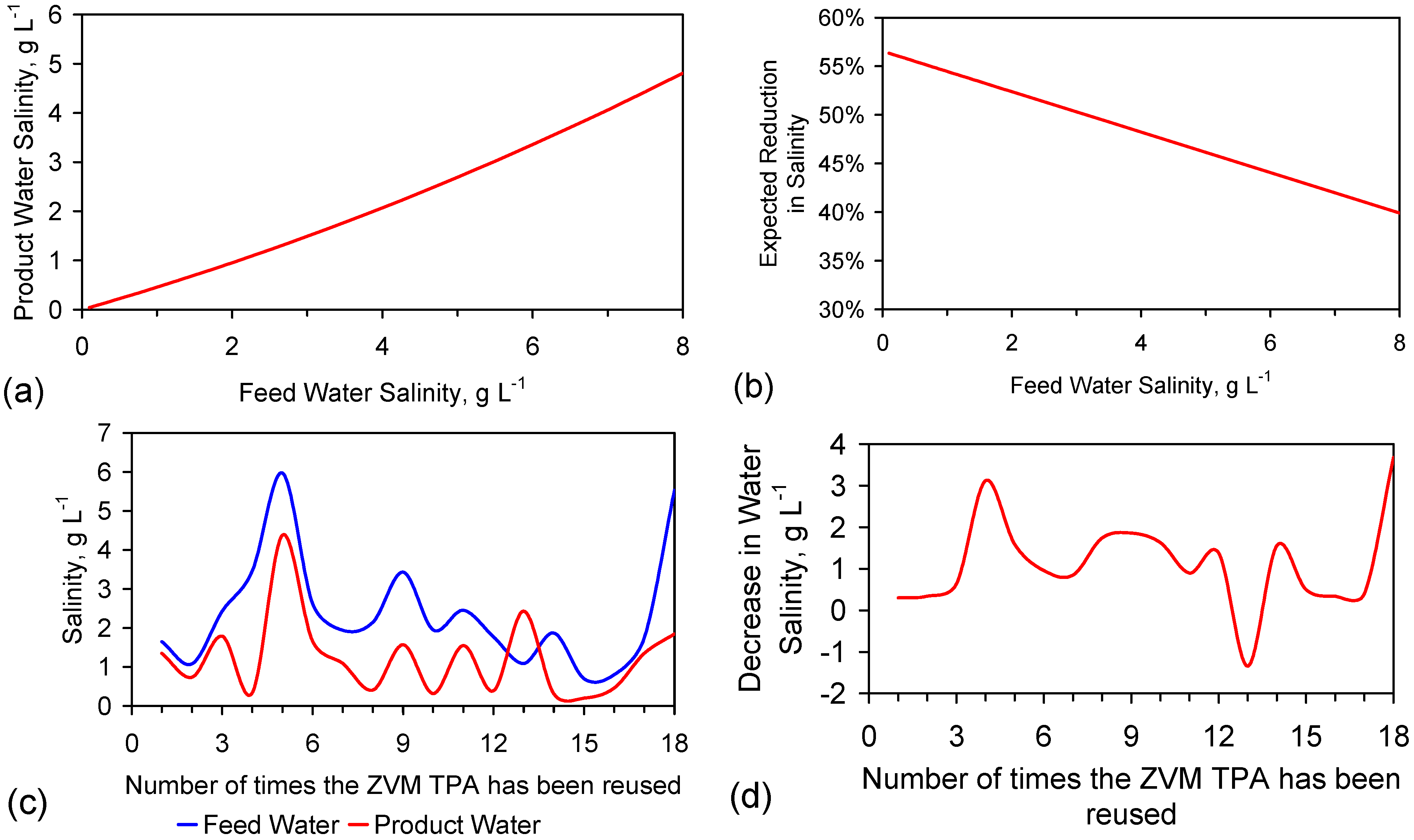
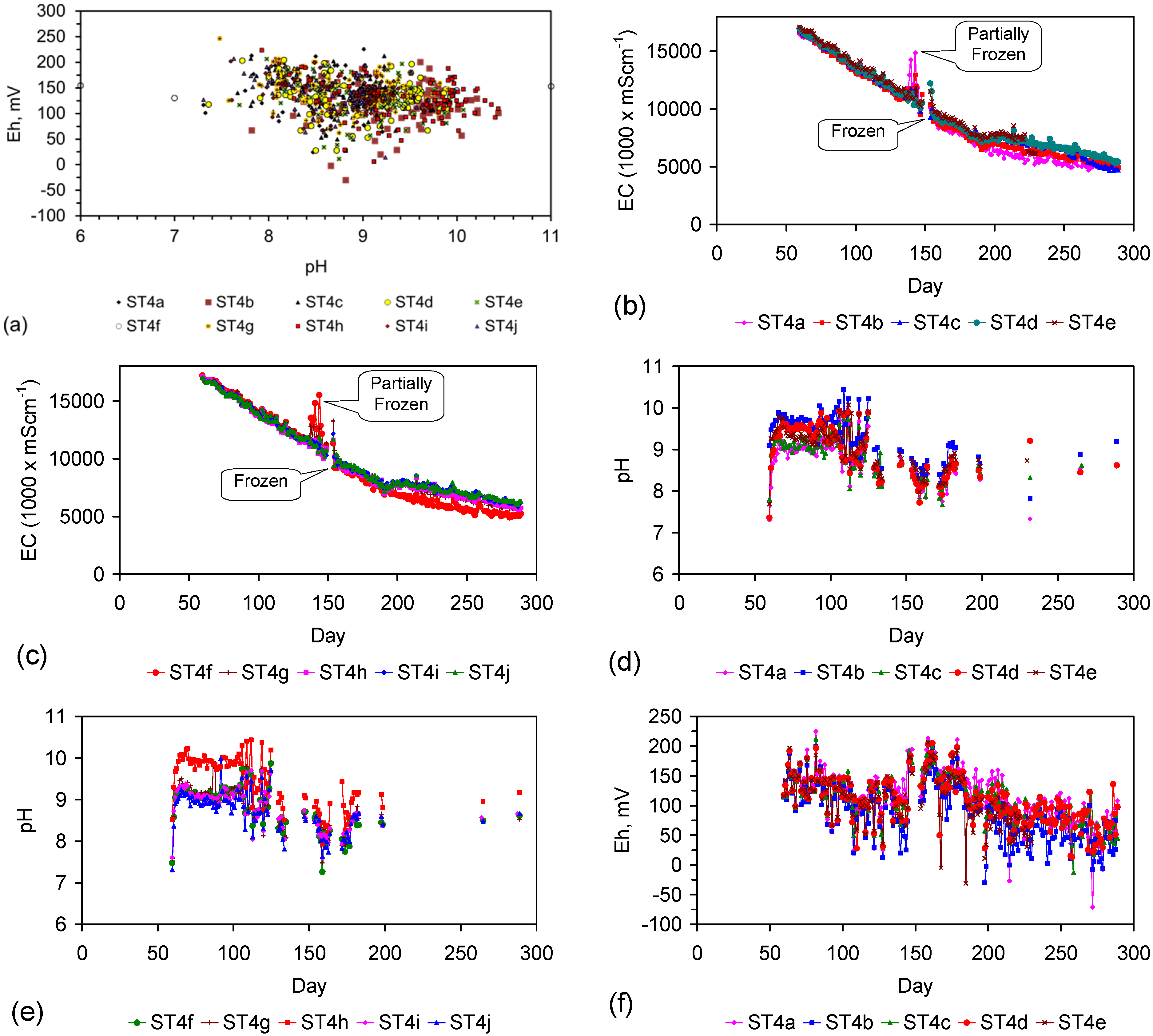
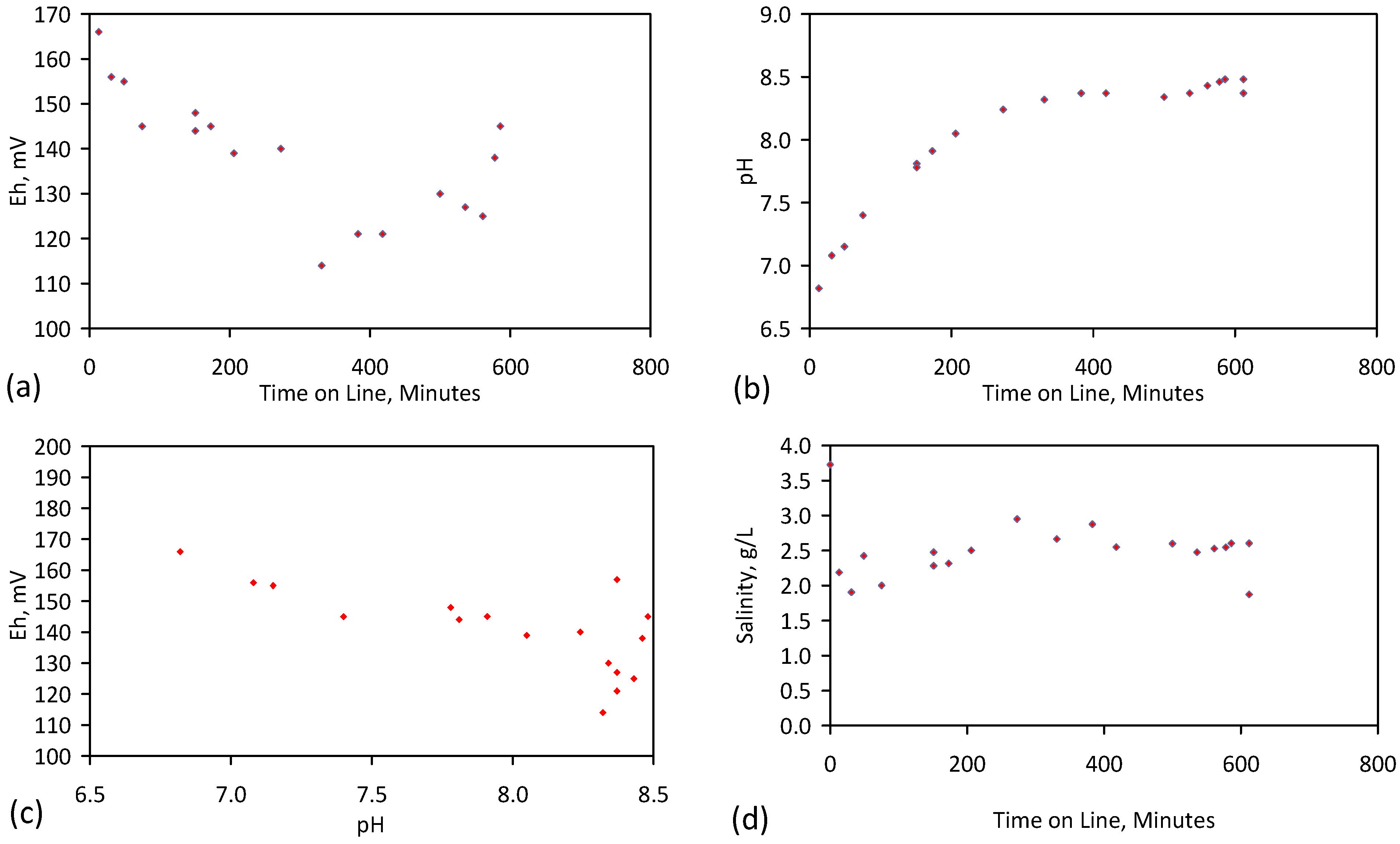
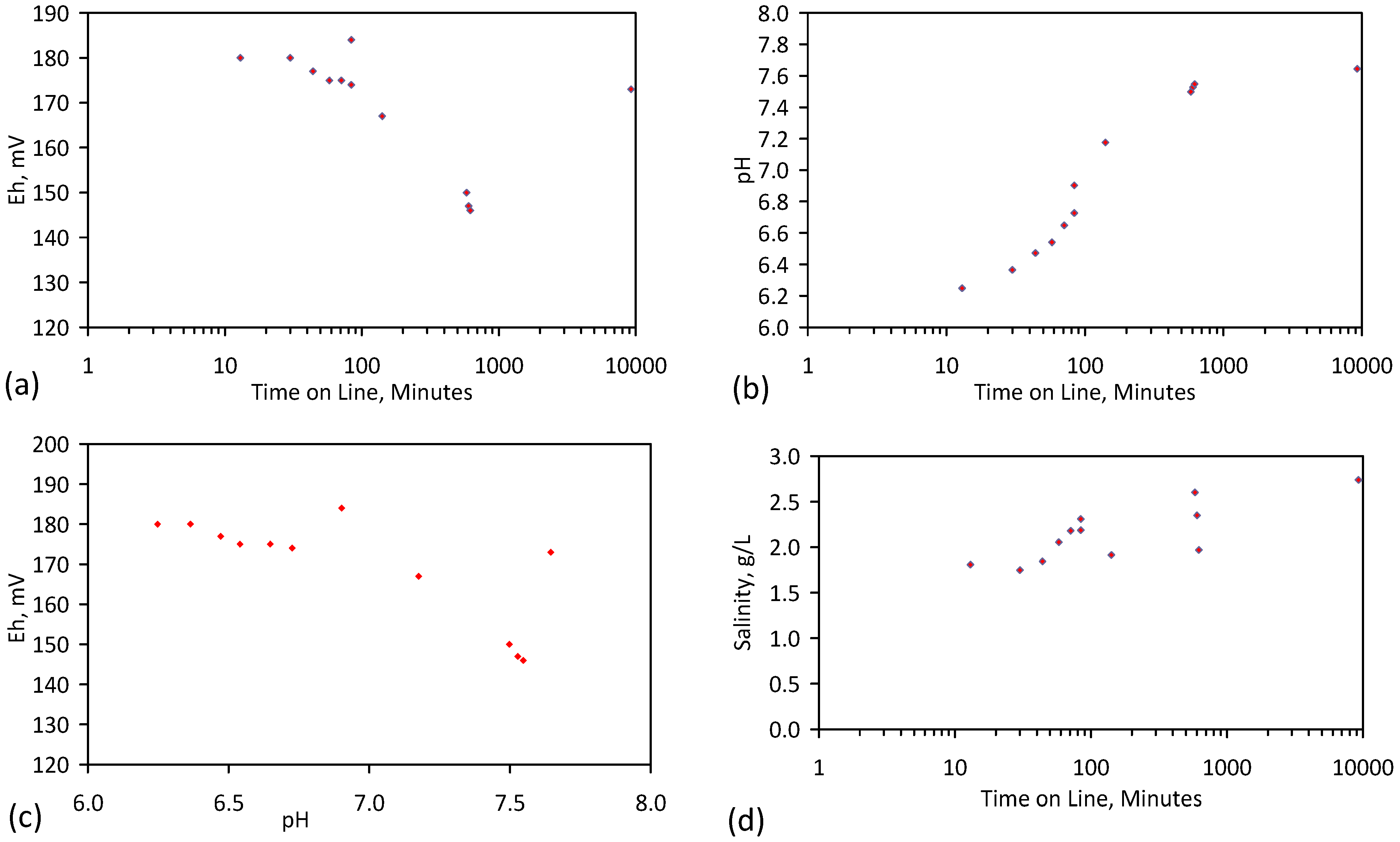
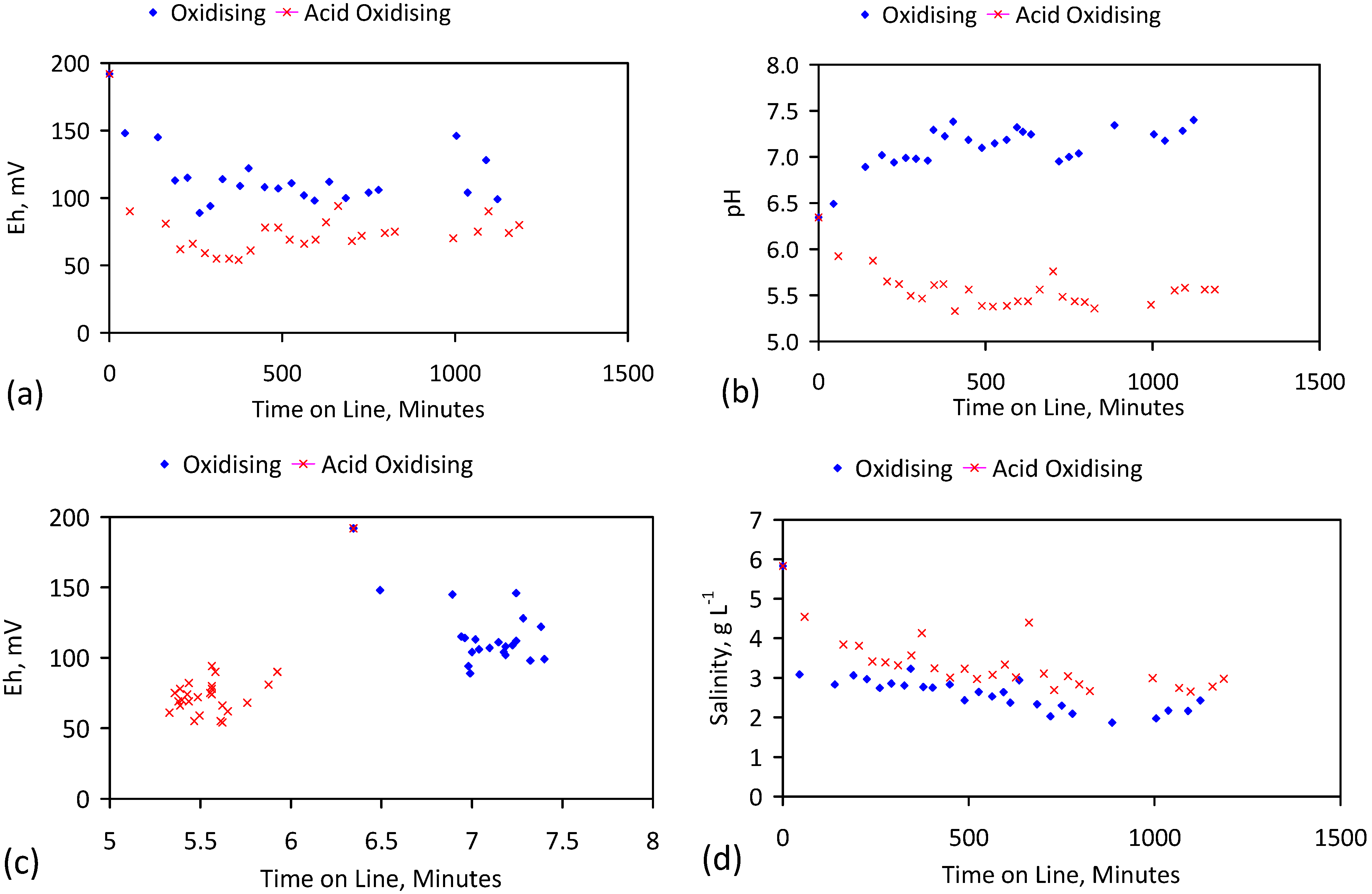
3. Interpretation of Salinity
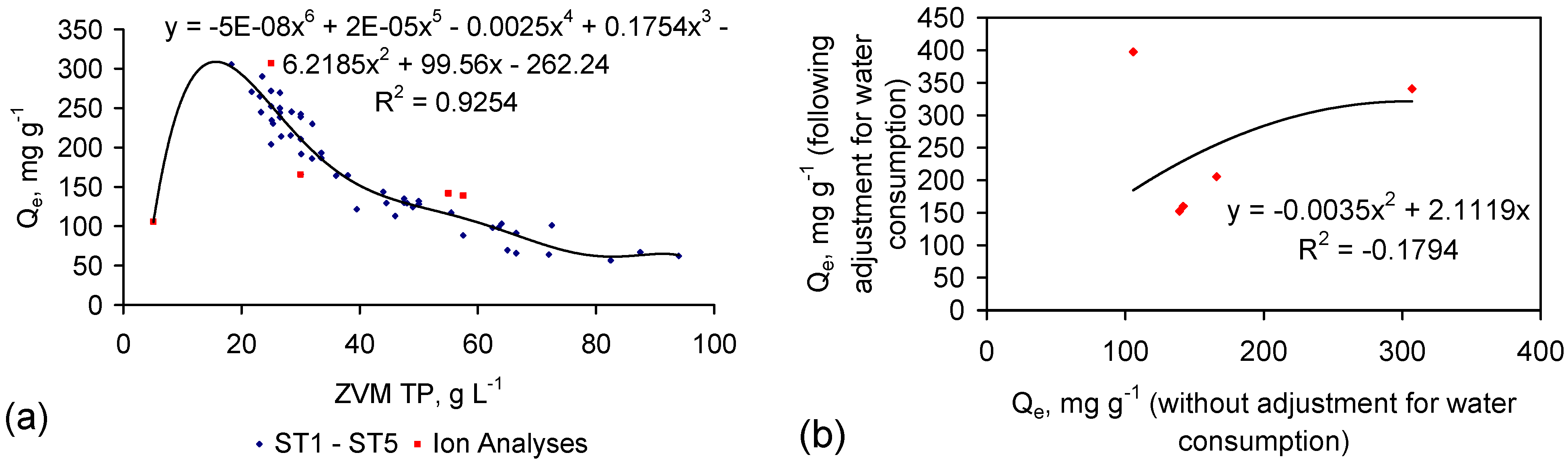
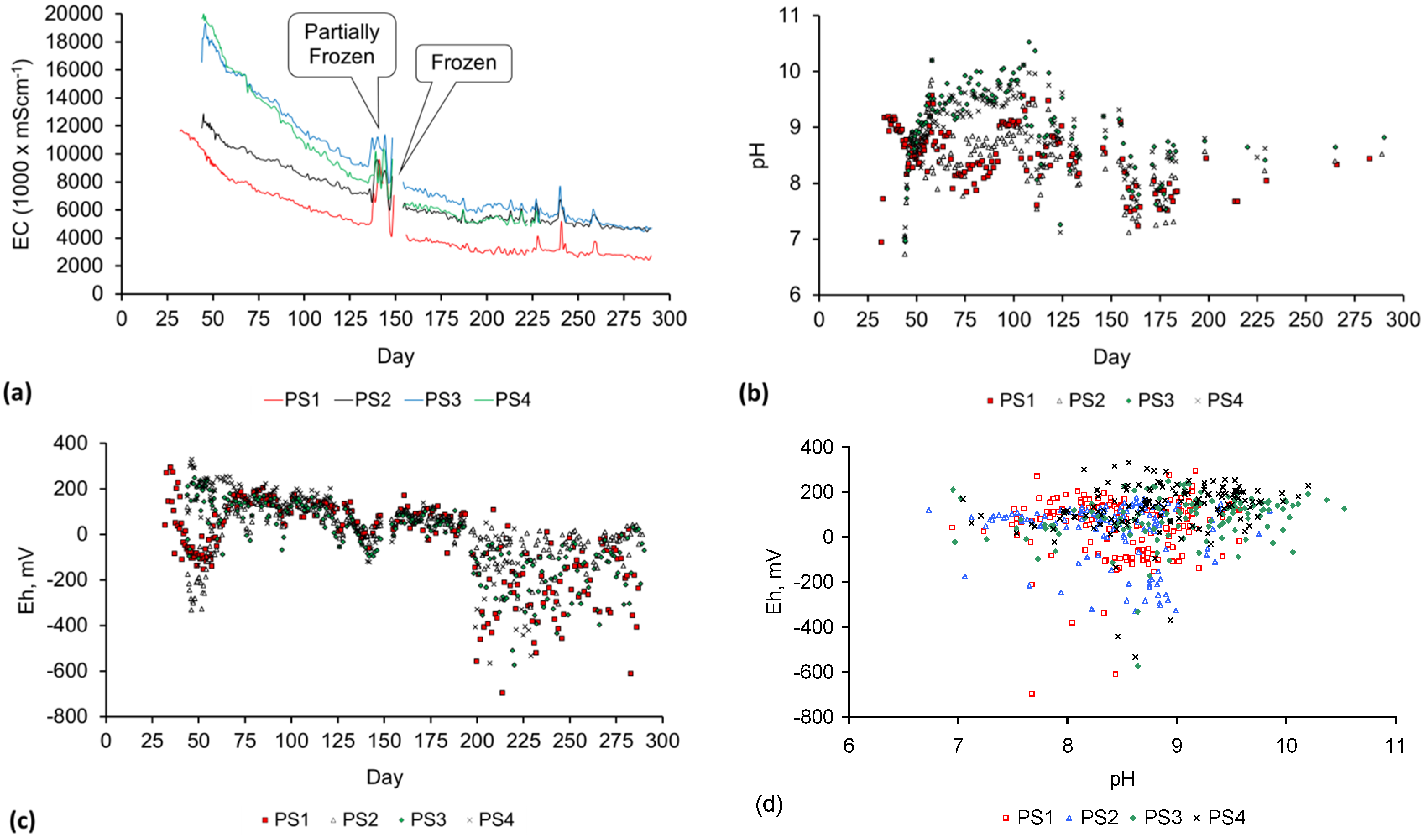
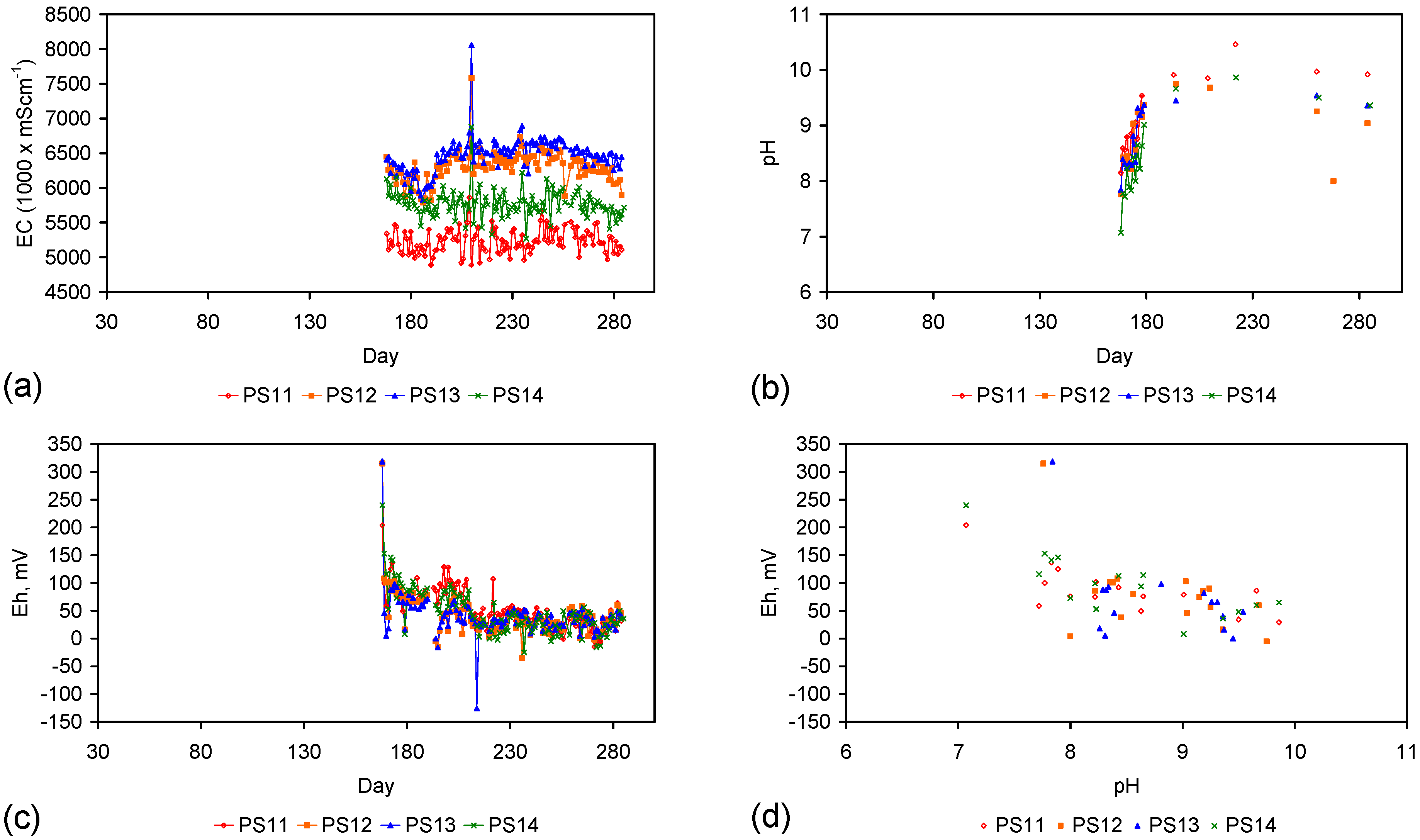
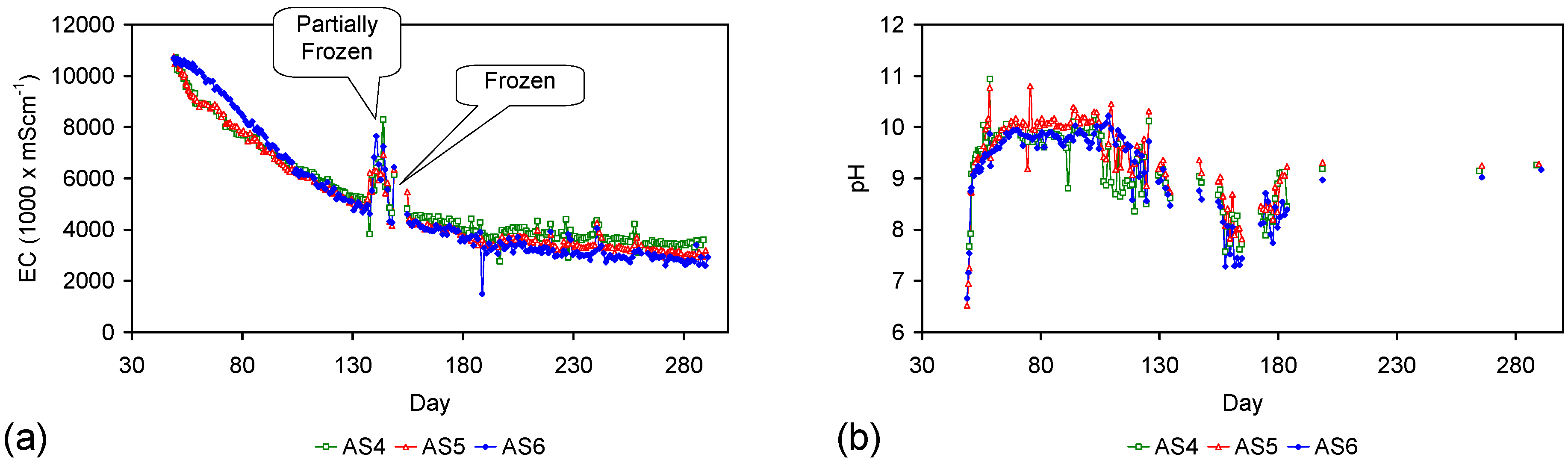
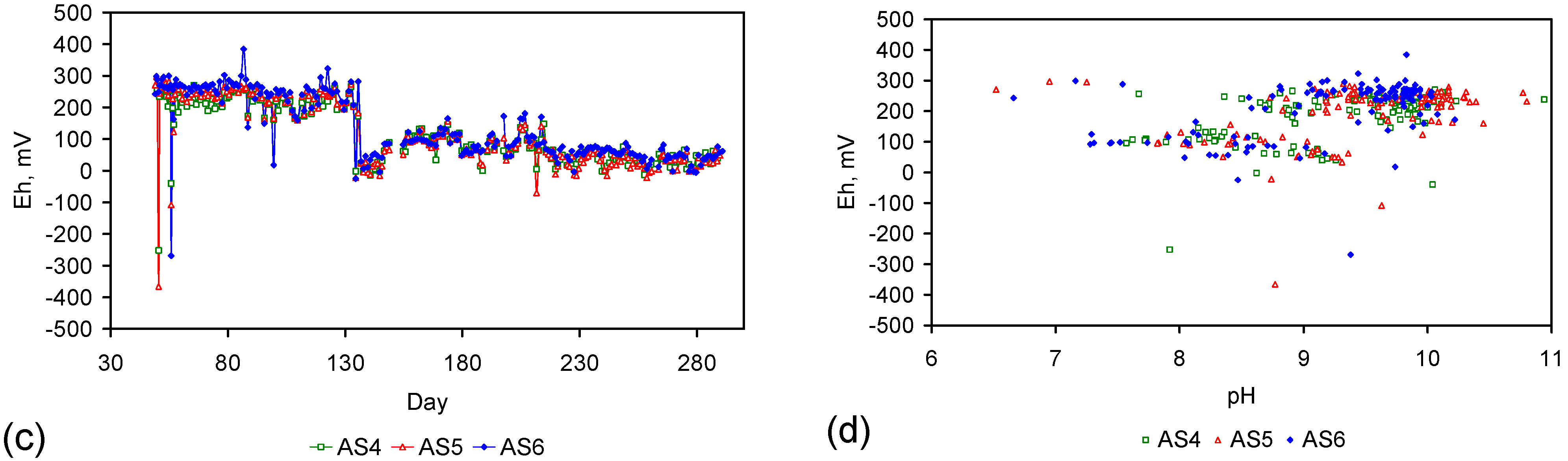
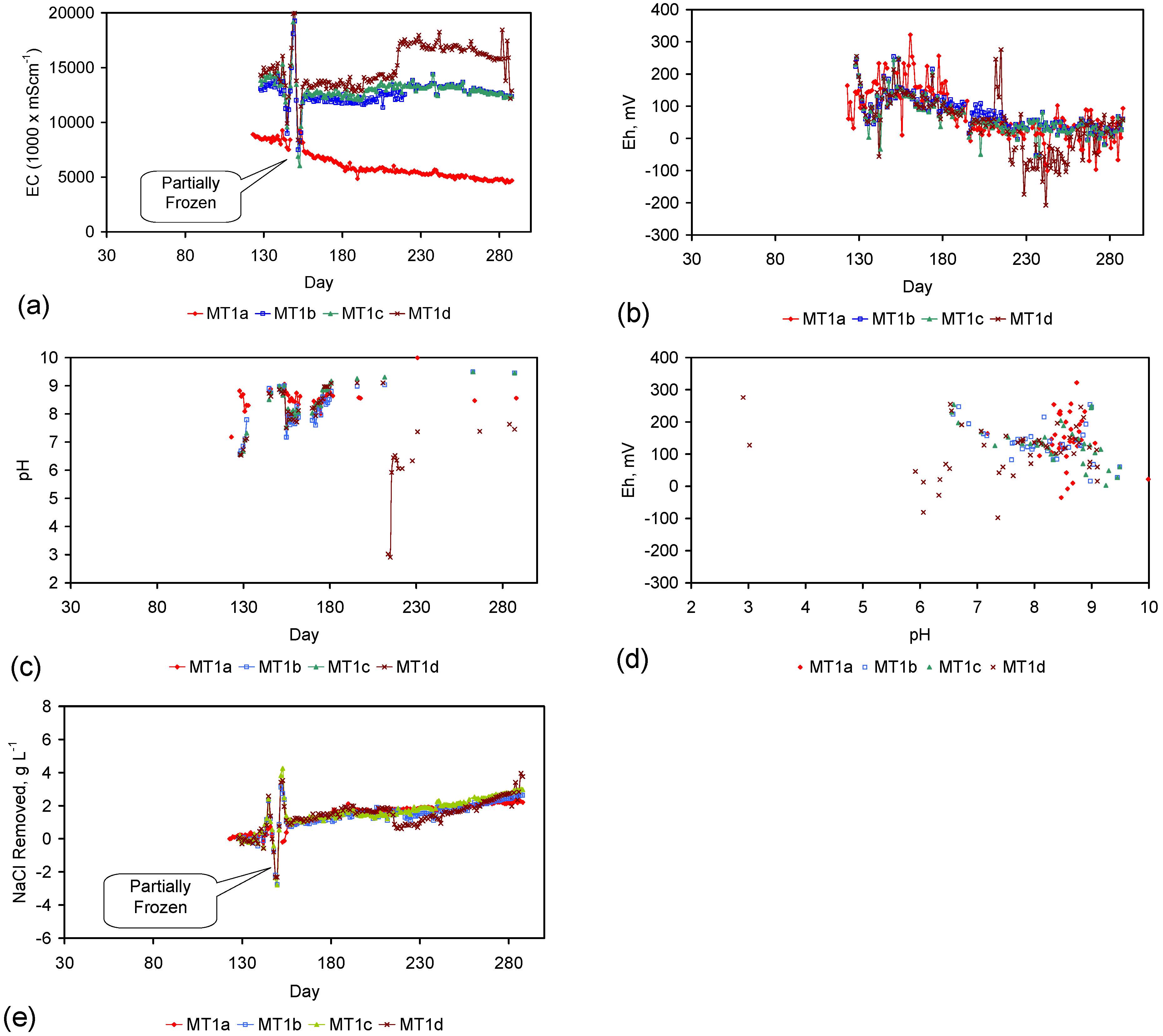
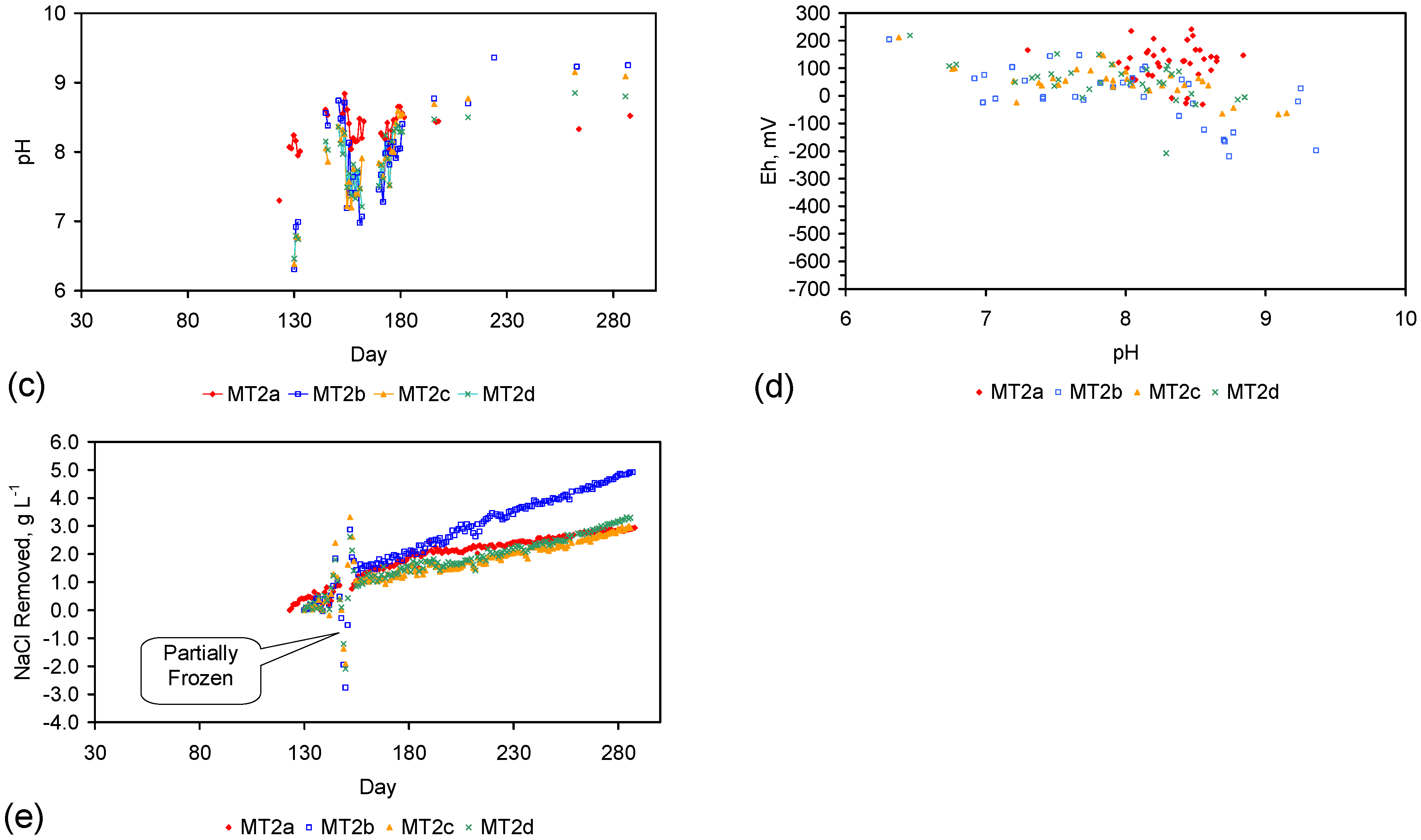
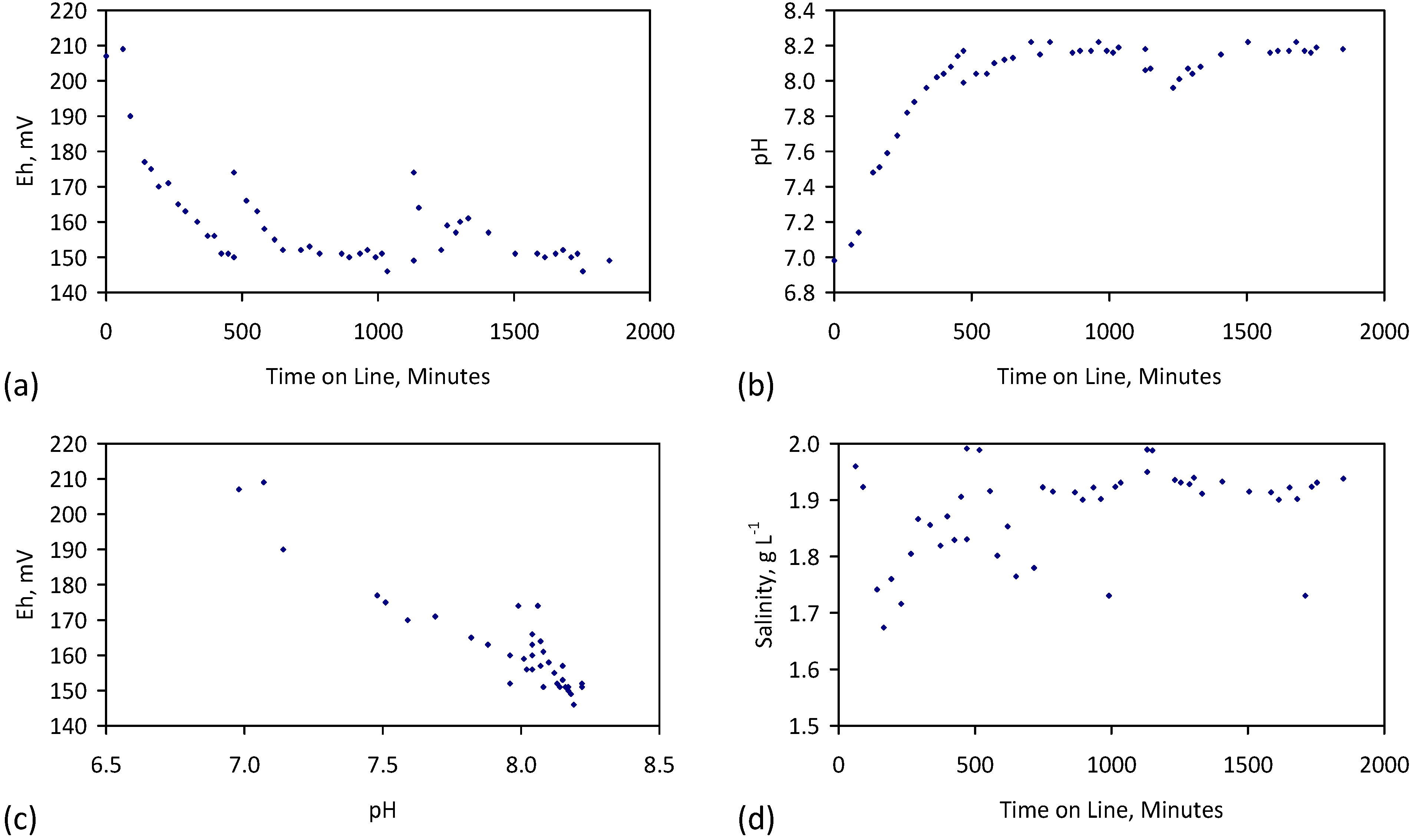
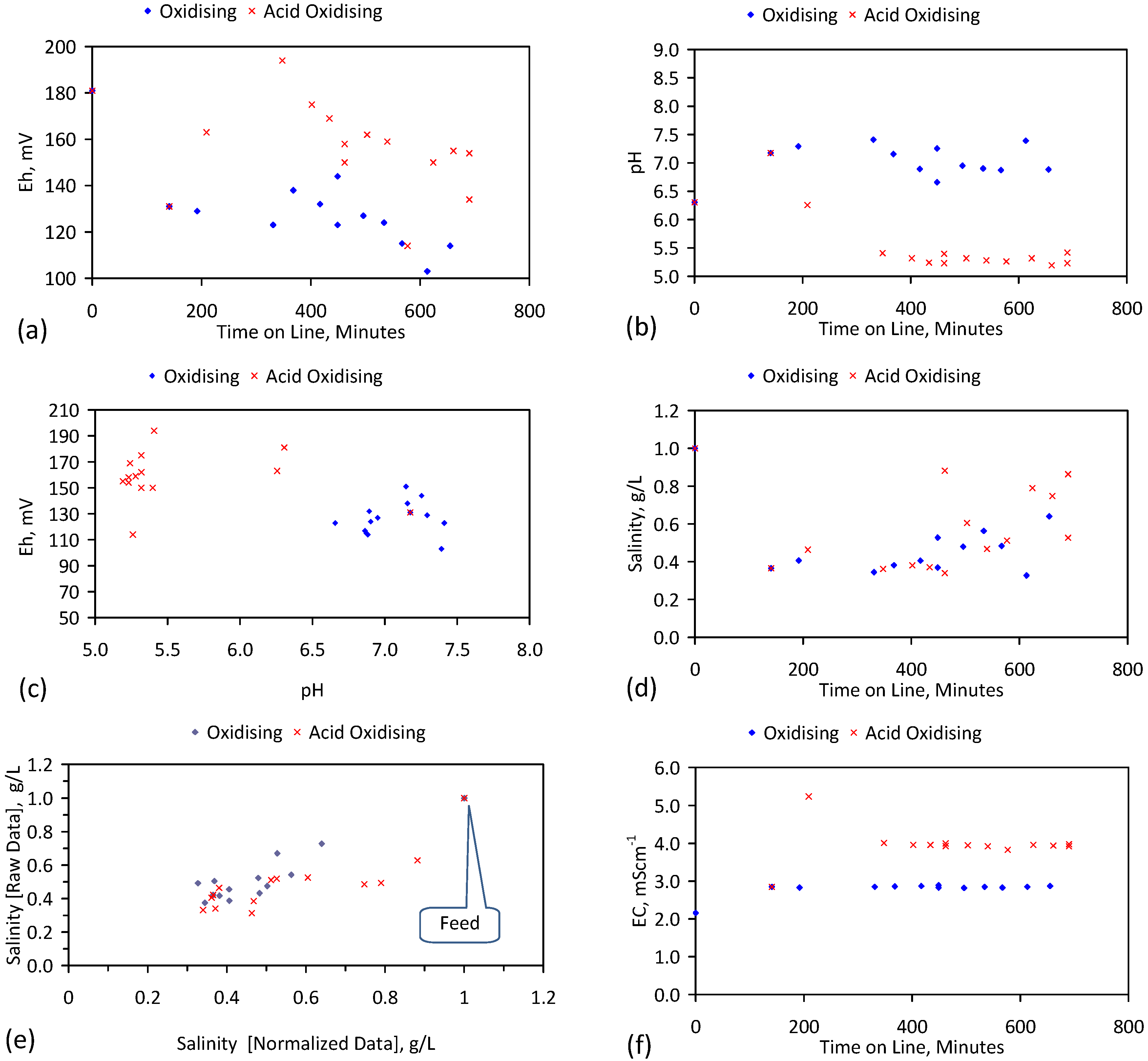
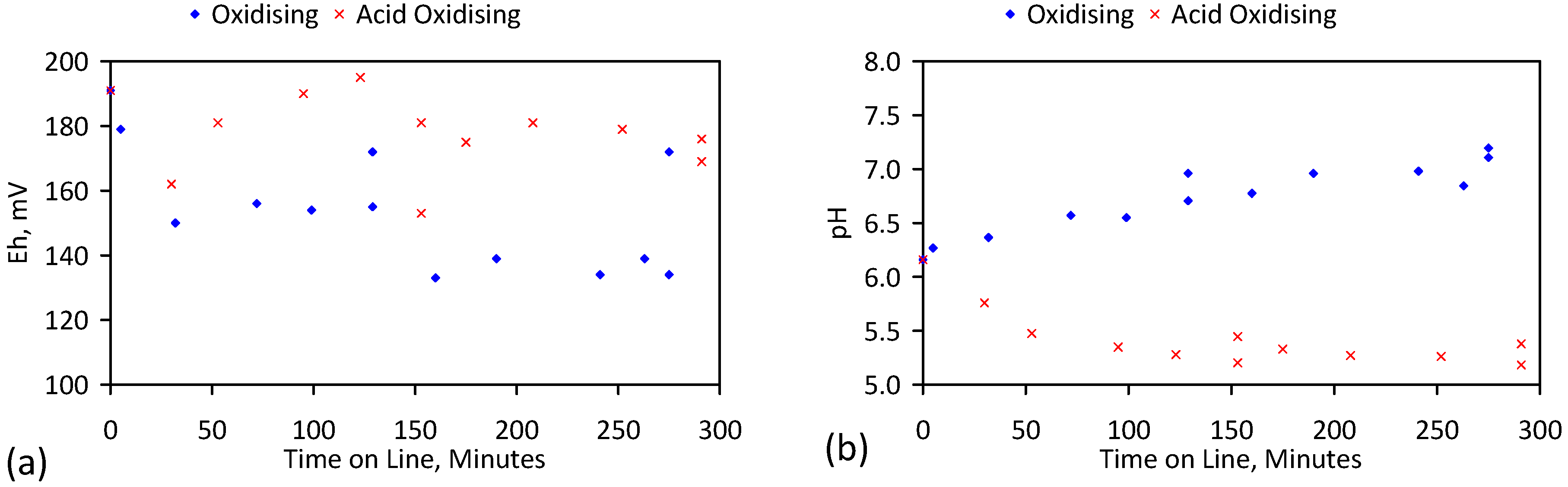
4. Results: ZVM TP
- (1)
- The weight of NaCl removed/unit weight ZVM TP (Qe);
- (2)
- The ZVM TP loading in the water body (weight of ZVM TP/unit volume of water, Pw);
- (3)
- The time, t, taken to achieve the required level of desalination;
- (4)
- The amount of water consumed during desalination;
- (5)
- The number of times the ZVM TP can be reused;
- (6)
- The residual value of the ZVM TP;
- (7)
- The cost of the ZVM TP.
| Trial | Feed Water | Feed Water Na + K + Cl | Product Water | Product Water Na + K + Cl | Number of Days | Reduction in Water Volume | Salinity Reduction | EC Reduction | Qe | Gross Salinity Reduction | Gross Qe | ZVM TP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC mS·cm−1 | mg·L−1 | mS·cm−1 | mg·L−1 | % | mg·L−1 | mS·cm−1 | mg g−1 | mg·L−1 | mg g−1 | g·L−1 | ||
| MT1b | 14.65 | 7,823.30 | 12.53 | 6,614.80 | 100 | 17% | 1,208.50 | 2.12 | 28.1 | 2,359.48 | 54.87 | 43 |
| MT2b | 12.66 | 6,475.88 | 10.93 | 5,922.57 | 98 | 21% | 553.31 | 1.73 | 4.56 | 1,785.20 | 14.72 | 121.3 |
| MT3d | 7.23 | 3,465.60 | 6.46 | 3,308.2 | 98 | 17% | 157.40 | 0.77 | 2.5 | 733.03 | 11.65 | 62.9 |
| MT4d | 7.84 | 3988.07 | 6.57 | 3,149.62 | 57 | 9% | 838.45 | 1.27 | 9.31 | 1,112.47 | 12.35 | 90.1 |
| ST3b | 18.84 | 11,049.49 | 6.73 | 3,374.48 | 126 | 25% | 7,675.01 | 12.11 | 307 | 8,518.63 | 340.75 | 25 |
| ST3f | 18.84 | 11,049.49 | 6.66 | 3,052.11 | 126 | 25% | 7,997.38 | 12.18 | 139.08 | 8,760.41 | 152.35 | 57.5 |
| ST6d | 16.59 | 9,089.82 | 15.74 | 8,550.01 | 120 | 17% | 539.81 | 0.85 | 105.85 | 2,027.51 | 397.55 | 5.1 |
| ST8e | 12.35 | 6,380.24 | 10.95 | 5,814.14 | 98 | 6% | 566.10 | 1.4 | 15.51 | 903.32 | 24.75 | 36.5 |
| PS4 | 19.71 | 10,001.69 | 5.05 | 2,195.88 | 150 | 45% | 7,805.81 | 14.66 | 141.92 | 8,793.96 | 159.89 | 55 |
| PS5 | 15.18 | 7,609.68 | 5.32 | 2,633.33 | 150 | 45% | 4,976.35 | 9.86 | 165.88 | 6,161.35 | 205.38 | 30 |
| PS7 | 14.05 | 7,391.68 | 4.7 | 1,941.37 | 198 | 45% | 5450.30 | 9.35 | 2180.12 | 6,323.93 | 2529.57 | 2.5 |
| PS11 | 7.84 | 3,988.07 | 5.43 | 2,664.32 | 57 | 7% | 1,323.75 | 2.41 | 49.95 | 1,515.58 | 57.19 | 26.5 |
| PS14 | 6.41 | 3,162.04 | 5.66 | 3,024.48 | 57 | 22% | 137.56 | 0.75 | 13.49 | 793.87 | 77.83 | 10.2 |
| PS15 | 57.49 | 23,707.28 | 31.12 | 17,284.81 | 79 | 33% | 6,422.47 | 26.37 | 47.93 | 12,212.88 | 91.14 | 134 |
| PS16 | 78.18 | 38,577.1 | 1.09 | 576.48 | 210 | 74% | 38,000.62 | 77.09 | 182.7 | 38,424.33 | 184.73 | 208 |
- (1)
- Incorporation into a Fe corrosion product. Green Rust One (Cl−), GR1, incorporates Cl− ions in its crystal structure. β-FeOOH requires the presence of Cl− ions to form. All other Fe corrosion products do not incorporate Cl− ions within their crystal structure. In this instance Qe < 70 mg NaCl removed g−1 ZVM [67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100].
- (2)
- Incorporation into the hydration shell of a Fe corrosion product (e.g., green rust, FeOOH) [96]. A hydrated amorphous FeOOH corrosion product containing 46 wt % H2O will contain 5 hydration shells. Radicals containing Na+ and Cl− can substitute for H2O in the hydration lattice [96]. This may potentially allow Qe to exceed 3 gNaCl removed g−1 ZVM [96].
- (3)
- Concentration of NaCl in the pore waters within the ZVM TP/ZVM TPA corrosion product bed, when the ZVM interacts with the overlying water body by diffusion. This may potentially allow Qe to exceed 20 gNaCl·removed·g−1 ZVM. This method of NaCl removal is investigated in the ZVM TP trials CSD1 and ZVM TPA trials E143, and E144 (Section 5).
4.1. Pre-Treatment
Example Calculation of the Relative Efficiency of a Pre-Treatment: ST1a, MT2b
- The size of the desalination tanks (water bodies) required to be reduced by 80% (e.g., from 1000 to 200 m3);
- The land take required for the desalination tanks to be reduced from 100–500 m2 to 50–150 m2.
- The expected value of [at+1] if the ZVM is used at a specific Pw, without pre-treatment;
- The expected value of [at+1] following pre-treatment without considering water losses;
- The expected value of [at+1] following pre-treatment after consideration of water losses.
| Trial | Expected without Pre-Treatment | With Pre-Treatment | Adjusted for Water Consumption | Improvement Due to Pre-Treatment | Improvement Due to Pre-Treatment |
|---|---|---|---|---|---|
| Observed | Observed | Adjusted for Water Consumption | |||
| ST1a | 0.00020 | 0.12000 | 0.15000 | 60,000% | 75,000% |
| MT2b | 0.00134 | 0.02000 | 0.10000 | 1498% | 7491% |
- The surface area, as, and Pw of the ZVI used in the reference desalination data set using untreated ZVI (Section 1.2.1 and Section 2.3.2 [21]);
- The as and Pw of the ZVI used in the control desalination data using untreated ZVI (Section 2.3.2 [6]);
- Qe and Pw for the individual trial (Table 1, Figure C1, Figure C2, Figure C3, Figure C4, Figure C5, Figure C6, Figure C7, Figure C8, Figure C9, Figure C10, Figure C11, Figure C12, Figure C13, Figure C14, Figure C15 and Figure C16).

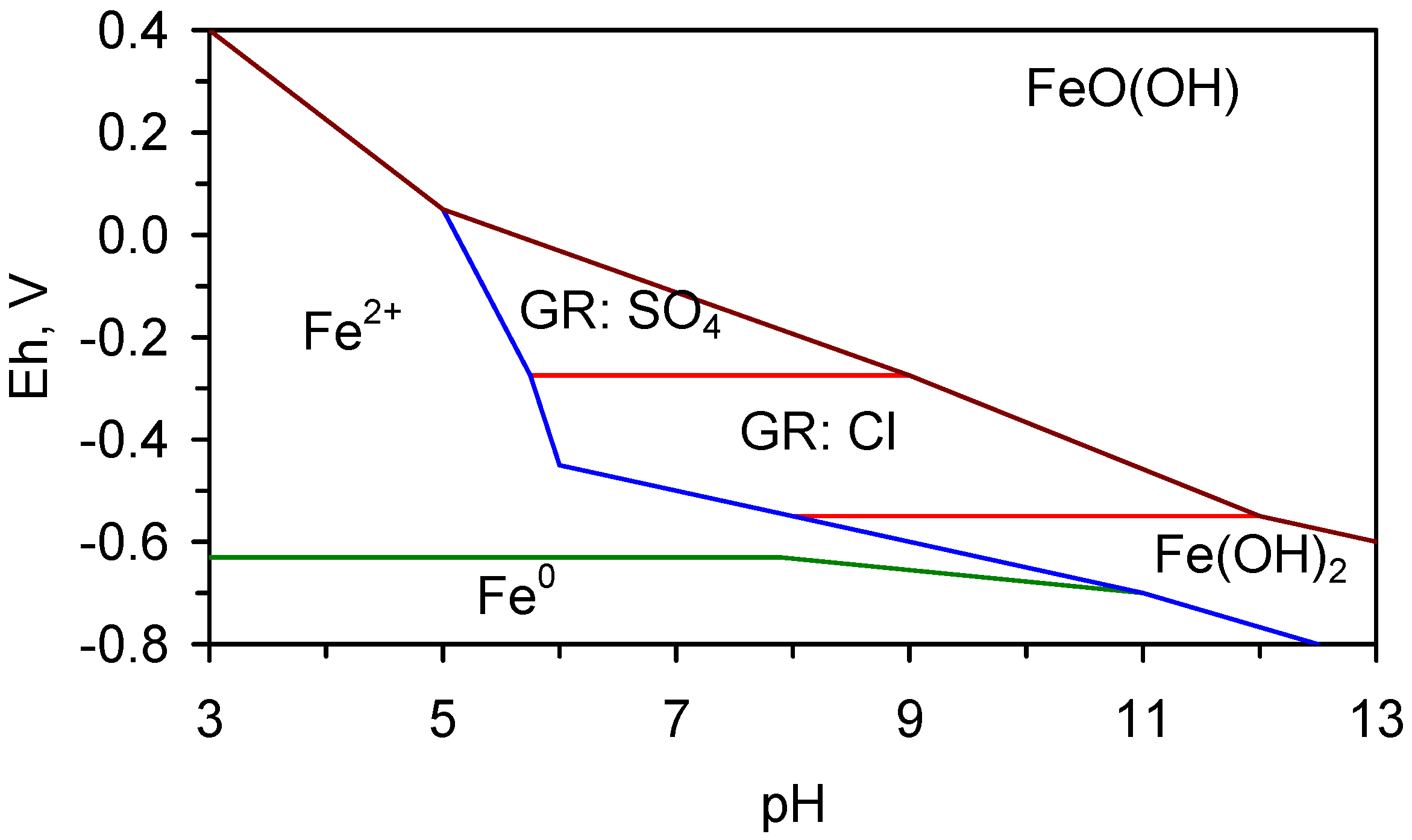
4.2. Iron Corrosion in Saline Water
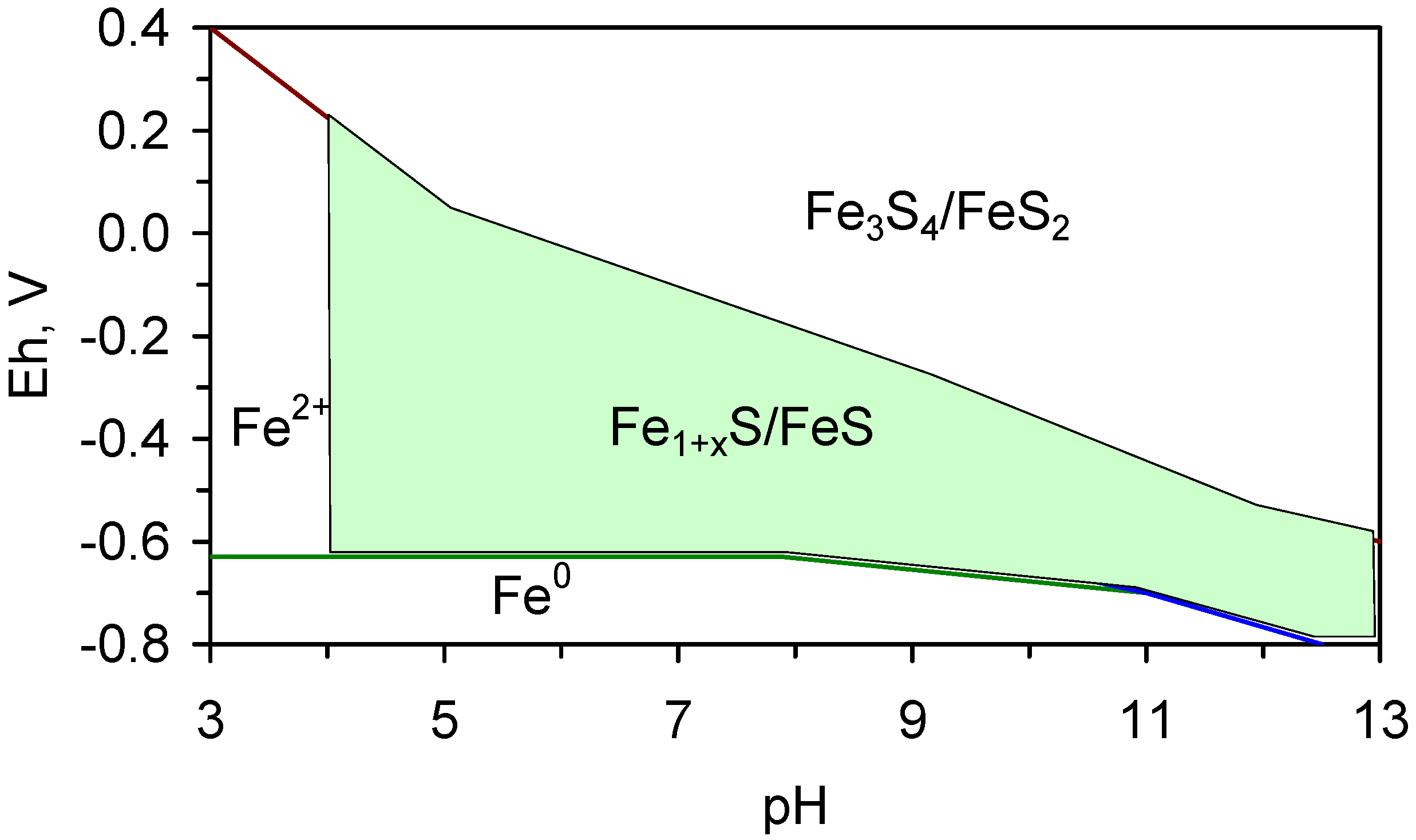
4.2.1. Pourbaix Relationships
4.2.2. Fe0 Corrosion Series
- transforms directly to β-FeOOH (akaganeite), or,
- adopts the reaction series, GR1 → α-FeOOH (goethite) → β-FeOOH, or,
- adopts the reaction series GR1 → γ-FeOOH (lepidocrocite) → α-FeOOH → β-FeOOH
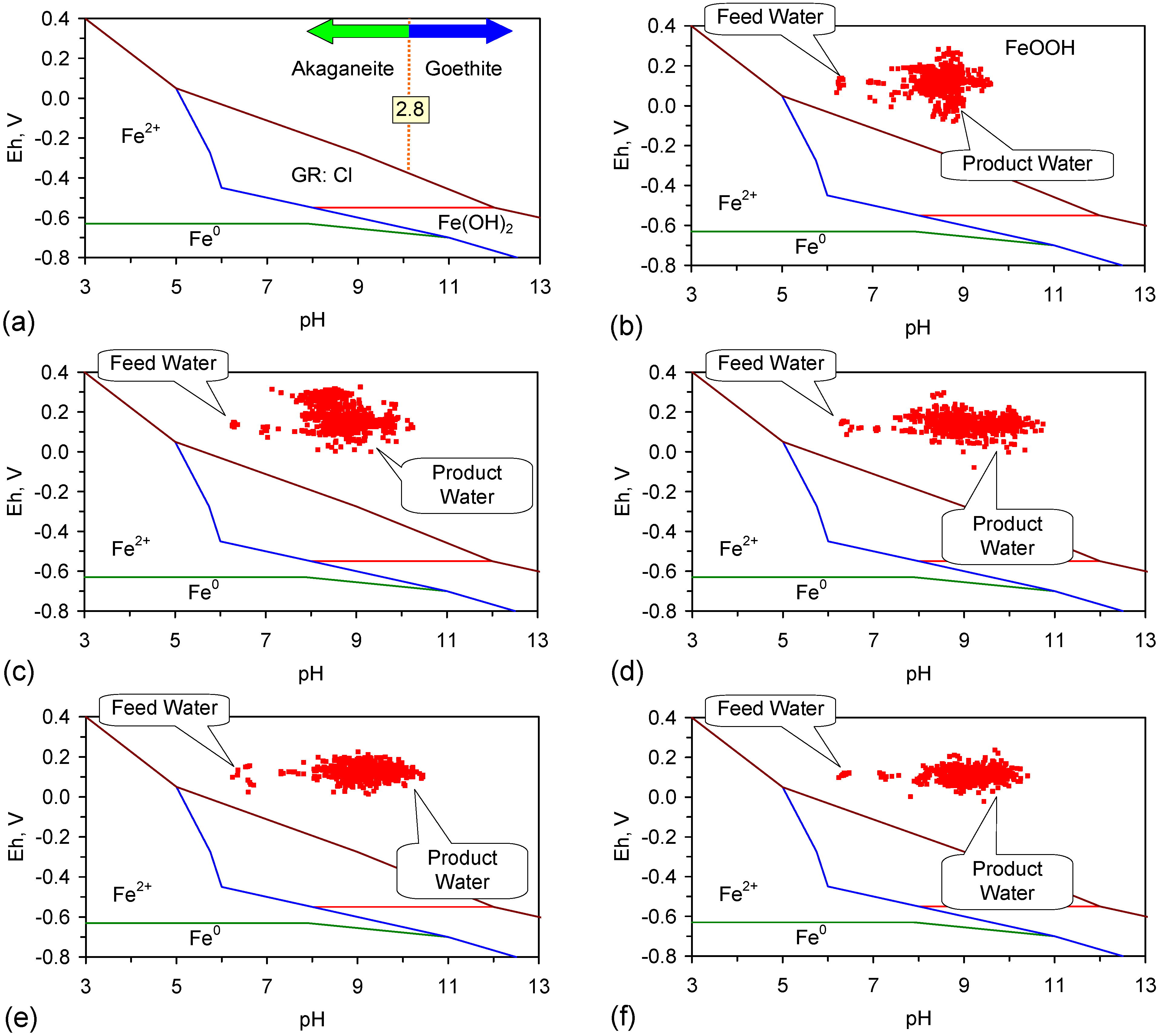
4.2.3. Transformation from α-FeOOH → β-FeOOH
- 1
- 2
- 3
- 4
- R ≥ 8, the only equilibrium FeOOH species which will be present is β-FeOOH [71].
4.2.4. Dominant Corrosion Species Associated with ZVM TP
4.2.5. NaCl Removal in the Hydrated Layers Surrounding ZVM TP
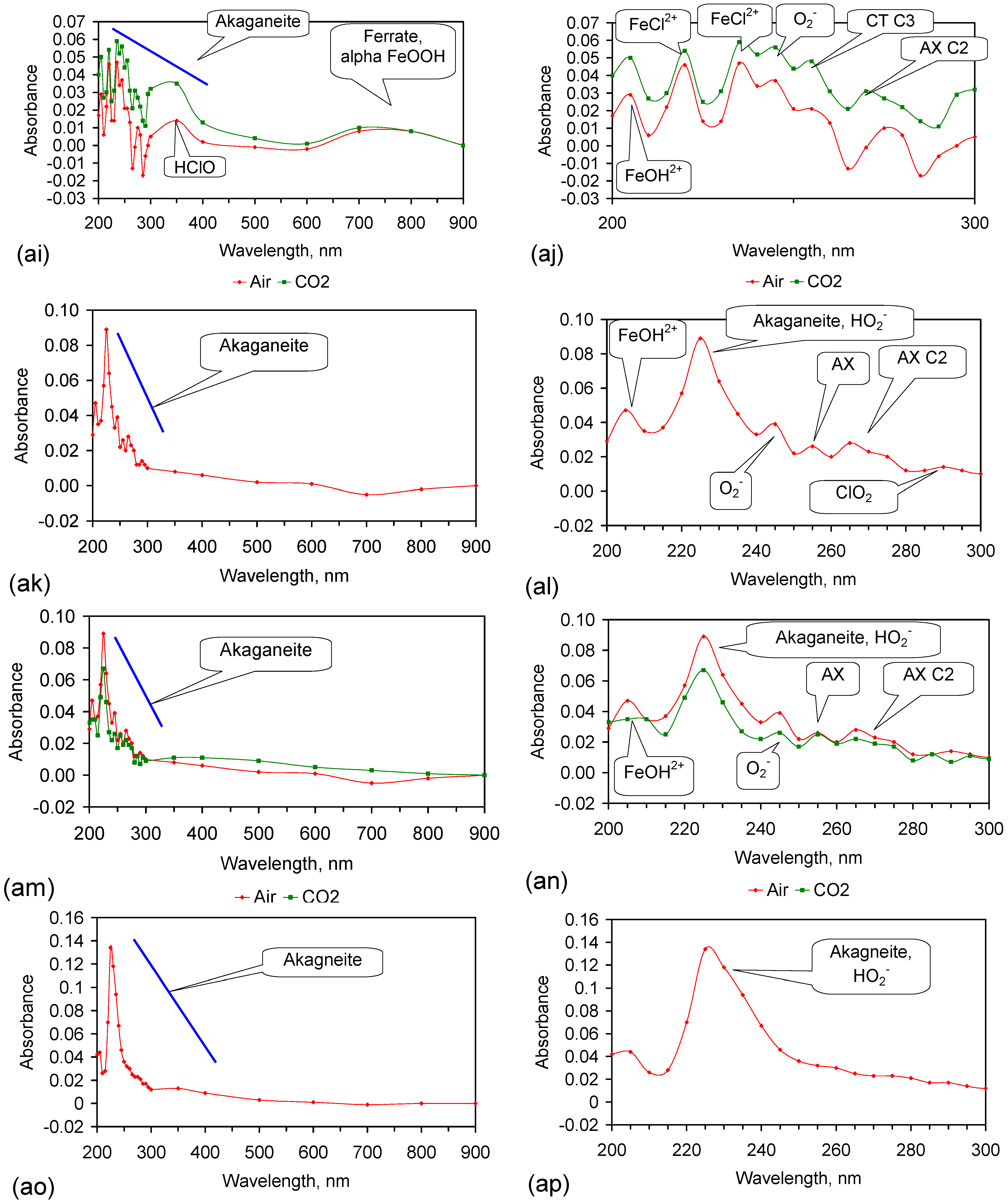
4.2.6. Identification of FeOOH or Other Fe Species Involved in NaCl Removal
| Species | Hydrated Ligand Field Transition Fe3+ | Ligand Field Transition Fe3+ | Ligand Field Transition Fe3+ | Ligand Field Transition Fe3+ | Excitation to an Fe-Fe Pair | Fe(II)-Fe(III) Intervalence and Fe(III) Absorption | Fe(II)-Fe(III) Intervalence | Excitation to an Fe-Fe Pair | Excitation to an Fe-Fe Pair | Excitation to an Fe-Fe Pair |
|---|---|---|---|---|---|---|---|---|---|---|
| Hematite | - | 300 | 370 | 430 | 485 | - | - | 555 | - | 900 |
| Ferrate | - | - | - | 450 | - | - | 550 | 800 | - | |
| Akaganeite (hydrated) | 225 | - | 350 | - | 500 | - | - | - | - | - |
| Akaganeite (low hydration) | - | - | - | - | 500 | - | - | - | - | - |
| Goethite | - | 300 | - | 450 | - | - | - | 590 | 760 | 910 |
| Lepidocrocite | - | 300 | 350 | 420 | 485 | - | - | - | - | - |
| GR2–SO4 | - | - | 320 | 410 | - | 550 | 690 | - | - | - |
| GR1–Cl | - | - | 350 | 485 | - | 550 | 690 | - | - | - |
4.2.7. Significance of pH Change
- An increase in surface protonation (H+, (–OH2)+), with an increase in Cl− ions adsorbed on the FeOOH surface [84].
- The competition between Cl− ions and OH− ions for the protonated sites decreasing due to both a decreased relative availability of OH− and increased surface protonation resulting in an increase in the number of available adsorption sites [84].
- The replacement of blocking OH− ions and H2O at the entrances to tunnels within the β-FeOOH by Cl− ions [84].
4.2.8. Significance of Water Consumption
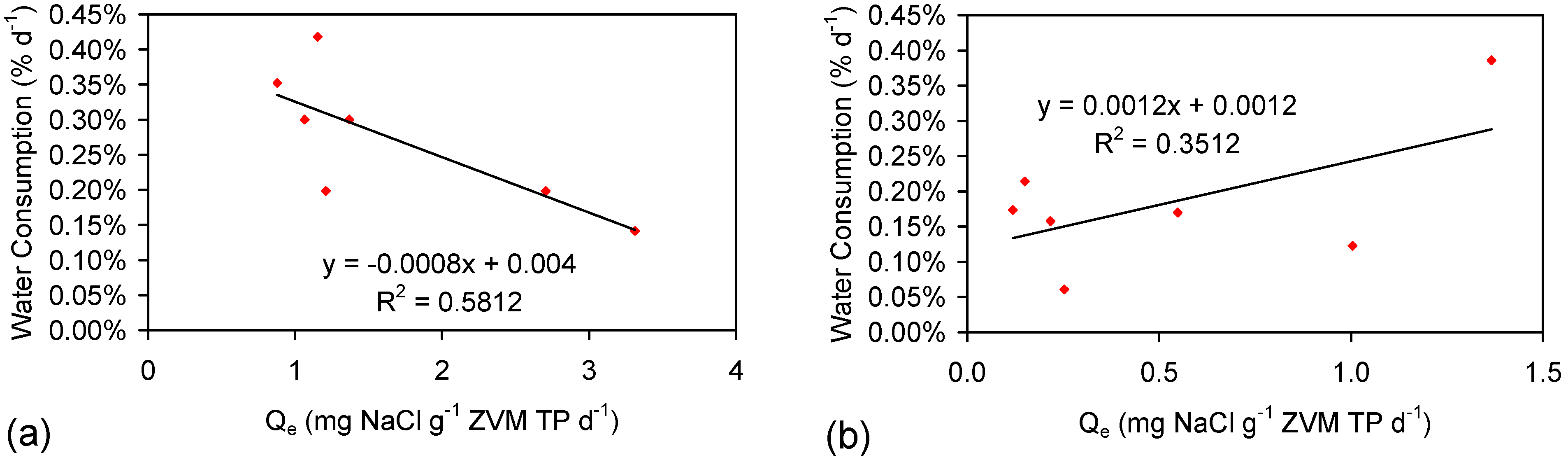
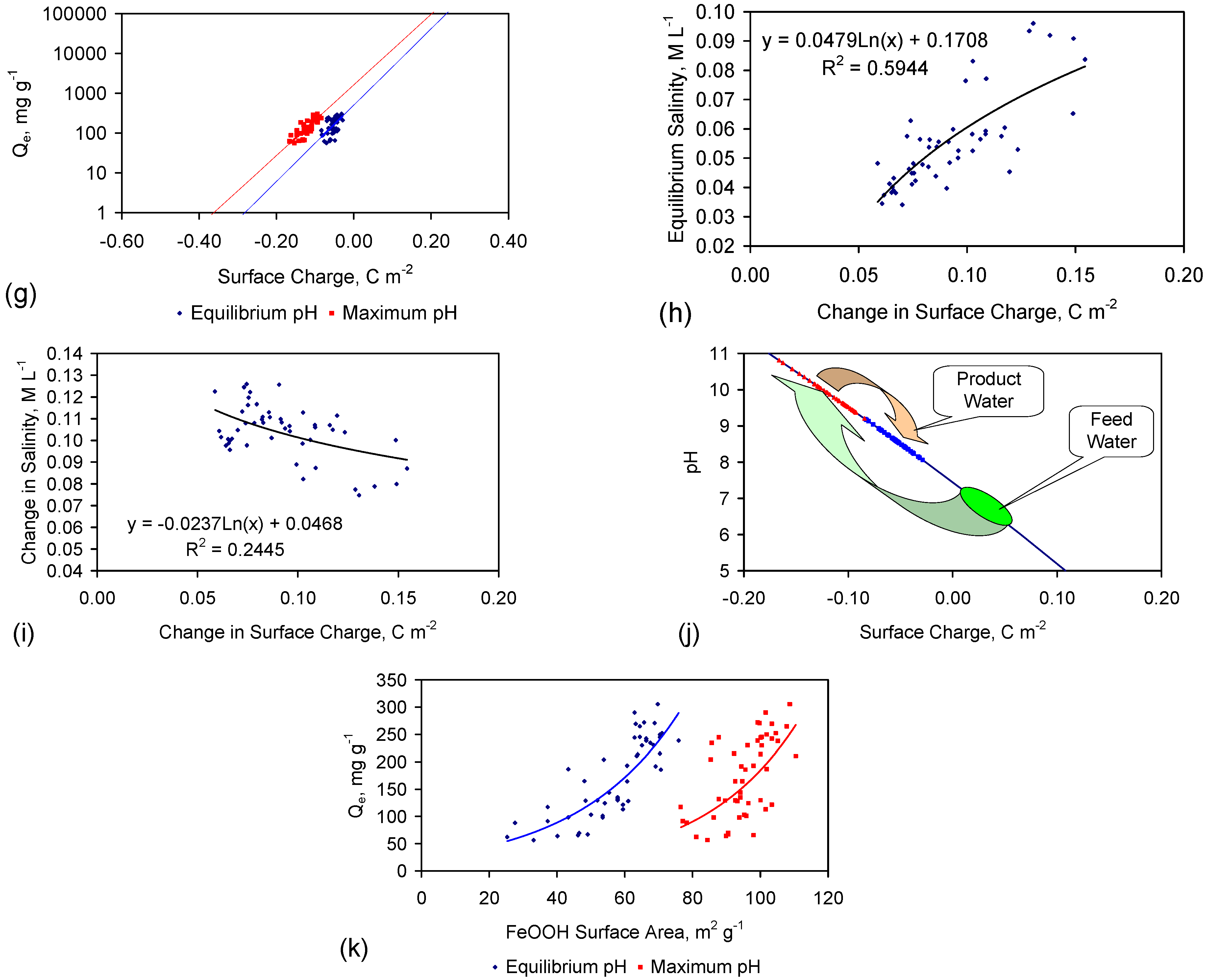
4.2.9. Significance of Surface Charge
- Protonation: FeOH0.5− + H+ = FeOH20.5+;
- Cl− capture: FeOH20.5+ + Cl− = [(FeOH20.5+)(Cl−)];
- Na+ capture: FeOH0.5− + Na+ = [(FeOH0.5−)(Na+)].
4.2.10. Recovery of Adsorbed NaCl from ZVM TP
- recover the Na+ and Cl− ions separately from the used ZVM TP;
- recover the charge held in the ZVM TP following desalination;
- regenerate the ZVM TP for reuse following desalination.
4.3. Removal of NaCl from Water in the Hydration Shells
Freudlich: Qe = KFCe1/n
Redlich-Peterson: Qe = [ACe]/[1 + BCeg]
Qe (mg/g) = [(C0 − Ce)V]/m = V[(C0 − Ce)/m] KR = 1/[1 + KLC0]
Removal Efficiency (%) = 100 − 100Ce/C0
4.3.1. Characteristics of NaCl Removal by Adsorption in the Hydration Shells
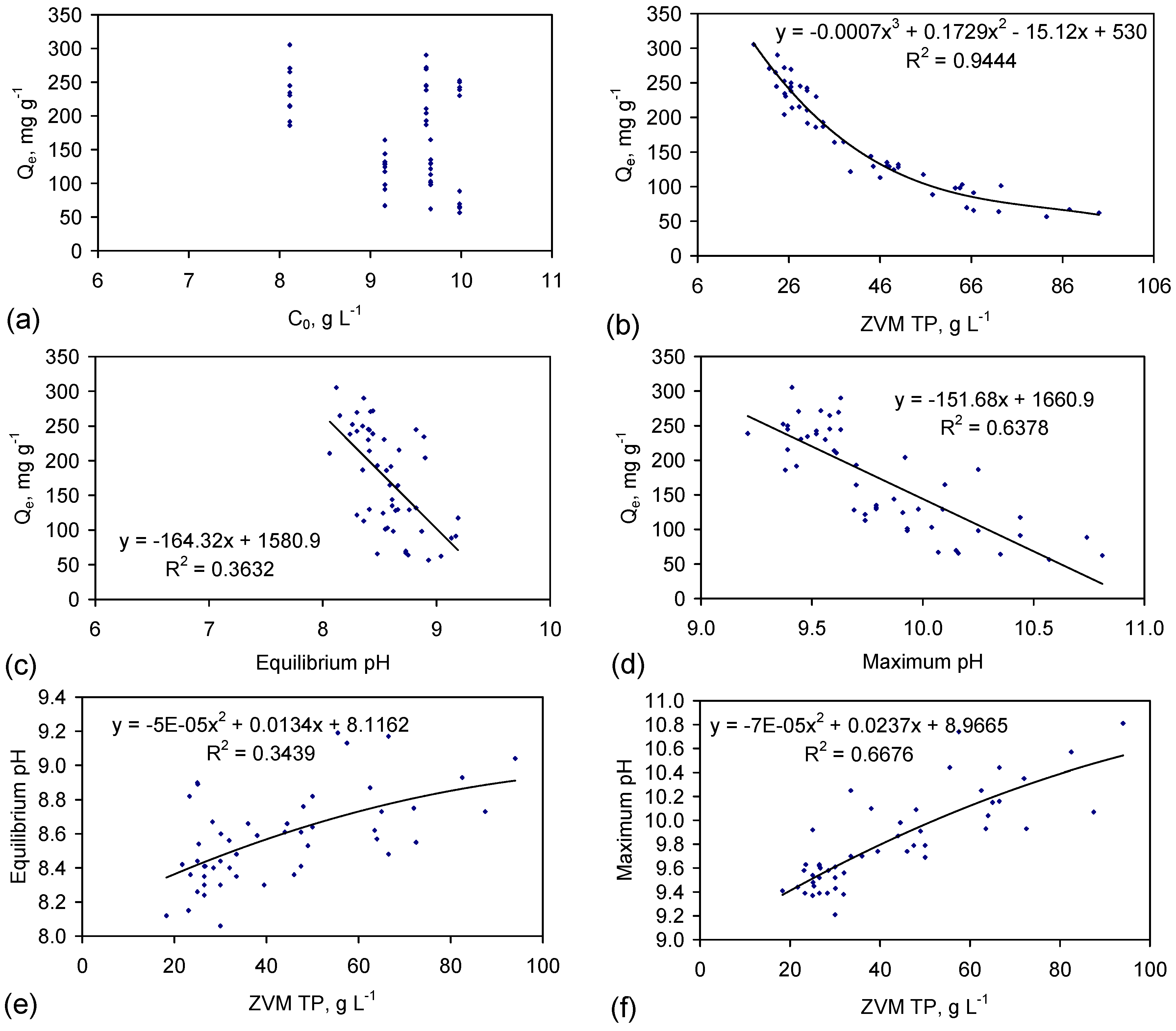
4.3.2. Impact of Water Consumption

4.3.3. Impact of ZVM Particle Size
4.4. Desalination Associated with NaCl Concentration in the Pore Waters within the ZVM TP Bed
4.4.1. Osmotic Pressure
- No Cl− is observed in the first co-ordination shell of Fe in the polymers when OH:Fe > 2 [110].
4.4.2. Role of Hydration Shells
- terminal surface (no hydration, e.g., FeOH0.5−): (OH)–(OH)–Fe–O–O–Fe–R (where R = a repeat of the stoichiometric atomic layer sequence, or tethering surface. R can included hydrated layers);
- interface terminal surface (no hydration, e.g., FeOH20.5+): (H2O)–(H2O)–(OH2)–(OH)–Fe–O–O–Fe–R
- double hydrated terminal surface: (H2O)–(H2O)–(OH)–(OH)–Fe–O–O–Fe–R
- double hydrated interface terminal surface: (H2O)–(H2O)–(OH2)–(OH)–Fe–O–O–Fe–R
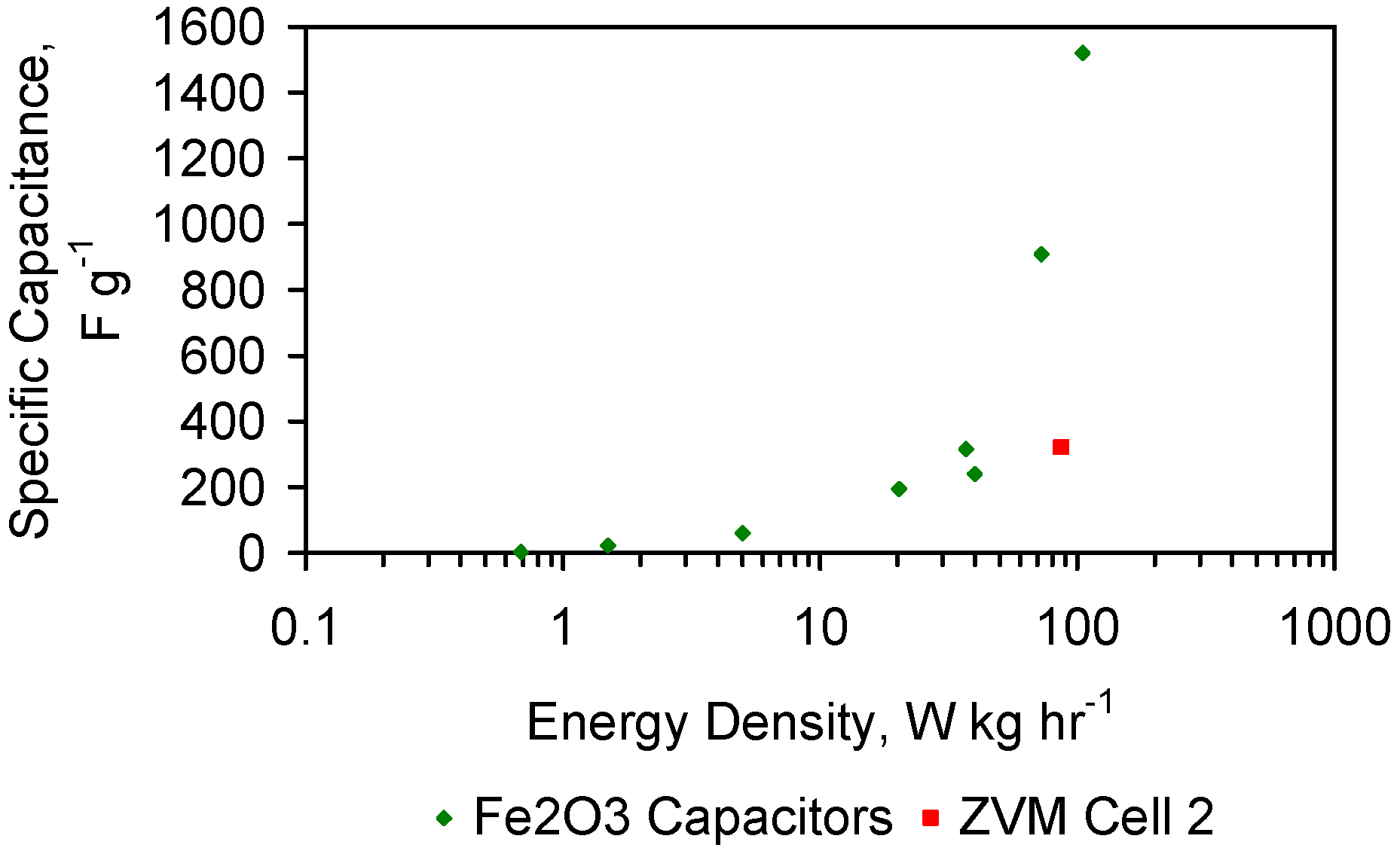
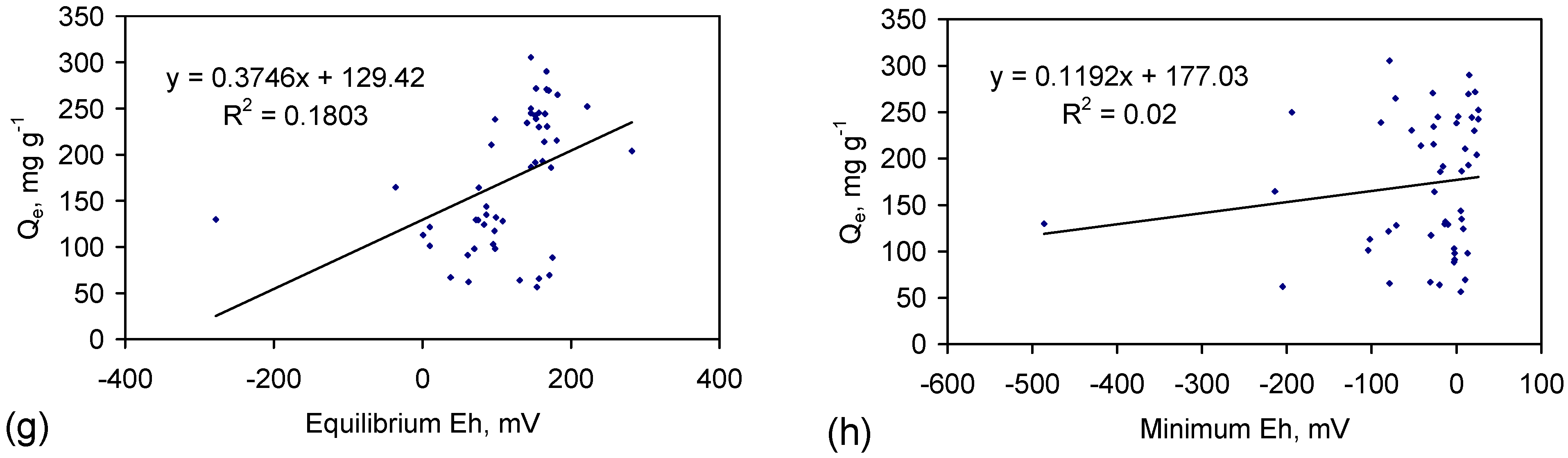
4.5. Role of Surface Charge and Capacitance in Assessing Desalination Efficiency
- the observed surface charge and capacitance associated with desalination;
- the capacitance characteristics of ZVM TP prior to use for desalination;
- the capacitance characteristics of the ZVM TP following desalination.
4.5.1. Observations Made during Desalination
- the proton uptake increases with both increasing salinity and decreasing pH; (e.g., at 0.1 M (5.844 g·L−1) NaCl proton uptake ([H+]ads (μM NaCl)−1·m−2 = −0.7006pH + 4.3174; at 0.01 M (0.5844 g·L−1) NaCl proton uptake ([H+]ads (μM NaCl)−1·m−2 = −0.7006pH + 3.8174 [116]), M = moles NaCl·L−1; m−2 refers to the BET surface area;
- the surface charge (C·m−2) increases (at 0.1 M (5.844 g·L−1) NaCl) with decreasing pH (e.g., Surface charge, C·m−2 = −0.0009pH2 − 0.033pH + 0.2957 [116]).
4.5.2. Impact of Changes in Surface Charge on Qe during Desalination

4.5.3. Measured Capacitance of the ZVM TP Prior to Usage for Desalination

4.5.4. Relationship between Capacitance and Desalination
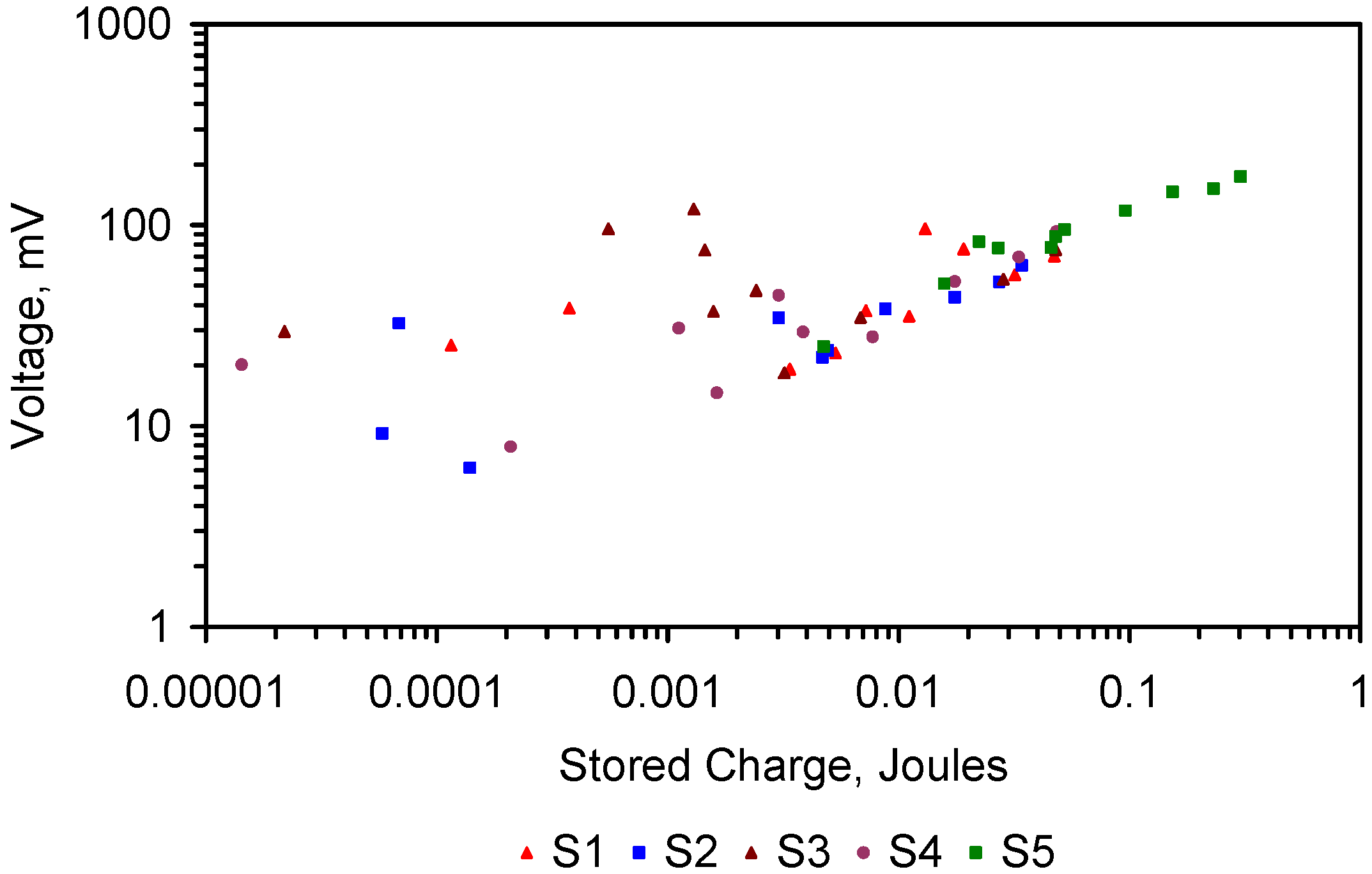
4.5.5. Capacitance
- Cell 1 represents the typical capacitance associated with discharged ZVM TP prior to placement in saline water.
- Cell 2 demonstrates that in saline water (following, or during desalination), the presence of NaCl around the ZVM TP has the effect of charging the cell and increasing its specific capacitance. These features are quantified in Table 4.
| Variable | Cell 1 | Cell 2 | |
|---|---|---|---|
| Current | mA | 0.08 exp(−0.24x) | 4.33 exp(−0.0119x) |
| R2 | 0.8463 | 0.408 | |
| Voltage | mV | 947.23 exp(−0.0216x) | 781.47 exp(−0.0028x) |
| R2 | 0.858 | 0.494 | |
| i | mA | 0.016 | 2.860 |
| m | g | 56.000 | 11.000 |
| (ΔE/Δt) | 0.01531 | 0.00081 | |
| Cs | F·g−1 | 0.018 | 321.208 |
| ΔV | V | 0.199 | 0.734 |
| E | Wh·kg−1 | 0.00036 | 86.411 |
| td | s | 12,469 | 4452 |
| P | kW·kg−1 | 0.00000003 | 0.01941 |
4.5.6. Voltage/Current Changes Associated with the Cessation of Desalination

4.5.7. ZVM TP Pellet Capacitance Following Desalination
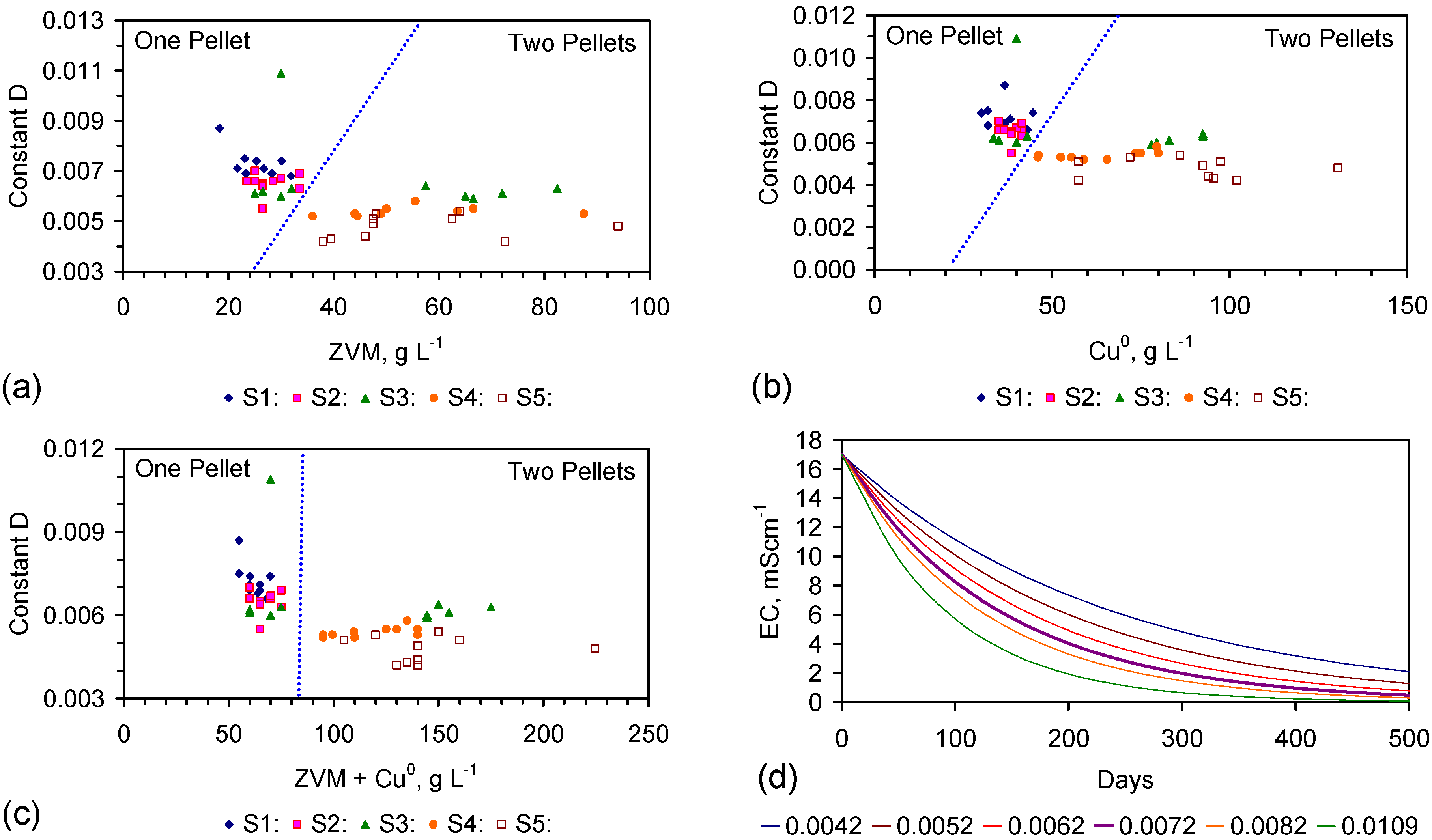
4.5.8. Desalination Model Associated with ZVM TP

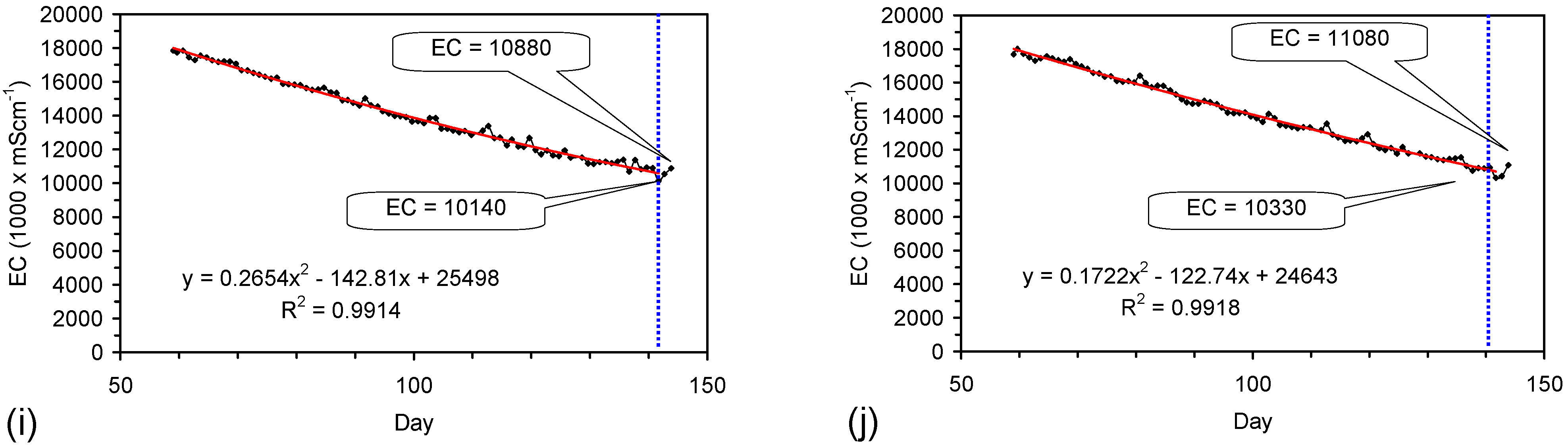
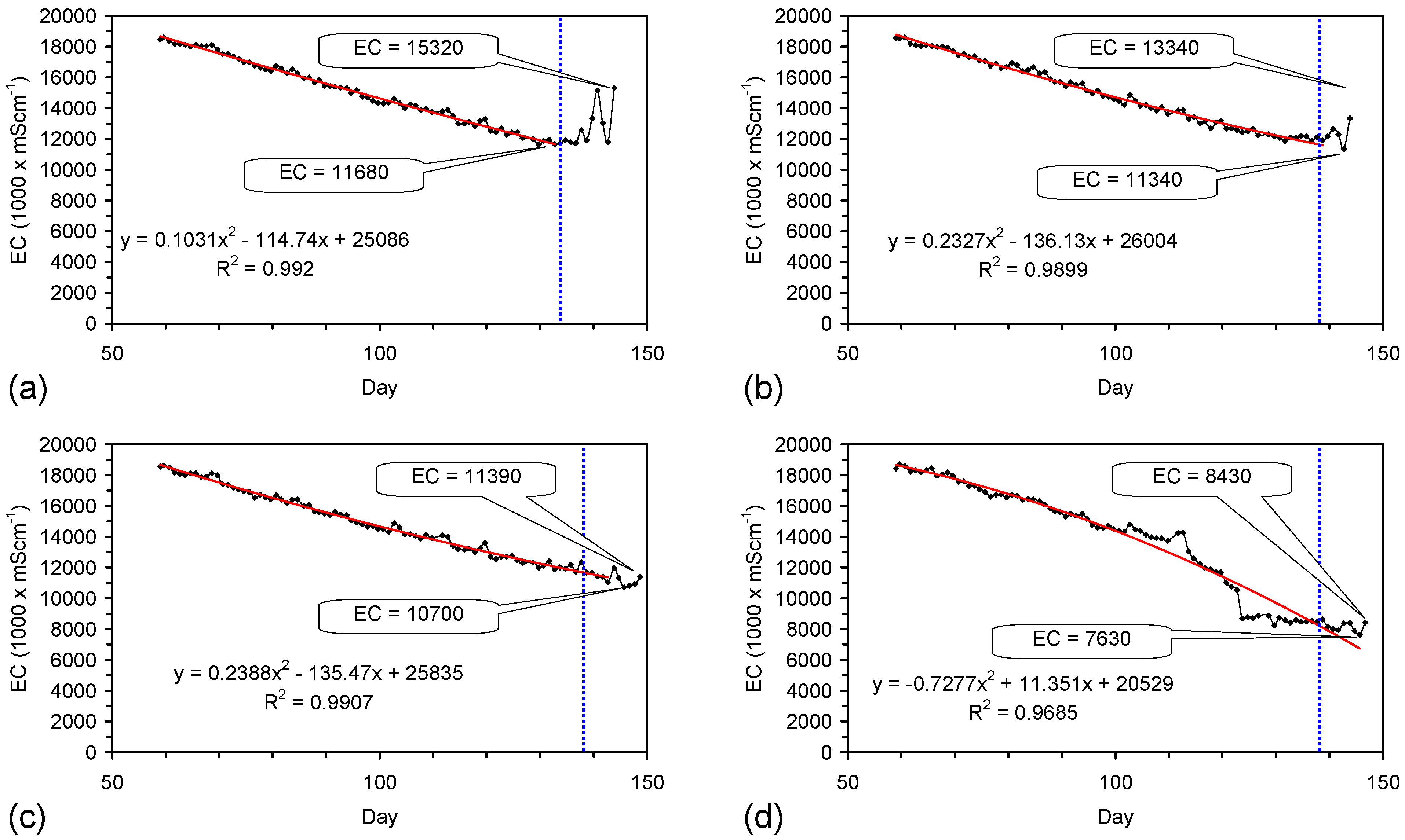
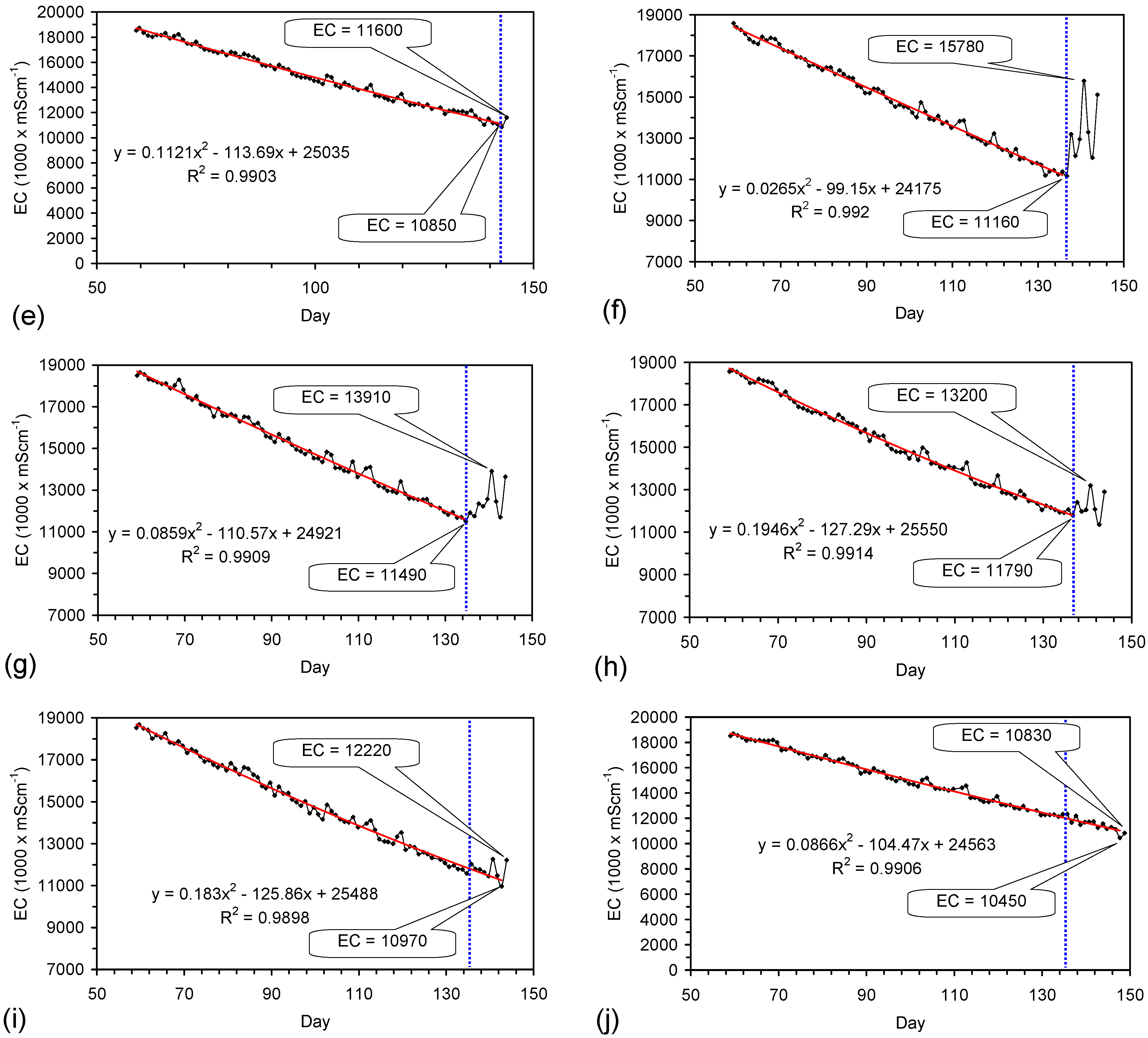
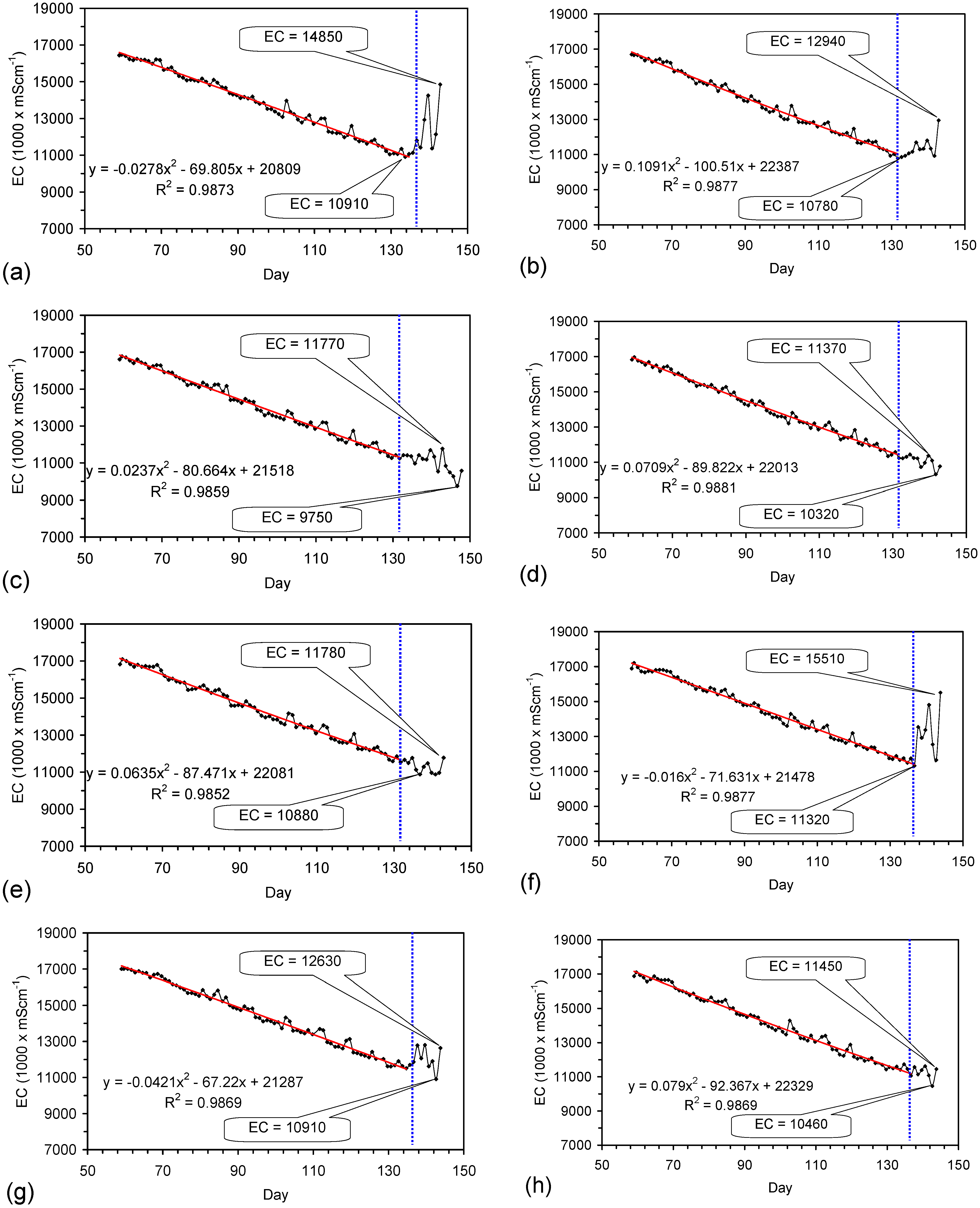
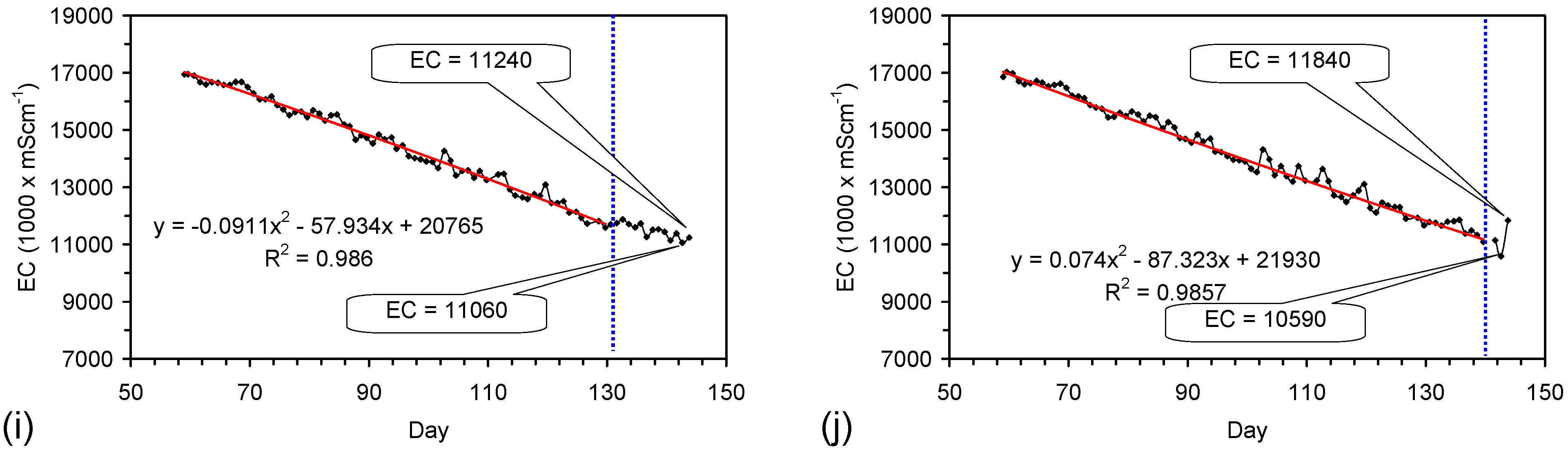
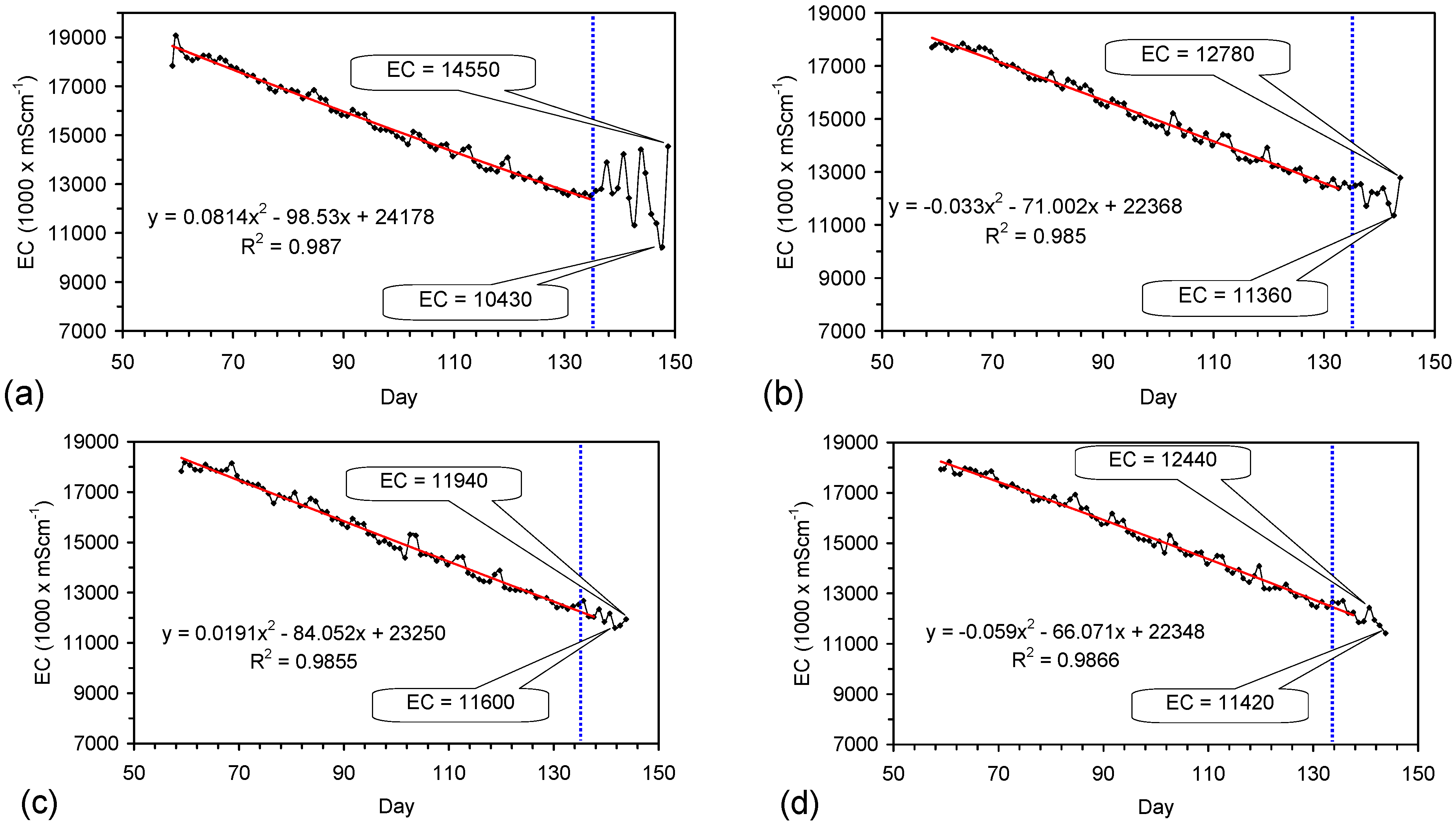
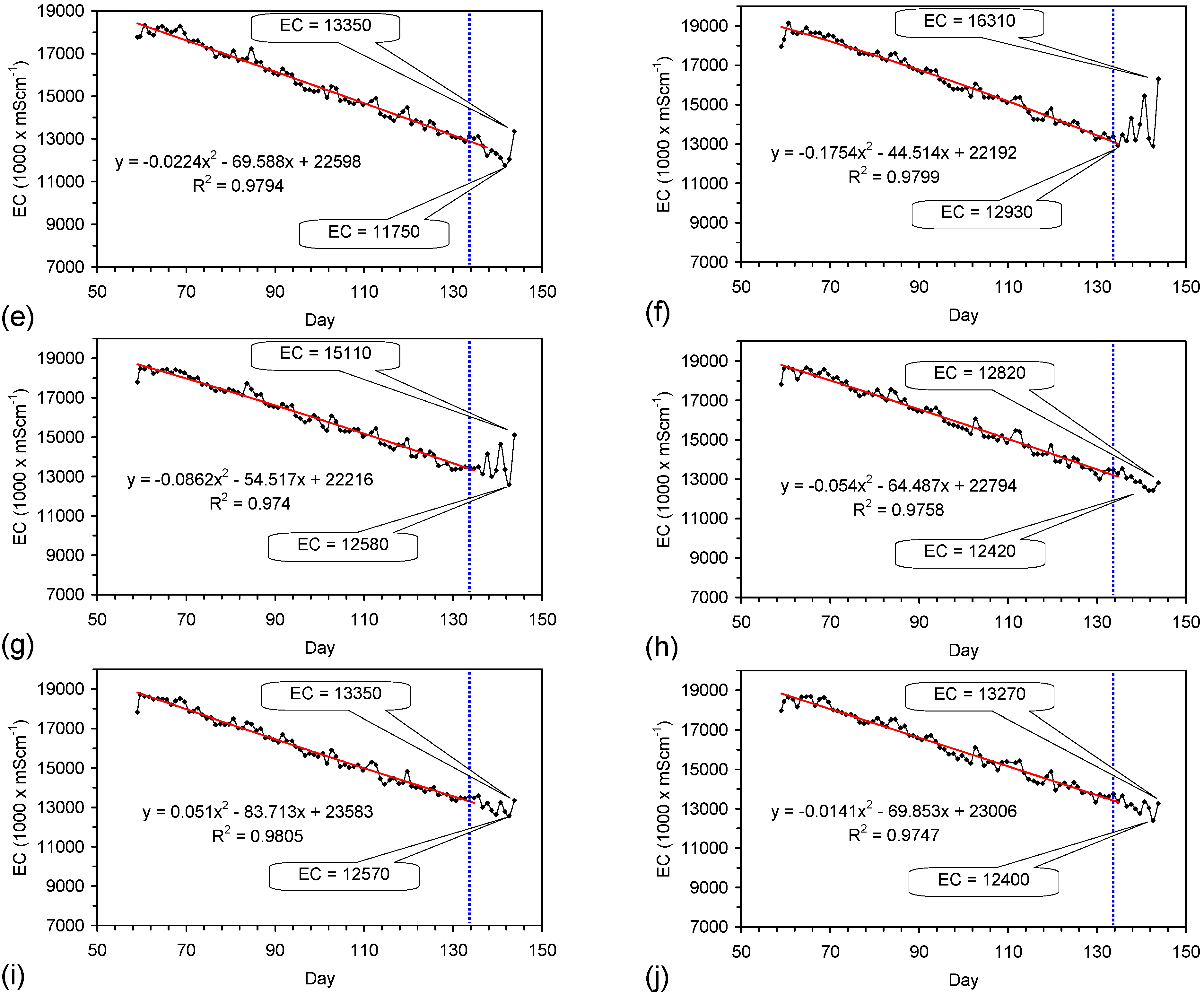
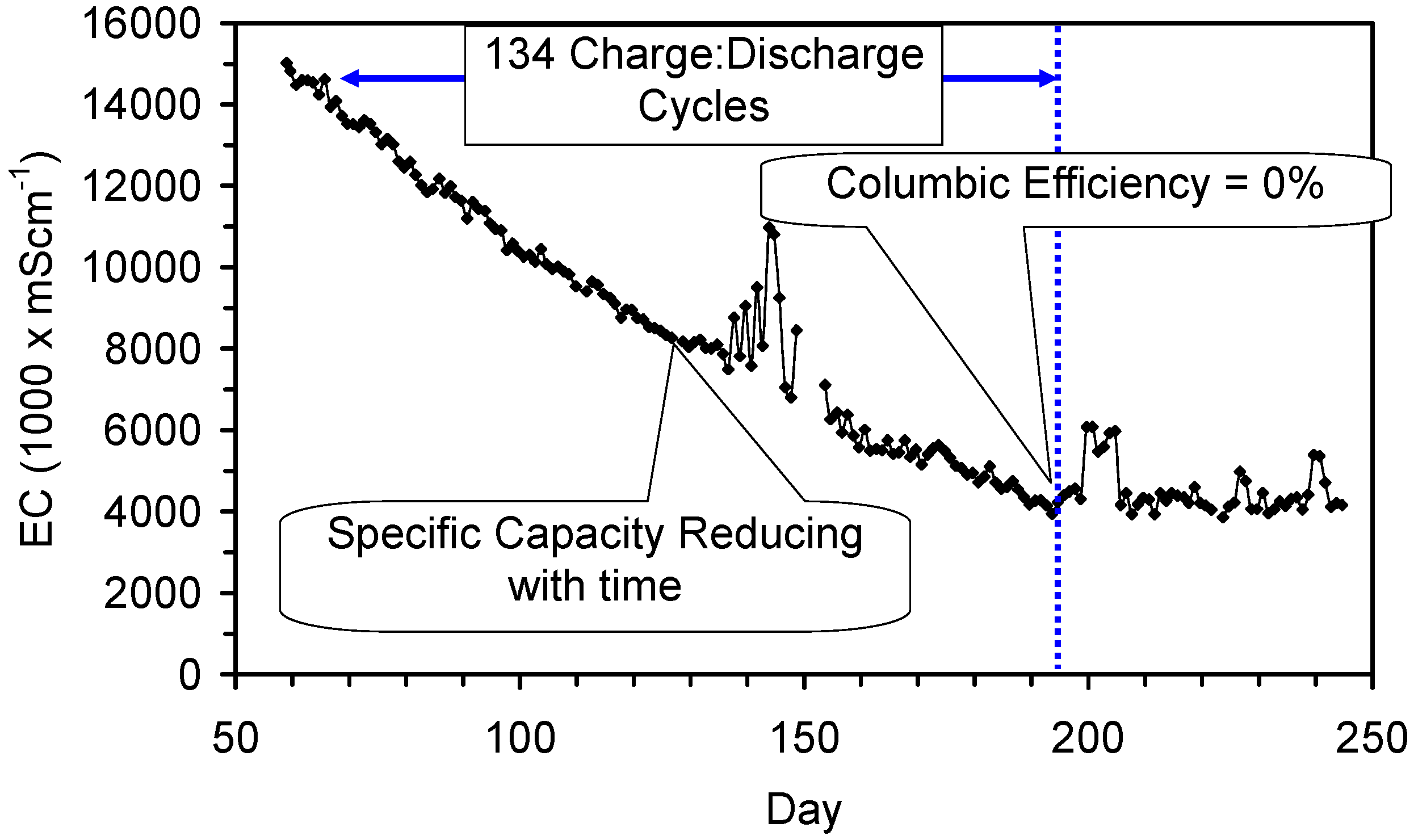
4.6. Statistical Analysis of the Change in Salinity with Time
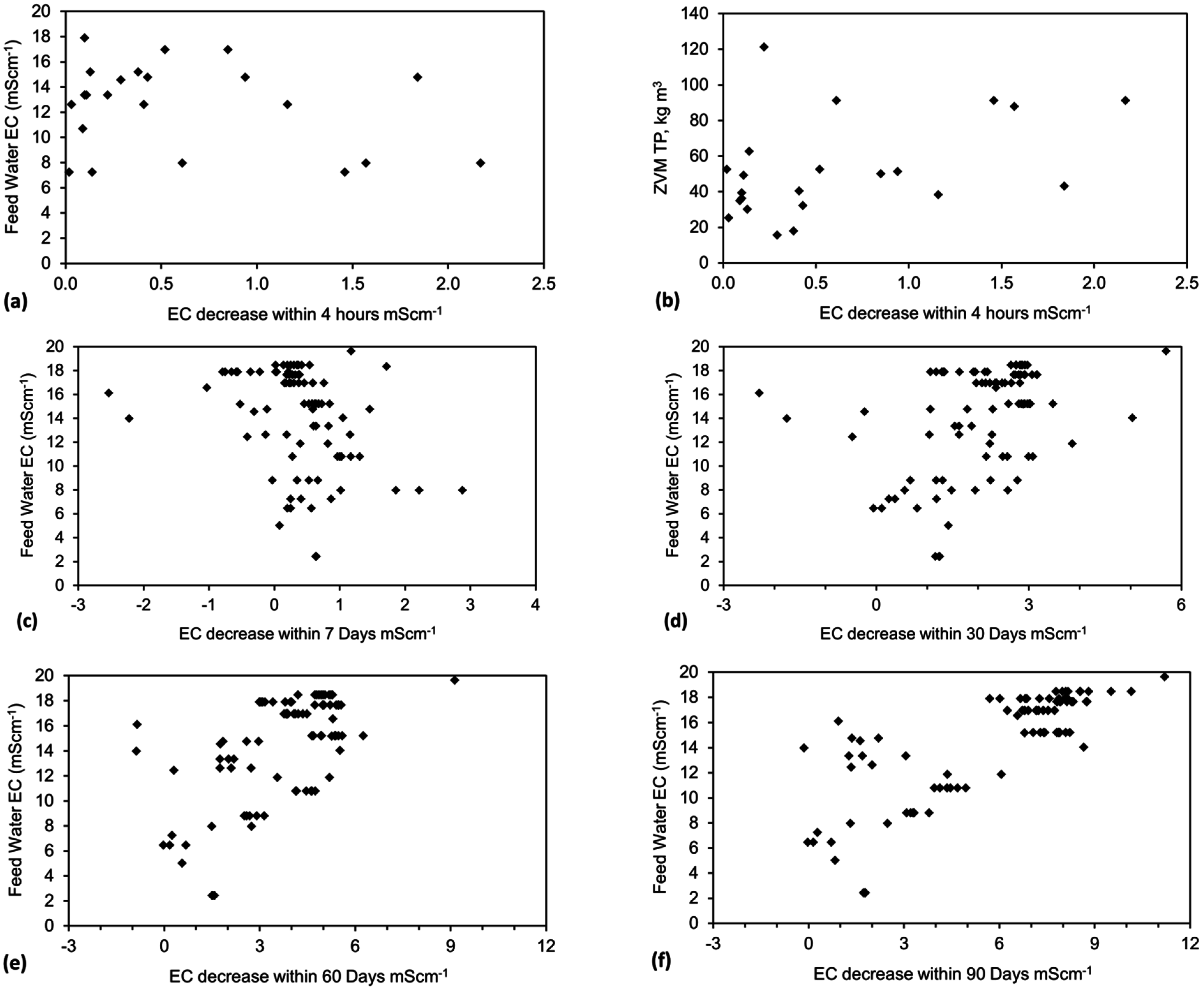
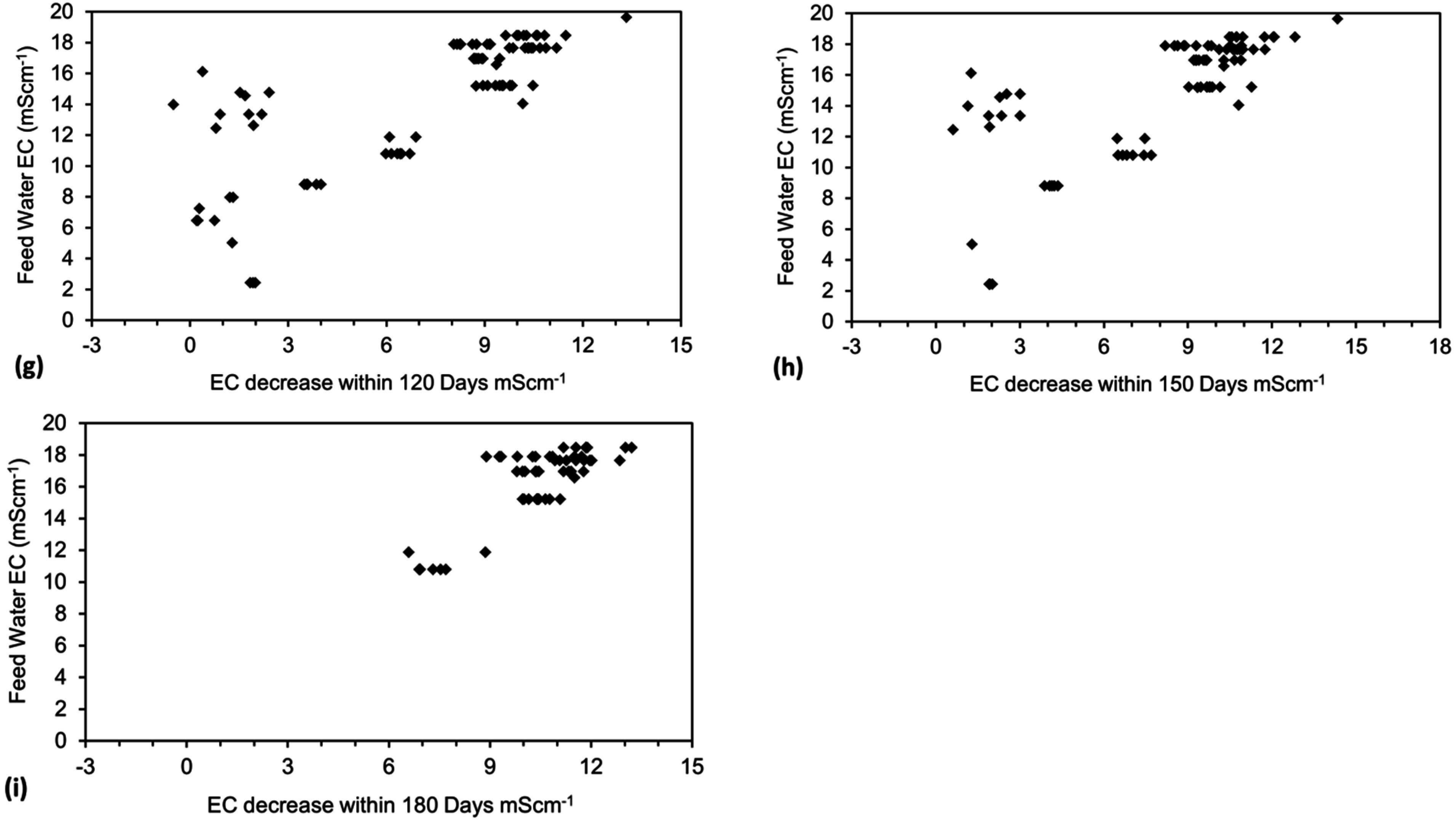
4.7. Cost of Desalination Using ZVM TP
4.7.1. Economics of ZVM TP without Reuse or Regeneration
4.7.2. Reuse of the ZVM TP

4.7.3 Reuse of ZVM TP as an Iron Sponge
In water: H2S (g) + H2O = H2S (aq) = HS− + H+
2Fe3+ (e.g., Fe2O3) + HS− = 2Fe2+ (e.g., Fe3O4) + H+ + S (s)
2Fe3O4 (s) + 0.5O2 (air) = 3Fe2O3 (s)
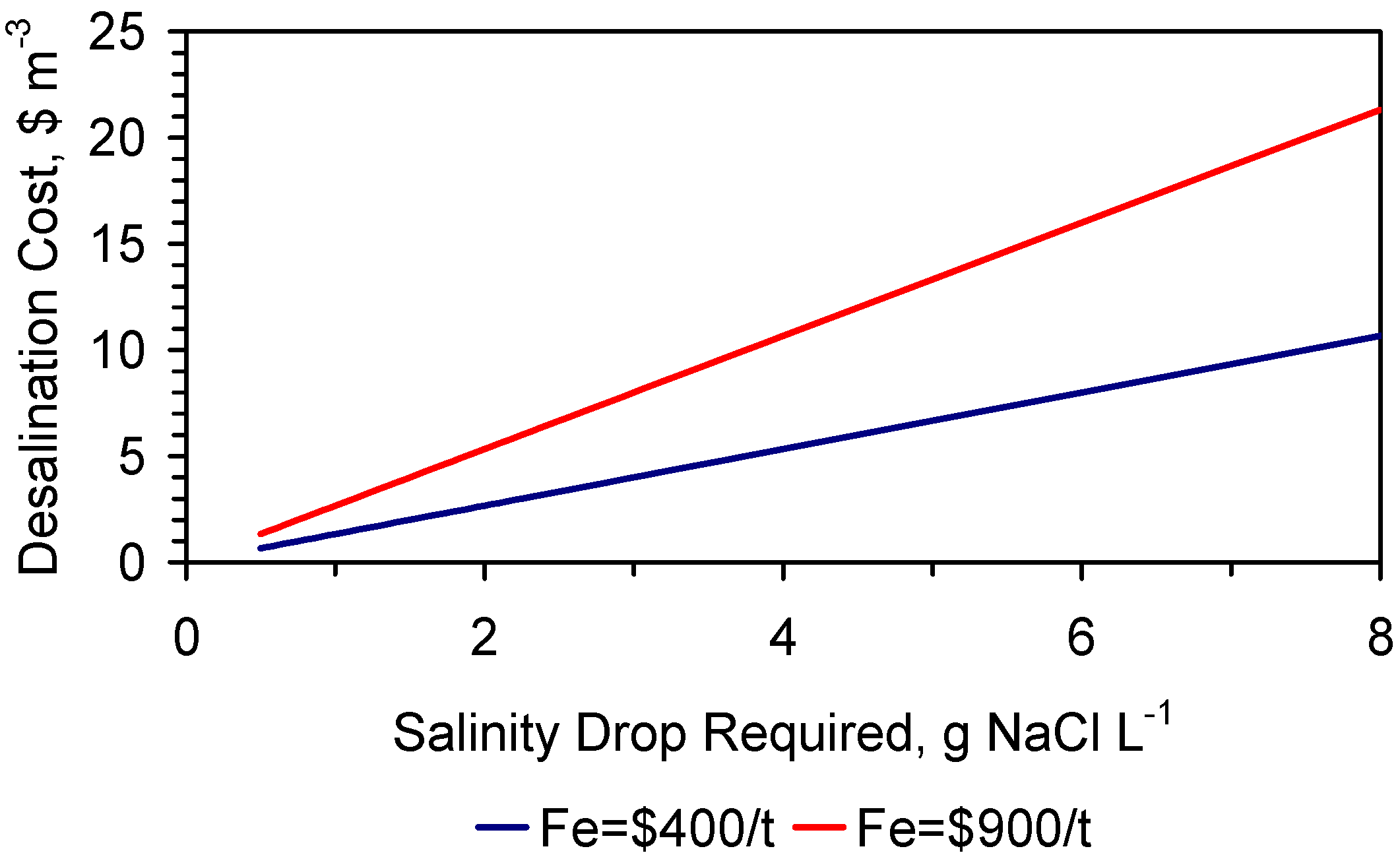
4.7.4. Reducing the Capital Costs Associated with Desalination
4.7.5. Improving the Economics of Partial Desalination by Increasing the Operating Temperature
ΔG0 = −RT·(lnKc)
ΔE0 = −RT/nF·(lnKc)
4.7.6. Relationship between EC (Salinity) Reduction and Temperature
- Trial CSD1a: The trial duration was 15 h during which time the water temperature was raised from 5 to 50 °C. Air was bubbled through the water at a rate of 55 mL·min−1.
- Trial CSD1b: The trial duration was 124 h during which time the water temperature was raised from 5 to 44 °C. Air was bubbled through the water at a rate of 64 mL·min−1.
- Trial CSD1c: The trial duration was 30 h during which time the water temperature was raised from 7 to 39 °C. Air was bubbled through the water at a rate of 60 mL·min−1.
- Trial CSD1d: The trial duration was 11 h during which time the water temperature was raised from 7 to 36 °C. Air was bubbled through the water at a rate of 22 mL·min−1 for 4.7 h followed by 15 mL·min−1 80% N2 + 20% CO2.
- increases the rate of desalination;
- allows the ZVM TP to be reused to sequentially partially desalinate batches of saline water;
- allows the salinity of the product water to reduce below 0.2 gNaCl·L−1.
| Trial | Feed Water | EC, mS·cm−1 | Salinity, g·L−1 | Product Water | EC, mS·cm−1 | Salinity, g·L−1 | NaCl Removed, g·L−1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Eh, V | pH | Eh, V | pH | ||||||
| CSD1a | 0.277 | 6.150 | 4.540 | 2.500 | 0.167 | 6.500 | 0.544 | 0.194 | 2.306 |
| CSD1b | 0.188 | 5.920 | 4.540 | 2.500 | 0.130 | 6.900 | 0.556 | 0.200 | 2.300 |
| CSD1c | 0.088 | 6.700 | 4.760 | 2.625 | 0.219 | 6.020 | 1.830 | 0.888 | 1.737 |
| CSD1d | 0.174 | 5.970 | 5.450 | 3.000 | 0.233 | 5.440 | 3.670 | 1.882 | 1.118 |
- The ZVM TP modifies the salinity of the water adjacent to the ZVM TP by removing NaCl from the overlying water body.
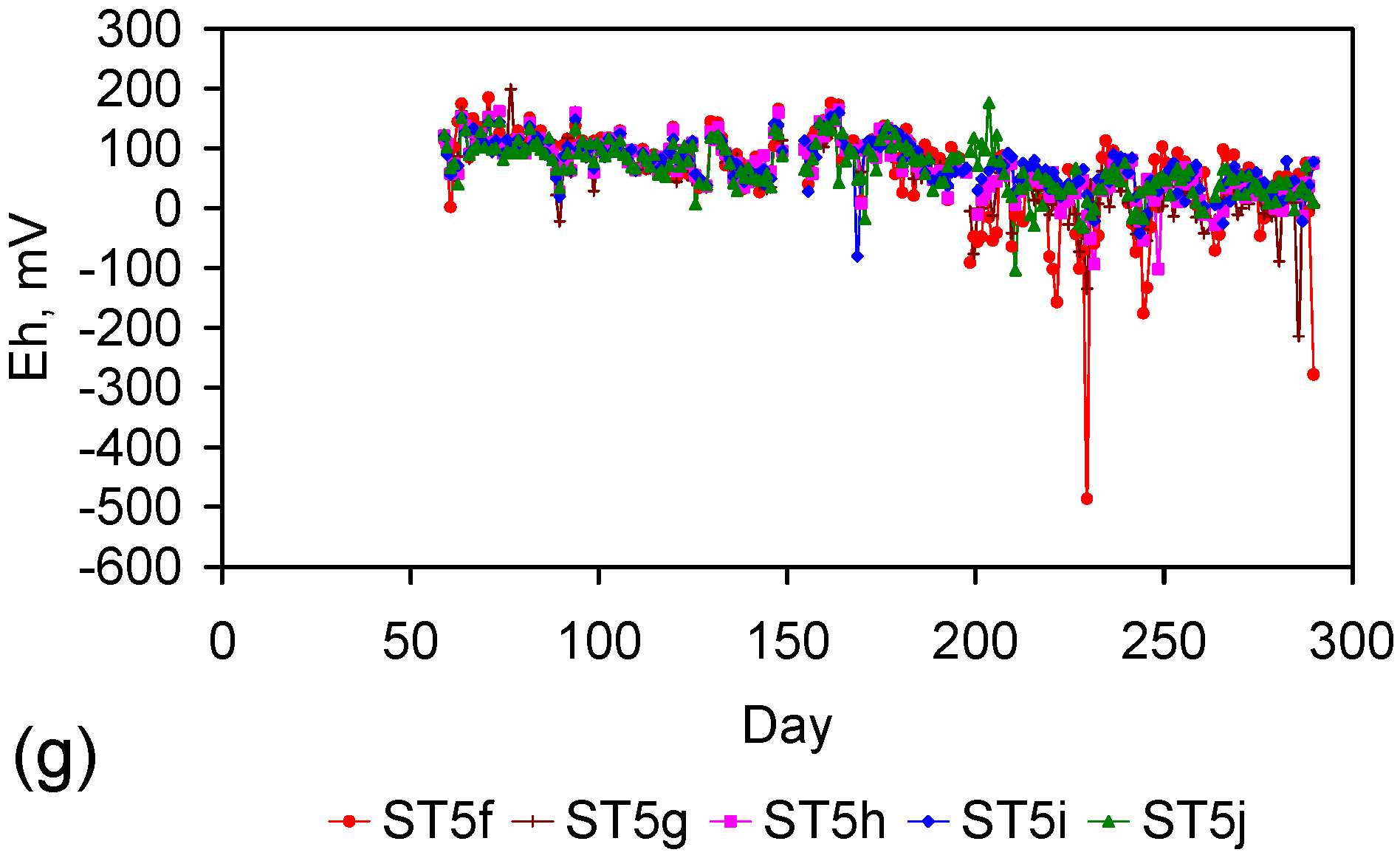
- The removal of NaCl can occur when the water body is static (e.g., ST1a–ST5j series of trials) or when the bulk of the water body is in constant movement due to gas bubbling and the water column connecting the ZVM TP to the moving water body is static.
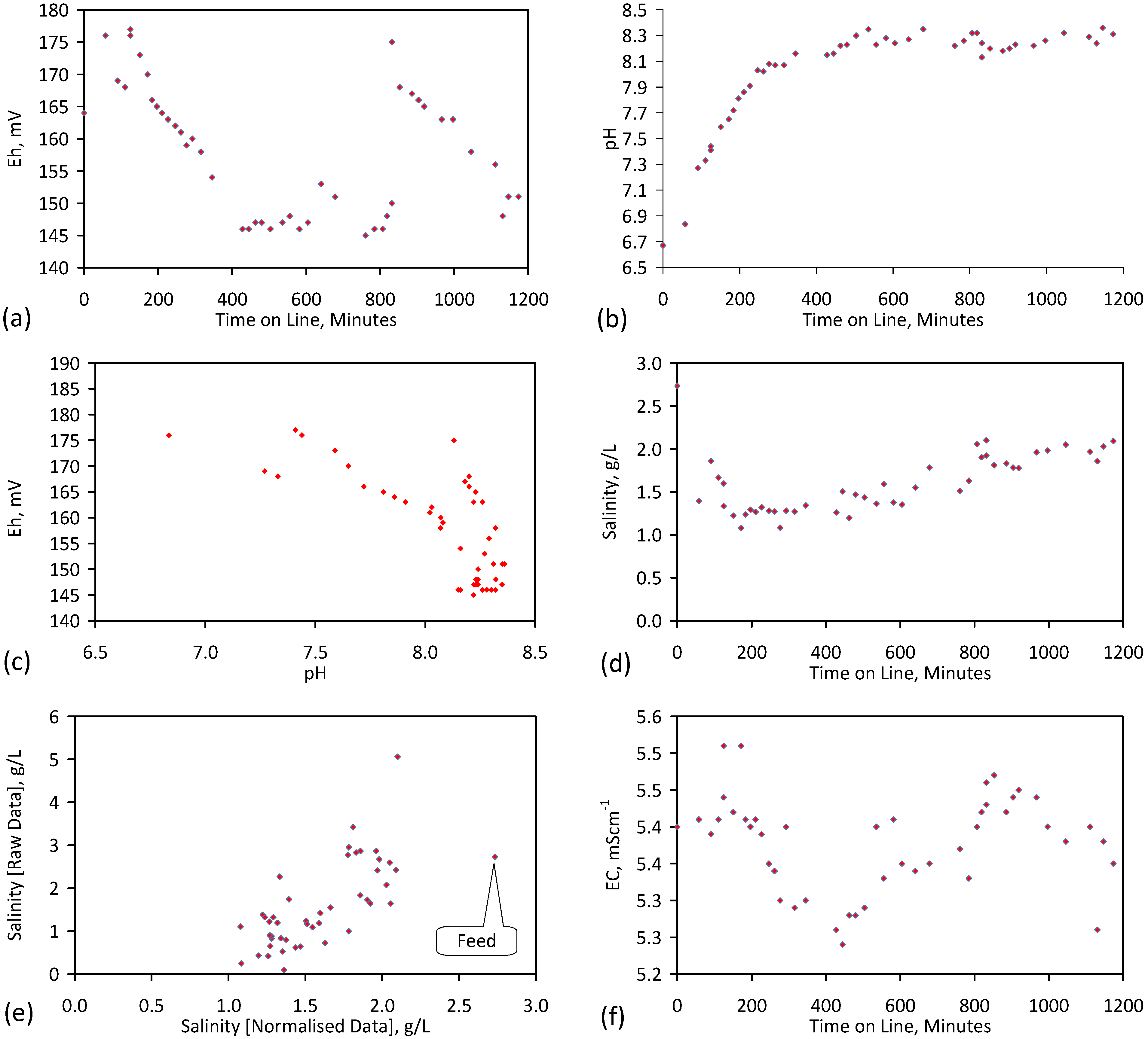
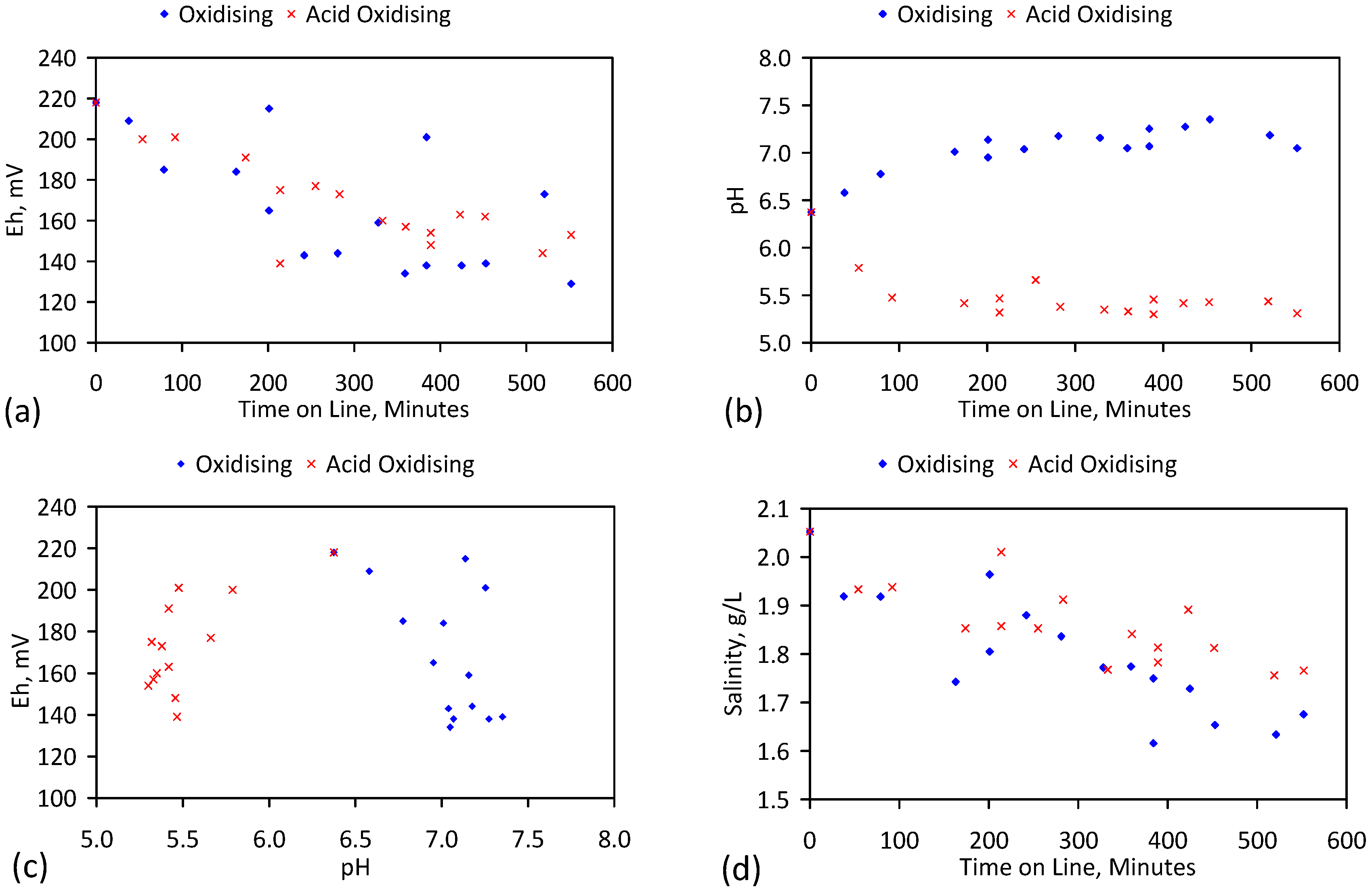
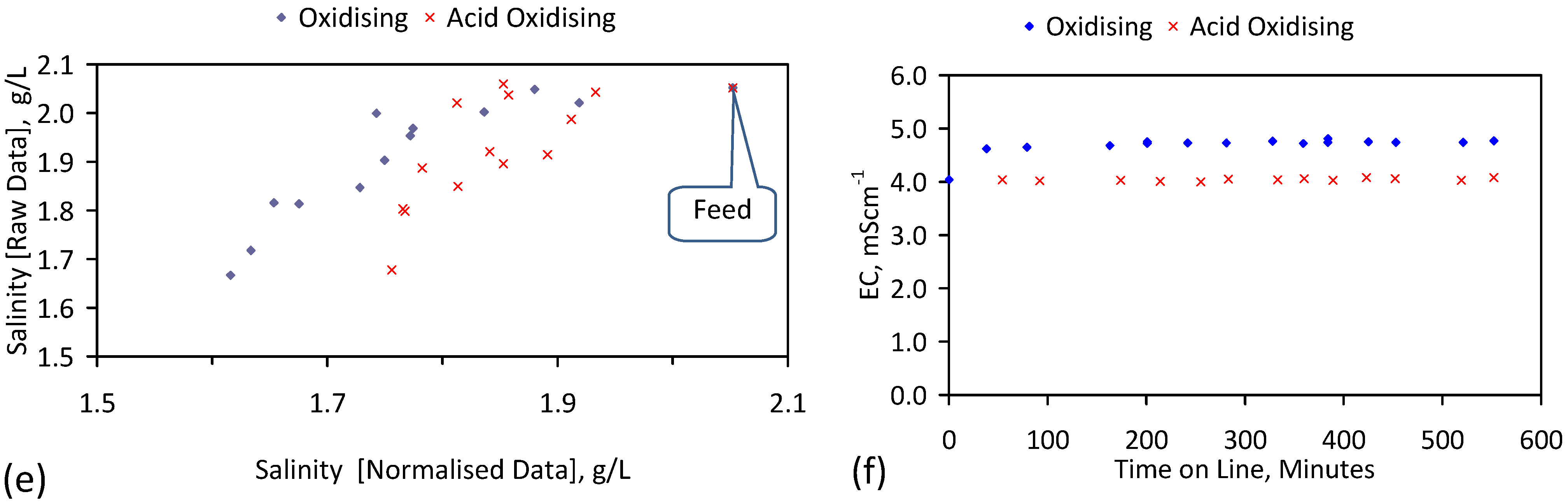
5. Results: ZVM TPA
- The main water body was kept in a state of constant movement by an acid oxygenating (N2:CO2), or oxygenating (N2:O2) gas which was bubbled through the water;
- The ZVM TP was held in a container which was separated from the main water body by a static water column.
5.1. Desalination Using ZVI + Ion Exchange Material (ZVM TPA)
5.2. Oxygenation and CO2
5.2.1. Oxygenated Water
Na+ + 0.5O22− + nH2O + e− = [(NaO)(H2O)n]•1−
Na+ + 0.5O2− + nH2O + 1.5e− = [(NaO)(H2O)n]•1−
Cl− + (1 + n)H2O = [(ClO)(H2O)n]•1− + 2H+ + e−
Cl− + Na+ + 0.5O2 + (1 + n + m)H2O = [(NaO)(H2O)n]•1− + [(ClO)(H2O)m]•1− + 2H+
5.2.2. Acid Oxygenated Water
Na+ + CO2 + (2 + n)H2O = [(HCO3)(NaO)(H2O)n]•1− + 3H+ + 2e−
Cl− + CO2 + (2 + n)H2O = [(CO3)(ClO)(H2O)n]•1− + 4H+ + 4e−
Cl− + CO2 + (2 + n)H2O = [(HCO3)(ClO)(H2O)n]•1− + 3H+ + 4e−
Cl− + Na+ + 2CO2 + (4 + n + m)H2O = [(CO3)(NaO)(H2O)n]•1− + [(CO3)(ClO)(H2O)m]•1− + 8H+ + 6e−
Cl− + Na+ + 2CO2 + (4 + n + m)H2O = [(HCO3)(NaO)(H2O)n]•1−+ [(HCO3)(ClO)(H2O)m]•1−+6H+ + 6e−
5.2.3. Reaction Quotient Associated with Desalination in Oxygenated or Acid Oxygenated Water
5.2.4. Secondary Reaction Associated with Oxygenation by Air or Oxygen
2OH− = H2O2 + 2e−
OH− + H+ = H2O
5.2.5. Adverse Impact of CO2 Demonstrated by ZVM TP
CO2 + H2O = CO32− + 2H+
5.3. Primary Desalination Mechanism Associated with ZVM TPA
5.4. Initial Trial: E143
- Achieved Qe(apparent) = 0.738 gNaCl·g−1 Fe0; Qe(after adjustment for water consumption) = 1.735 gNaCl·g−1 Fe0;
- Processed 2.4 L water·g−1 Fe0;
- Processed 59.4 L feed water (3.96 gNaCl·L−1 average salinity) to produce 48.28 L product water (2.28 gNaCl·L−1).
- Demonstrated a partial desalination cost of $0.9 m−3 feed water (for a Fe0 cost of $400 t−1).
- Product water pH > feed water pH when the CO2 content of the feed gas is 0%, otherwise Product water pH < feed water pH.
- NaCl removal decreased with increasing usage of the ZVM TPA.
- NaCl removal is associated with relatively high water consumption.
- The NaCl removal reaction is partially reversible. Some trials (Table C8) demonstrated increases in product water salinity when one, or more, of the water Eh, pH and/or temperature changed;
- The product water composition is stable and initial pH values in the range 5–6, are changed after 6 weeks to pH values in the range 6–7 and remained stable at that level thereafter.
5.4.1. Munsell Analysis
- 0.3 L (light orange colored; Munsell Parameters: hue = 5YR; chroma = 12; value = 7) water was recovered from the manifold (on recovery: Eh = 0.222 V; pH = 5.31; EC = 44.75 mS·cm−1; after 4 weeks storage: Eh = 0.275 V; pH = 6.47; EC = 50.17 mS·cm−1).
- 0.1 L (deep orange colored; Munsell Parameters: hue = 5YR; chroma = 8; value = 4) water was recovered from the cartridge (on recovery: Eh = 0.263 V; pH = 5.15; EC = 425.4 mS·cm−1 (3.92 M NaCl·L−1); after 4 weeks storage: Eh = 0.225 V; pH = 6.48; EC = 350.37 mS·cm−1).
5.4.2. Halocline Analysis
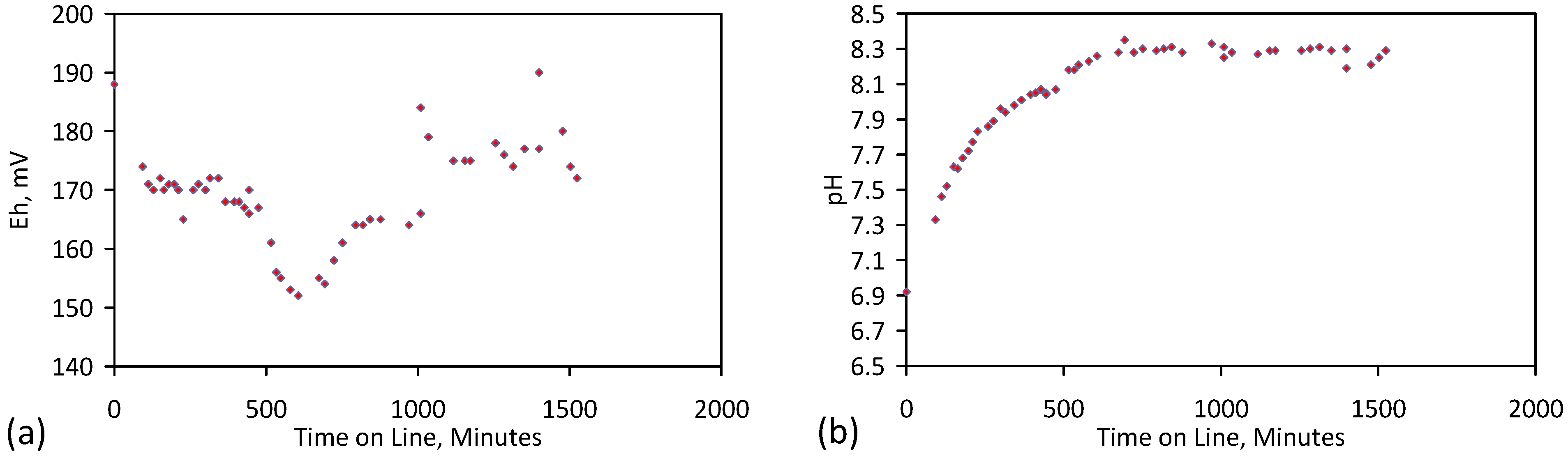
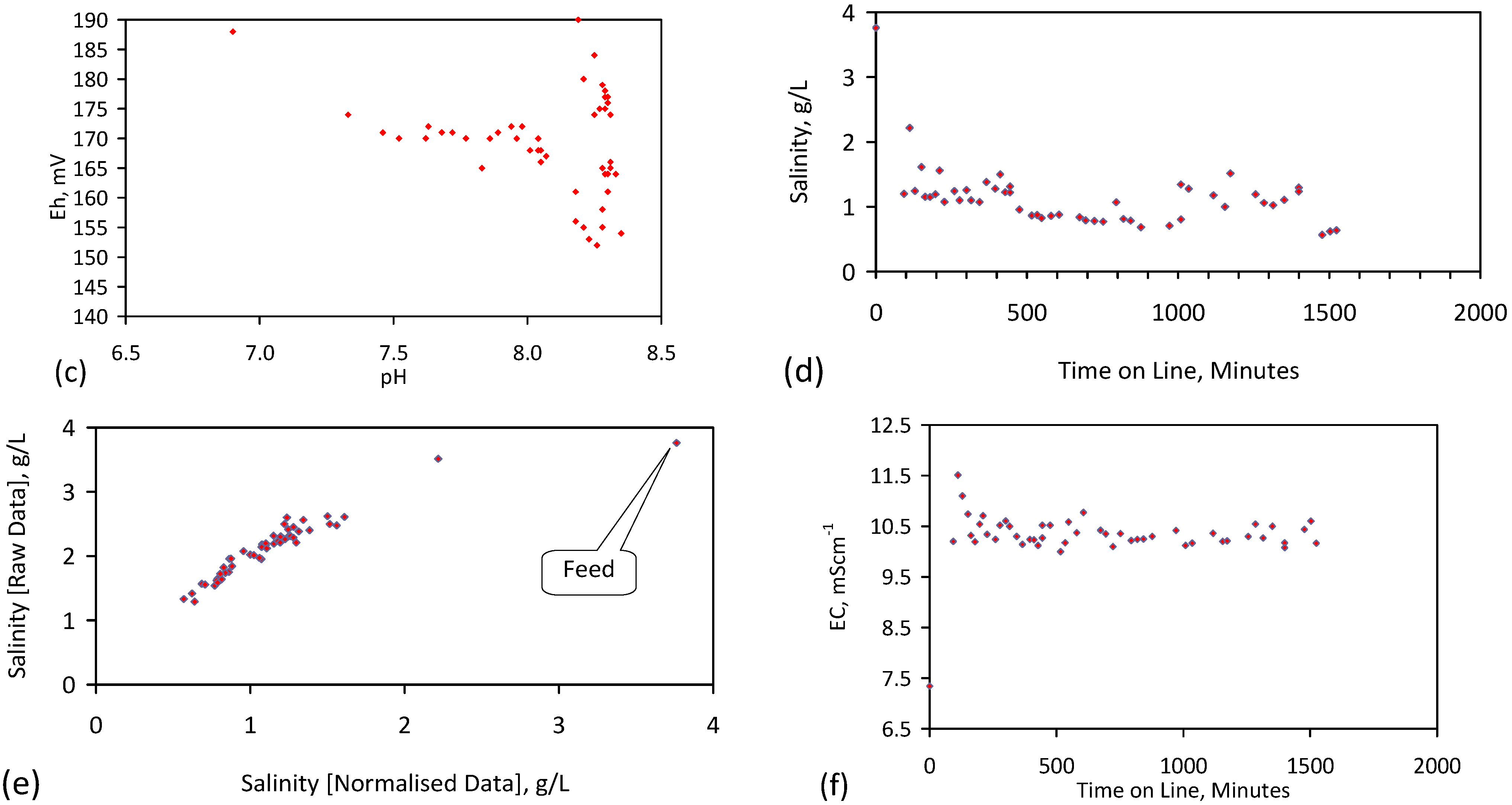
5.5. Long Duration Trial: E144
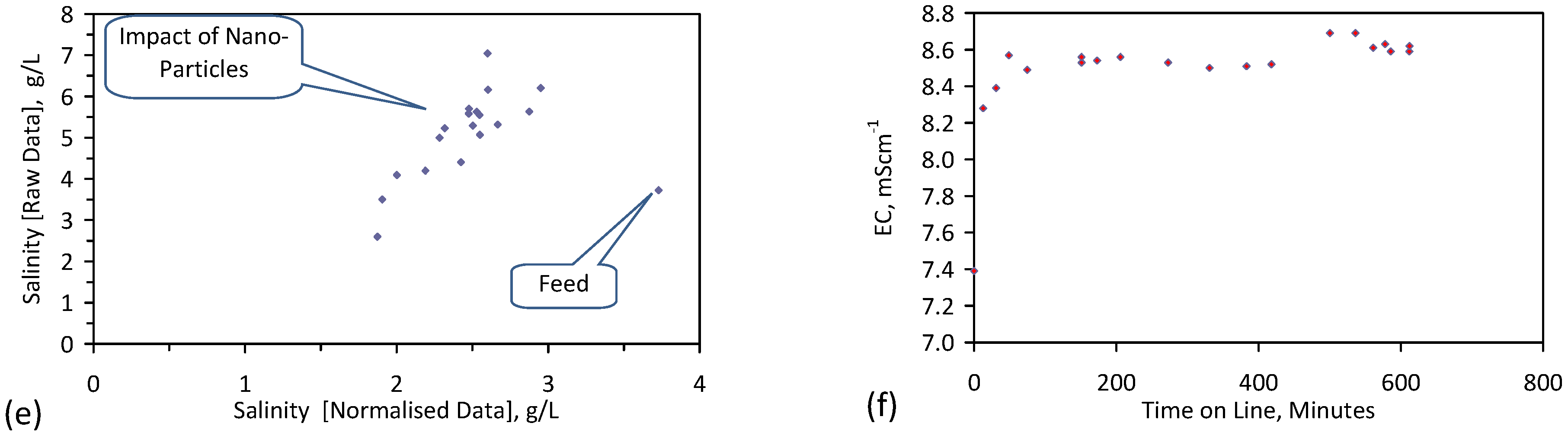
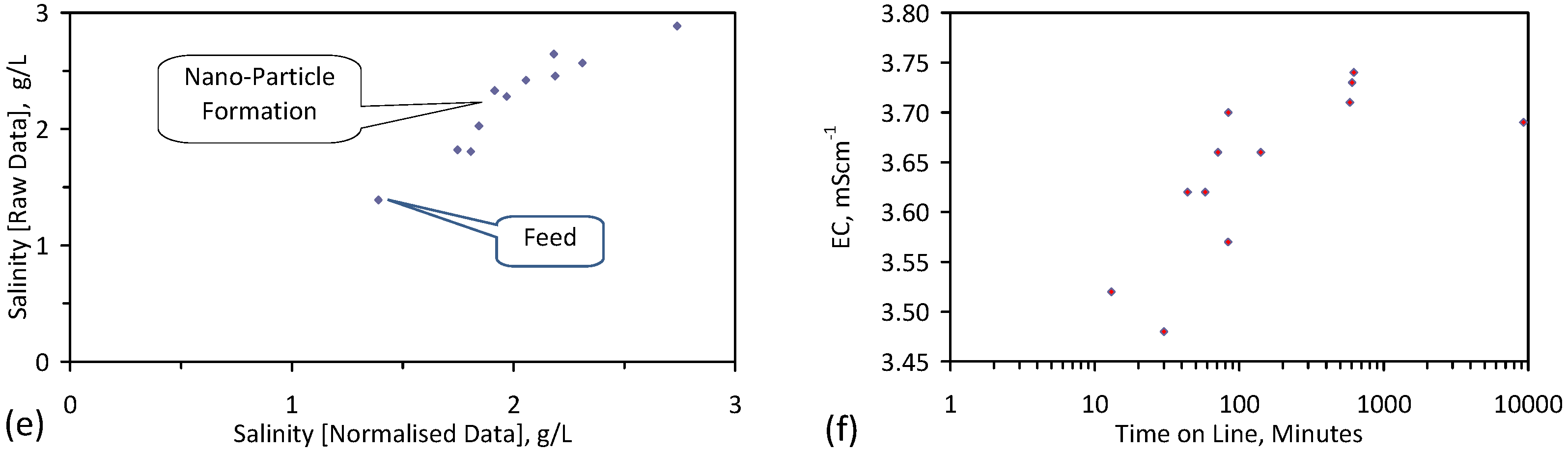
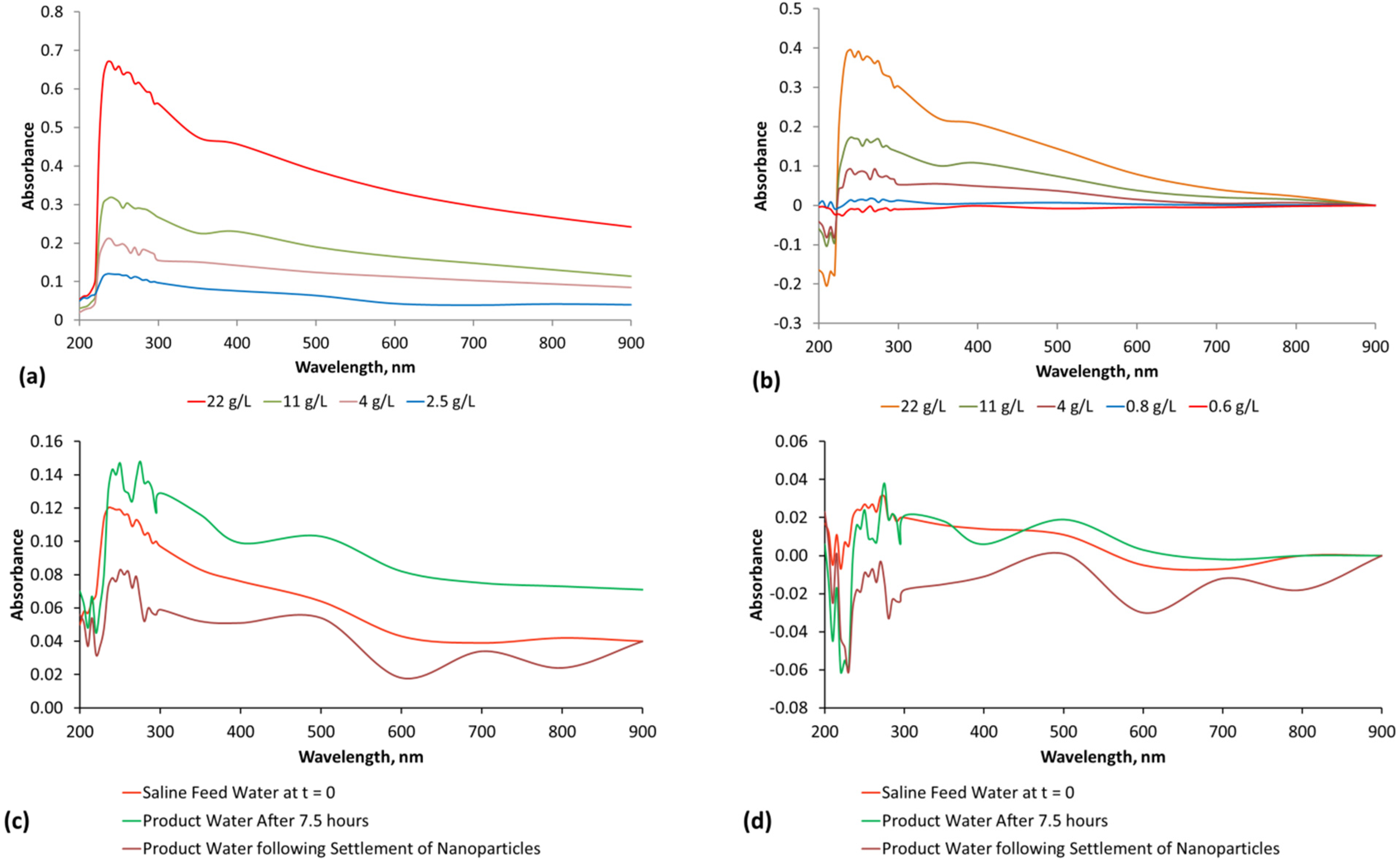
- Product water salinity based on EC overestimated the actual water salinity (calculated from UV-visible spectrometry) due to the formation of nano-particles in the water;
- Desalination using ZVM TPA has an optimum treatment time within the range 1 to 24 h, though the amount of NaCl removed increases with time.
- ZVM TPA can be reused
- 32.4 L of feed water (3.02 gNaCl·L−1) produced 24.15 L of product water (1.56 gNaCl·L−1)
- An achieved Qe(apparent) of 0.932 gNaCl·g−1 Fe0; Qe(after adjustment for water consumption) = 1.25 gNaCl·g−1 Fe0;
- Processimg of 0.63 L water·g−1 Fe0;
5.6. Initial Upscaled Trial: E145
5.7. Upscaled Trial: E146
5.8. Cost of ZVM TPA Desalination
6. Cations and Anions (Pollutants) Removed during Desalination by ZVM TP and ZVM TPA
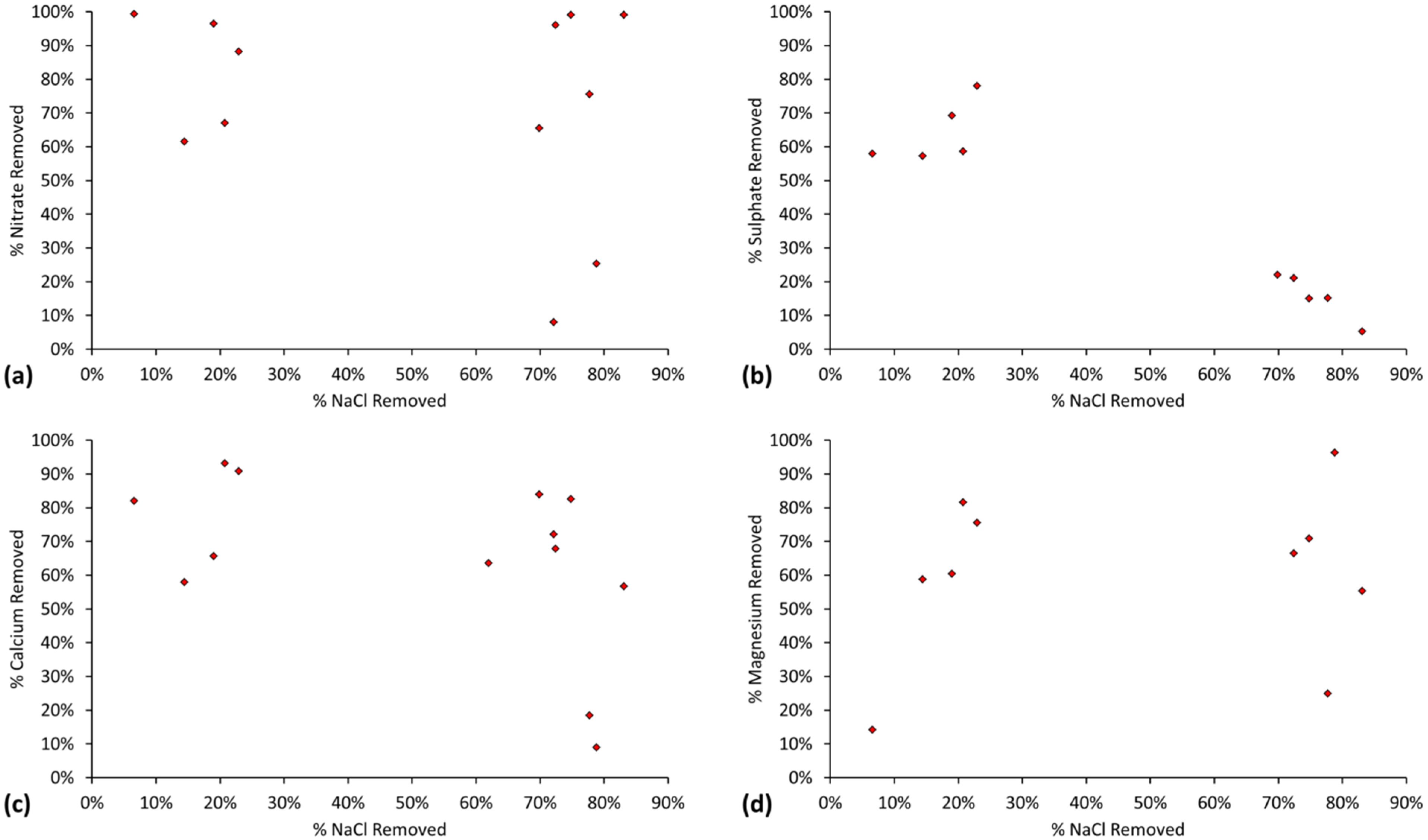
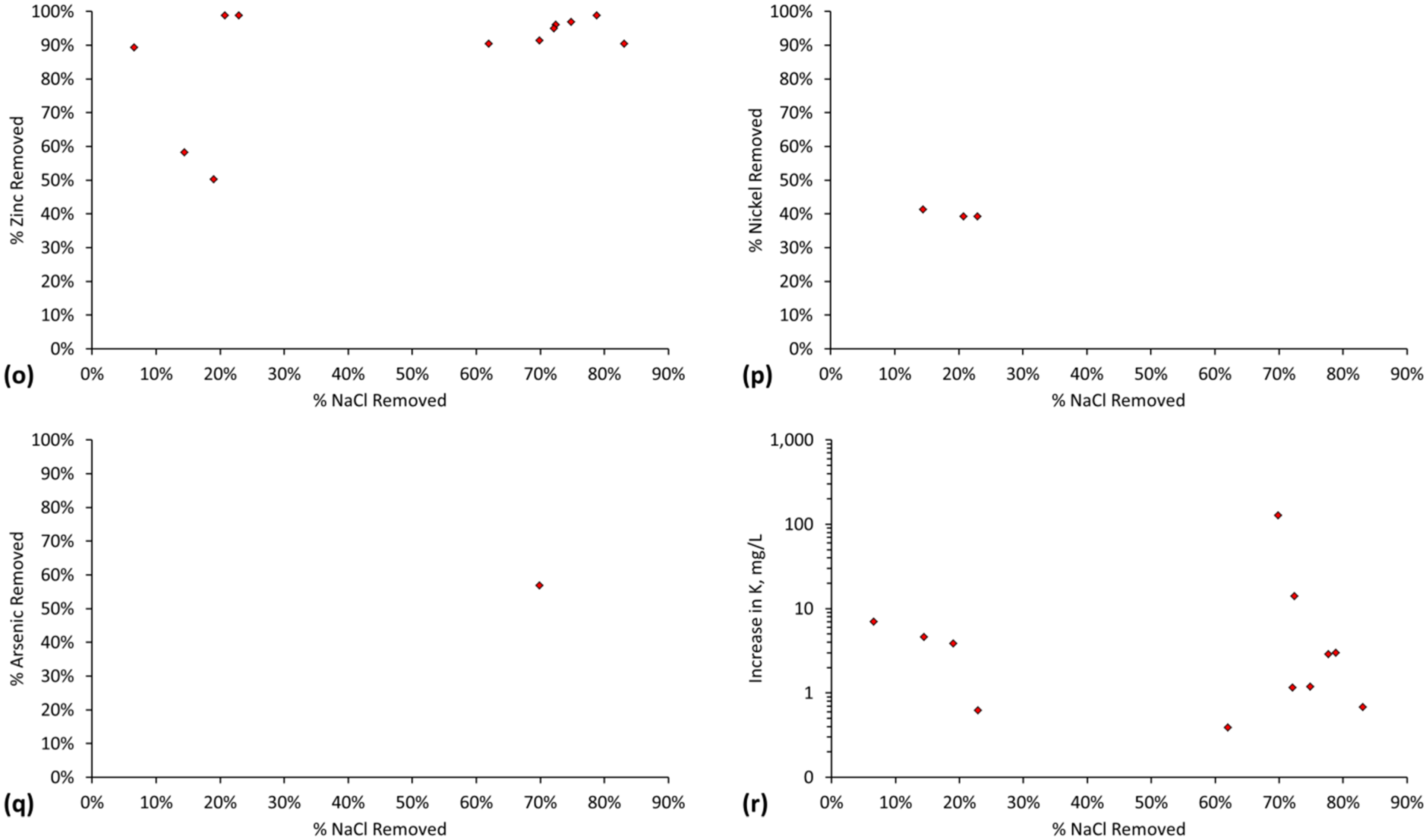
6.1. Cations and Anions (Pollutants) Removed during Desalination Using ZVM TP
6.2. Impact of Sulphates: ZVM TP and ZVM TPA
6.3. Impact of Sulphides: ZVM TP and ZVM TPA
6.4. Impact of Bicarbonates: ZVM TP and ZVM TPA
6.4.1. Trial E146: ZVM TPA
6.4.2. Trials PS15 and PS16: ZVM TP
- ZVM TP can be reused if it is held in an oxygen free environment.
- In an oxygen free environment, ZVM TP can increase the desalination rate [Constant D] to a value in the range 0.0128–0.0462. This compares with a [Constant D] of 0.004–0.011 using ZVM TP from the same batch in an oxygenated environment.
- Desalination using ZVM TP can occur when the water contains bicarbonate or carbonate ions.
| Water | Feed and Recovered Product Waters | pH | EC, mS·cm−1 | Storage Period, Years | Product Water Following Storage | pH | EC, mS·cm−1 | HCO3−, g·L−1 |
|---|---|---|---|---|---|---|---|---|
| Eh, V | Eh, V | |||||||
| PS15 | ||||||||
| Feed | 0.207 | 6.14 | 57.49 | - | - | - | - | - |
| Product | 0.094 | 9.49 | 31.12 | 1.11 | 0.220 | 8.9 | 35.70 | >10 |
| PS16 | ||||||||
| Feed | 0.207 | 6.14 | 78.16 | - | - | - | - | - |
| Product | −0.111 | 10.26 | 1.09 | 0.58 | 0.109 | 9.18 | 1.25 | 1.14 |
6.4.3. Desalination in an Alkaline Bicarbonate Saturated Anoxic Environment: ZVM TP
6.4.4. Impact of CaCO3 or MgCO3 in the Water: ZVM TP and ZVM TPA
6.4.5. Impact of Chlorinated Organic Compounds in the Water: ZVM TP and ZVM TPA
6.4.6. Bicarbonate/Carbonate Buffers: ZVM TP and ZVM TPA
6.4.7. Acid Bicarbonate Buffer: ZVM TP and ZVM TPA
- Table 5: HCO3−/pCO2 ratio = 0.004266:1; HCO3−/H2CO3 ratio = 0.1022; Feed water = 3 g·L−1; Product water = 1.88 g·L−1. Desalination time required = 16 h;
- E143b: HCO3−/pCO2 ratio = 0.1023:1; HCO3−/H2CO3 ratio = 2.74; Feed water = 4.44 g·L−1; Product water = 1.67 g·L−1. Desalination time required = 16.5 h;
- E143c: HCO3−/pCO2 ratio = 0.0109:1; HCO3−/H2CO3 ratio = 0.299; Feed water = 4.81 g·L−1; Product water = 2.11 g·L−1. Desalination time required = 15 h;
- E143d: HCO3−/pCO2 ratio = 0.0135:1; HCO3−/H2CO3 ratio = 0.343; Feed water = 5.37 g·L−1; Product water = 2.99 g·L−1. Desalination time required = 15.5 h;
- E143e: HCO3−/pCO2 ratio = 0.0229:1; HCO3−/H2CO3 ratio = 0.594; Feed water = 6.48 g·L−1; Product water = 4.95 g·L−1. Desalination time required = 15.5 h;
- 13th Reuse (Figure C33): HCO3−/pCO2 ratio = 0.00195:1; HCO3−/H2CO3 ratio = 0.063; Feed water = 1 g·L−1; Product water = 0.35 g·L−1. Desalination time required ≤ 3 h;
- 14th Reuse (Figure C34): HCO3−/pCO2 ratio = 0.00219:1; HCO3−/H2CO3 ratio = 0.072; Feed water = 1.1 g·L−1; Product water = 0.7 g·L−1. Desalination time required ≤ 4 h;
- 15th Reuse (Figure C35): HCO3−/pCO2 ratio = 0.00275:1; Feed water = 2.05 g·L−1; Product water = 1.6 g·L−1. Desalination time required ≤ 9 h;
- 16th Reuse (Figure C36): HCO3−/pCO2 ratio = 0.00245:1; Feed water = 5.83 g·L−1; Product water = 1.8 g·L−1. Desalination time required ≤ 17 h;
6.4.8. Alkali Bicarbonate Buffer: ZVM TP
6.5. Desalination of Water Containing Sulphates and Bicarbonates: ZVM TP and ZVM TPA
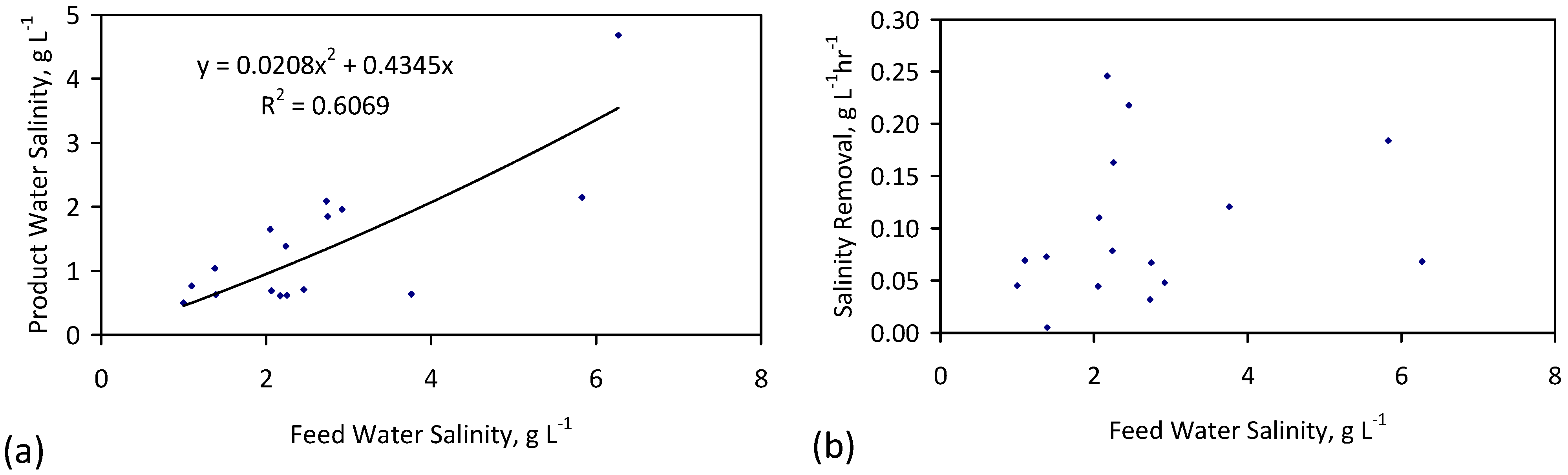
7. Desalination Economics
- (1)
- 2.65 gNaCl·L−1 can be desalinated to:
- (a)
- ZVM TPA—multiple use:1.55 g·L−1 (over 1–24 h, Trial E146) and 6 gNaCl·L−1 reduced (Trial E146) to 3.35 gNaCl·L−1) for a cost of less than $0.009–$0.026 m−3. This cost is based on the demonstrated 18 times reuse of the same ZVM TPA charge. The number of times a ZVM TPA charge can be reused needs to be demonstrated in field operation, but may be in the range 20–100 times. This would allow the desalination cost to fall within the range $0.002–$0.024 m−3.
- (b)
- ZVM TP—multiple use:0.791 g·L−1 (over 11–124 h, Trials CSD1a–CSD1d) for a cost of $2.28–$6.3 m−3. This cost is further reduced if the used ZVM TP is sold for sulphur removal, or is reprocessed for reuse. The number of times a ZVM TP charge can be reused needs to be demonstrated in field operation, but may be in the range 5–20 times. This allows the desalination cost to fall within the range $0.4–$6.1 m−3. ZVM TP can be reused for sulphur removal from natural gas. This reuse application can result in a residual ZVM TP value which is greater than the cost of ZVM TP purchase and use as a desalination agent.
- (c)
- ZVM TP—single use: 0.2 g·L−1 (over <15 h, Trial CSD1) for a cost of $4.55–$12.6 m−3; Reuse of the ZVM TP for sulphur removal can reduce the cost of producing potable water to <$0.0 m−3, depending on the resale value realised for the used ZVM TP.
- (2)
- ZVM TP—single use: 11.05 gNaCl·L−1 can be desalinated to 3.34 g·L−1 (over 126 days, Trial ST3b) for a cost of $5.6–$22.48 m−3. Reuse of the ZVM TP for sulphur removal can potentially reduce the cost of producing partially desalinated water to <$0.0 m−3, depending on the resale value realised for the used ZVM TP.
- (3)
- ZVM TP—single use: 38.6 gNaCl·L−1 can be desalinated to 0.55 g·L−1 (over 210 days, Trial PS16) for a cost of $67.6–$187.2 m−3. Reuse of the ZVM TP for sulphur removal can potentially reduce the cost of producing partially desalinated water to <$3.0 m−3, depending on the resale value realised for the used ZVM TP.
- Partial desalination of irrigation water for arable crops;
- Partial desalination of livestock feed water;
- Partial desalination of water for municipal activities and grey water applications;
- Emergency water for people and livestock in areas affected by natural or anthropogenic disasters;
- Desalination of saline water in impoundments (including flowback water from shale gas wells and reject brine from RO desalination plants);
7.1. Irrigation Market
Ratios Used to Assess Irrigation Water Quality
7.2. Cost of Irrigation Water
7.2.1. ZVM TP
7.2.2. ZVM TPA
7.3. Potential Use of ZVM TPA to Boost Rice Yields
| Item | No Desalination | Partial Desalination | ||
|---|---|---|---|---|
| Irrigation, m3·ha−1 | 5000 | 10,000 | 5000 | 10,000 |
| Feed Water salinity, g·L−1 | 6 | 6 | 6 | 6 |
| Irrigation Water Salinity, g·L−1 | 6 | 6 | 3.35 | 3.35 |
| Rice Yield, t·ha−1 | - | - | - | - |
| Low | 6 | 6 | 6 | 6 |
| High | 24 | 24 | 24 | 24 |
| Yield Decrement | 100% | 100% | 40% | 40% |
| Actual rice yield, t·ha−1 | - | - | - | - |
| Low | 0 | 0 | 3.6 | 3.6 |
| High | 0 | 0 | 14.4 | 14.4 |
| Production Cost, $·ha−1 | - | - | - | - |
| Low | 300 | 300 | 345 | 390 |
| High | 400 | 400 | 500 | 620 |
| Rice price, $·t−1 | - | - | - | - |
| Low | 300 | 300 | 300 | 300 |
| High | 400 | 400 | 400 | 400 |
| Net Sales Revenue, $·ha−1 | - | - | - | - |
| Low | −400 | −400 | 580 | 460 |
| High | −300 | −300 | 5415 | 5370 |
7.4. Economic Implications of Corrosion Scale
Fe(OH)2 = Fe(OOH) + H+ + e−
Species formed or removed = (1/MW(species) × F/en × en/Moles Species)/Charge
en = Moles e− associated with removal of 1 mole of the contaminant species
7.5. Applicability to Specific Sites
8. Conclusions
- pH range = 2–12,
- Electrical conductivity range of the feed water = 2–78 mS·cm−1,
- Salinity range of the feed water = 1–39 gNaCl·L−1,
- Carbonate range in the feed water = 10 mg·L−1 to >10 g·L−1; sulphate ≤ 10.2 mg·L−1.
- Temperature range considered = −8 to 50 °C.
- No external energy, or
- Pressurized (<0.1 MPa) air, or
- Pressurized (<0.1 MPa) acidic gas (CO2), or
- Pressurized (<0.1 MPa) anoxic gas (N2), or
- Pressurized (<0.1 MPa) anoxic, acidic, reducing gas (H2 + CO + CO2 + CH4 + N2).
- Increase with increasing temperature;
- Increase with decreasing particle size (and increased particle BET surface area);
- Are optimised using Pw = 0.3–20 gFe0·L−1;
- Are optimised by maintaining the ZVM TP/TPA in a static water body, while allowing the overlying water body to be circulating and pressurised using a reducing gas, or an oxidising gas.
Acknowledgments
Conflicts of Interest
Appendix A. Microbiota, Cations and Anions that Can Be Removed by ZVM
- (1)
- cations (M) present as: ions (Mn+), hydroxides [M(OH)xn+/−], peroxides [M(OOH)n+/−], oxides [MyOxn+/−], and hydrides [MyHxn+/−], M is one or more of: Ac, Ag, Al, Am, As. Au, B, Ba, Be, Bi, Ca, Cd, Ce, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, Fe, Ga, Gd, Ge, Hf, Hg, Ho, In, Ir, K, La, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Np, Os, P, Pa, Po, Pr, Pt, Pu, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Tb, Tc, Te, Th, Ti, Tl, Tm, U, V, Y, Yb, Zn, Zr, and nitrogen based cations, e.g., NH4+. These ions are removed by precipitation, or by transfer into another ionic species.
- (2)
- anions (A) present as:
- (a)
- ions held in a a monoatomic or polyatomic form: H, F, Cl, Br, I, At, O, S, Se, Te, N, P, As, Sb, C, Si, Ge, B;
- (b)
- oxides, e.g., arsenates (AsO43−), acetates (C2H3O2−), bicarbonates (HCO3−), carbonates (CO32−), chlorites (ClO2−), chlorates (ClO3−), chromates (CrO42−), hypochlorites (ClO−), dichromates (Cr2O72−), hydrogen phosphates (HPO42−), nitrates (NO3−), nitrites (NO2−), N2O22−, perchlorates (ClO4−), permanganates (MnO4−), oxalates (C2O42−), phosphates (PO43−), sulphates (SO42−), sulphites (SO32−), thiosulphates (S2O32−), S2O62−, S2O82−, S4O62−, HS2O4−, or [Mz][On]y−;
- (c)
- nitrogen based anions, e.g., thiocyanates (CNS−), cyanides (CN−);
- (d)
- These ions are removed by precipitation, or by transfer into another ionic species.
- (3)
- Dissolved organic species:, e.g., chloromethane (CM), trichloromethane (TCM), dichloromethane (DCM), tetrachloromethane, perchloroethylene (PCE), trichloroethylene (TCE), dichloroethylene (DCE), vinyl chloride (VC), hexachloroethane, tetrachloroethane, trichloroethane, dichloroethane, chloropropane (and related species), chlorobutane (and related species), chlorobenzene, (and related species), ethylene dibromide (EDB), perchlorate, polychlorinated biphenyls (PCB’s), azo dyes, atrazine, cyclonite/hexogen (RDX), dinitrotoluene (DNT), nitrosodimethylamine (NDMA), nitrocellulose, tetramethylenetetranitramine (HMX), trinitrotoluene (TNT), disinfection by-products (DBP’s), fertilizers, pesticides, herbicides, fungicides, methyl tert-butyl ether (MTBE), aromatics, hydrocarbons, hormonal pollutants. These species are removed by reduction and fragmentation to one or more compounds or ions.
- (1)
- Alcaligenes eutrophus, Aspergillus versicolor, Bacillus cereuis, Bacillus subtilis var. niger, colliforms (e.g., Enterococcus faecium, Enterococcus faecilis), Cryptosporidium spp., Dehalococcoides spp., Daphnia magna, Dunaliella tertiolecta, Escherichia coli, Giardia spp., Hartmannella veriformis, Isochrysis galbbana, Klebsiella pneumoniae, Naeglaeria spp., Naeglaeria fowleri, Pseudokirchneriella subcapitata, Pseudomonas spp., Pseudomonas fluorescens, Pseudomonas aeruginosa, Salmonella typhimurium, Salmonella enterica, Salmonella paratyphi, Salmonella spp., Shigella spp., Staphylococcus aureus, Streptococci spp., Tetrahymena pyriformis, Thalassiorsria pseudonana, Vibrio parahaemolyticus, Vibrio cholerae,
- (2)
- Aichi virus, adenovirus 41, MS-2, Hepatitis A, norovirus, phiX174/FX174, rotavirus, T1, f2 virus, fecal coliforms, fecal streptococci, fungi, prions, viruses, protozoa, bacteria, algae.
Appendix B. Agricultural Significance of Partial Desalination

Appendix C. Trial Results
| Water | Cl | N(NO3) | S(SO4) | P(PO4) | F | N(NO2) | Product Water (% Feed Water) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |||||
| Fresh Spring Water | ||||||||||
| Base Water | 11.67 | 11.28 | 4.16 | <0.10 | 0.024 | 0.04 | - | |||
| PS4 | ||||||||||
| Feed Water | 6004.17 | 11.28 | 4.16 | <0.10 | 0.024 | 0.04 | - | |||
| Product Water | 1434.2 | 0.12 | 3.12 | <0.10 | 0.03 | 2.48 | 55.00% | |||
| PS5 | ||||||||||
| Feed Water | 4568.84 | 11.28 | 4.16 | <0.10 | 0.024 | 0.04 | - | |||
| Product Water | 1613.63 | 0.73 | 2.32 | <0.10 | <0.020 | 3.17 | 55.00% | |||
| PS11 | ||||||||||
| Feed Water | 2464.2 | 1.38 | 1.7 | 0.229 | <0.020 | 2.49 | - | |||
| Product Water | 1554.33 | 1.53 | 2.78 | <0.10 | 0.046 | 2.7 | 92.80% | |||
| PS14 | ||||||||||
| Feed Water | 1914.05 | 10.81 | 4.46 | <0.10 | 0.024 | 3 | - | |||
| Product Water | 1822.42 | 0.13 | 4.84 | <0.10 | 0.024 | 3.22 | 78.30% | |||
| ST3b | ||||||||||
| Feed Water | 6519 | 8.41 | 4.5 | <0.010 | 0.023 | 2.2 | - | |||
| Product Water | 2060 | 0.16 | 1.32 | <0.010 | 0.017 | 1.31 | 75.00% | |||
| ST3f | ||||||||||
| Feed Water | 6519 | 8.41 | 4.5 | <0.010 | 0.023 | 2.2 | - | |||
| Product Water | 2066 | 3.69 | 2.48 | <0.010 | 0.025 | 2.08 | 75.00% | |||
| ST6d | ||||||||||
| Feed Water | 5457.6 | 11.28 | 4.16 | <0.10 | 0.024 | 0.04 | - | |||
| Product Water | 5269.3 | 3.33 | 4.27 | <0.10 | <0.020 | 2.34 | 82.60% | |||
| ST8e | ||||||||||
| Feed Water | 3983.85 | 10.04 | 4.72 | <0.10 | <0.020 | 0.29 | - | |||
| Product Water | 3631.88 | 0.09 | 4.74 | <0.10 | 0.034 | 0.02 | 94.20% | |||
| MT1b | ||||||||||
| Feed Water | 4769 | 11.03 | 4.86 | <0.010 | 0.015 | 1.06 | - | |||
| Product Water | 3937 | 4.61 | 4.58 | <0.010 | 0.067 | 2.21 | 82.60% | |||
| MT2b | ||||||||||
| Feed Water | 4037.39 | 10.5 | 4.51 | <0.10 | <0.020 | <0.020 | - | |||
| Product Water | 3700.83 | 0.53 | 4.49 | <0.10 | <0.020 | 0.08 | 79.20% | |||
| MT3d | ||||||||||
| Feed Water | 2092.1 | 10.81 | 4.55 | 0.436 | <0.020 | 2.4 | - | |||
| Product Water | 1982.02 | 9.77 | 7.35 | <0.10 | 0.079 | 2.43 | 82.60% | |||
| MT4d | ||||||||||
| Feed Water | 2464.2 | 1.38 | 1.7 | 0.229 | <0.020 | 2.49 | - | |||
| Product Water | 1873.3 | 1.39 | 5.5 | <0.10 | 0.041 | 2.42 | 91.30% | |||
| PS15 | ||||||||||
| Feed Water | 16,243.98 | 27.55 | 10.15 | <0.10 | 0.06 | 0.09 | - | |||
| Product Water | 10,076.22 | 3.94 | 6.04 | <0.10 | 0.13 | 2.47 | 66.80% | |||
| PS16 | ||||||||||
| Feed Water | 23,162.04 | 26.03 | 9.59 | <0.10 | 0.06 | 0.09 | - | |||
| Product Water | 348.03 | 0.64 | 1.52 | 0.15 | 0.03 | 0.20 | 26.50% | |||
| Water | K | Ca | Mg | Na | Al | Fe | Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/L | µg/L | µg/L | µg/L | ||||
| Fresh Spring Water | ||||||||||
| Base Water | 1.69 | 32.91 | 10.54 | 6.32 | <150.0 | <30.0 | 1.70 | |||
| PS4 | ||||||||||
| Feed Water | 1.69 | 32.91 | 10.54 | 3995.83 | <150.0 | <30.0 | 1.70 | |||
| Product Water | 15.71 | 10.79 | 16.45 | 899.63 | <150.0 | 47.6 | 2.10 | |||
| PS5 | ||||||||||
| Feed Water | 1.69 | 32.91 | 10.54 | 3039.15 | <150.0 | <30.0 | 1.70 | |||
| Product Water | 10.11 | 20.52 | 7.58 | 1009.59 | 173.4 | <30.0 | 5.50 | |||
| PS11 | ||||||||||
| Feed Water | 1.80 | 9.27 | 2.13 | 1522.07 | <150.0 | <30.0 | 2.80 | |||
| Product Water | 2.37 | 3.64 | 44.29 | 1107.61 | <150.0 | <30.0 | 2.10 | |||
| PS14 | ||||||||||
| Feed Water | 2.67 | 31.13 | 9.6 | 1245.32 | <150.0 | <30.0 | 2.80 | |||
| Product Water | 4.92 | 6.94 | 3.58 | 1197.14 | <150.0 | <30.0 | 1.80 | |||
| ST3b | ||||||||||
| Feed Water | 3.49 | 26.65 | 7.65 | 4527 | <150.0 | <30.0 | 8.00 | |||
| Product Water | 5.48 | 3.26 | 2.49 | 1309 | <150.0 | <30.0 | 13.00 | |||
| ST3f | ||||||||||
| Feed Water | 3.49 | 26.65 | 7.65 | 4527 | <150.0 | <30.0 | 8.00 | |||
| Product Water | 7.76 | 17.63 | 7.38 | 1293 | <150.0 | <30.0 | 5.90 | |||
| ST6d | ||||||||||
| Feed Water | 1.69 | 32.91 | 10.54 | 3630.53 | <150.0 | <30.0 | 32.90 | |||
| Product Water | 5.53 | 32.48 | 9.58 | 3275.18 | <150.0 | <30.0 | 69.90 | |||
| ST8e | ||||||||||
| Feed Water | 5.89 | 31.26 | 9.5 | 2390.85 | <150.0 | 34.7 | 14.20 | |||
| Product Water | 6.97 | 14.36 | 4.5 | 2175.29 | <150.0 | <30.0 | 3.30 | |||
| MT1b | ||||||||||
| Feed Water | 2.30 | 31.16 | 9.54 | 3052 | <150.0 | 42.1 | 2.90 | |||
| Product Water | 156.80 | 6.08 | 49.23 | 2521 | 377 | <30.0 | 2.40 | |||
| MT2b | ||||||||||
| Feed Water | 4.18 | 30.17 | 9.09 | 2434.31 | <150.0 | <30.0 | 4.30 | |||
| Product Water | 23.05 | 12.27 | 3.85 | 2198.69 | 2085.2 | <30.0 | 2.90 | |||
| MT3d | ||||||||||
| Feed Water | 2.80 | 30.55 | 9.42 | 1370.7 | <150.0 | <30.0 | 5.10 | |||
| Product Water | 7.04 | 33.65 | 0.41 | 1319.14 | 10,720 | <30.0 | 1.40 | |||
| MT4d | ||||||||||
| Feed Water | 1.80 | 9.27 | 2.13 | 1522.07 | <150.0 | <30.0 | 2.80 | |||
| Product Water | 3.24 | 2.84 | 35.75 | 1273.08 | <150.0 | <30.0 | 2.00 | |||
| PS15 | ||||||||||
| Feed Water | 4087.42 | 80.39 | 25.75 | 8406.66 | <150.0 | <30.0 | 4.15 | |||
| Product Water | 3266.23 | 25.51 | 6.23 | 4197.07 | <150.0 | <30.0 | 23.90 | |||
| PS16 | ||||||||||
| Feed Water | 3.89 | 75.94 | 24.33 | 15,411.17 | <150.0 | <30.0 | 3.92 | |||
| Product Water | 5.24 | 7.46 | 1.82 | 223.21 | 288.8 | <30.0 | 4.00 | |||
| Water | P | S | B | Ba | Cd | Co | Cr |
|---|---|---|---|---|---|---|---|
| mg/L | mg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| Fresh Spring Water | |||||||
| Base Water | <0.005 | 4.31 | 29.40 | 135.60 | <0.2 | <0.2 | <2.0 |
| PS4 | |||||||
| Feed Water | <0.005 | 4.31 | 29.40 | 135.60 | <0.2 | <0.2 | <2.0 |
| Product Water | 0.01 | 3.3 | 12.00 | 15.10 | <0.2 | 1.30 | <2.0 |
| PS5 | |||||||
| Feed Water | <0.005 | 4.31 | 29.40 | 135.60 | <0.2 | <0.2 | <2.0 |
| Product Water | 0.01 | 2.55 | <10 | <15 | <0.2 | 1.50 | <2.0 |
| PS11 | |||||||
| Feed Water | 0.25 | 1.89 | <10 | 16.40 | <0.2 | 1.80 | <2.0 |
| Product Water | 0.04 | 3.15 | 25.80 | <15 | <0.2 | 1.40 | <2.0 |
| PS14 | |||||||
| Feed Water | 0.03 | 4.51 | 22.20 | 117.10 | <0.2 | 1.50 | <2.0 |
| Product Water | 0.01 | 4.8 | <10 | <15 | <0.2 | 1.50 | <2.0 |
| ST3b | |||||||
| Feed Water | 0.07 | 4.59 | 14.90 | 103.10 | 0.9 | 3.30 | <2.0 |
| Product Water | 0.01 | 1.56 | 59.40 | <15.0 | 1.2 | 1.20 | <2.0 |
| ST3f | |||||||
| Feed Water | 0.07 | 4.59 | 14.90 | 103.10 | 0.9 | 3.30 | <2.0 |
| Product Water | 0.02 | 2.63 | 17.50 | <15.0 | 1.0 | 1.60 | <2.0 |
| ST6d | |||||||
| Feed Water | <0.005 | 4.31 | 29.40 | 135.60 | <0.2 | <0.2 | <2.0 |
| Product Water | 0.01 | 4.31 | <10 | 68.50 | <0.2 | 3.90 | <2.0 |
| ST8e | |||||||
| Feed Water | 0.01 | 4.8 | 28.90 | 118.70 | <0.2 | 2.20 | <2.0 |
| Product Water | 0.01 | 4.9 | 34.60 | <15 | <0.2 | 1.10 | <2.0 |
| MT1b | |||||||
| Feed Water | 0.04 | 4.79 | 20.80 | 102.80 | 0.4 | 1.30 | <2.0 |
| Product Water | 0.01 | 4.79 | 70.40 | <15.0 | 0.4 | 1.60 | <2.0 |
| MT2b | |||||||
| Feed Water | 0.02 | 4.66 | 15.80 | 115.40 | <0.2 | 2.40 | <2.0 |
| Product Water | 0.01 | 4.68 | 11.30 | <15 | <0.2 | 2.20 | <2.0 |
| MT3d | |||||||
| Feed Water | 0.47 | 4.66 | 36.00 | 118.90 | 0.3 | 0.80 | <2.0 |
| Product Water | 0.03 | 7.55 | 22.30 | <15 | <0.2 | 1.80 | <2.0 |
| MT4d | |||||||
| Feed Water | 0.25 | 1.89 | <10 | 16.40 | <0.2 | 1.80 | <2.0 |
| Product Water | 0.02 | 6.02 | 97.30 | <15 | <0.2 | 2.40 | <2.0 |
| PS15 | |||||||
| Feed Water | <0.005 | 10.52 | 71.82 | 331.20 | <0.2 | <0.2 | <2.0 |
| Product Water | 0.01 | 6.12 | 38.10 | 55.20 | 10.2 | 3.60 | <2.0 |
| PS16 | |||||||
| Feed Water | <0.005 | 9.94 | 67.80 | 312.90 | <0.2 | <0.2 | <2.0 |
| Product Water | <0.005 | 3.74 | 109.60 | <15 | <0.2 | 0.20 | <2.0 |
| Water | Cu | Ni | Pb | Si | Sr | Zn | As |
|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/L | mg/L | µg/L | µg/L | µg/L | |
| Fresh Spring Water | |||||||
| Base Water | 77.7 | <3 | <10 | 5.21 | 144.9 | 37.4 | |
| PS4 | |||||||
| Feed Water | 77.7 | <3 | <10 | 5.21 | 144.9 | 37.4 | |
| Product Water | <20 | <3 | <10 | 0.05 | 76 | <4 | |
| PS5 | |||||||
| Feed Water | 77.7 | <3 | <10 | 5.21 | 144.9 | 37.4 | |
| Product Water | 54.3 | <3 | <10 | 0.09 | 54.2 | 33.8 | |
| PS11 | |||||||
| Feed Water | <20 | <3 | <10 | 1.43 | 28.5 | 137.5 | |
| Product Water | <20 | <3 | <10 | 0.9 | 4.3 | 14.1 | |
| PS14 | |||||||
| Feed Water | 122.1 | <3 | <10 | 4.77 | 136.1 | 125.6 | |
| Product Water | <20 | <3 | <10 | 0.04 | 54 | <4 | |
| ST3b | |||||||
| Feed Water | 45.1 | 3.7 | <10 | 3.99 | 112.4 | 337.6 | 2.3 |
| Product Water | <20.0 | <3.0 | <10 | 0.29 | 15.6 | <4.00 | 5.4 |
| ST3f | |||||||
| Feed Water | 45.1 | 3.7 | <10 | 3.99 | 112.4 | 337.6 | 2.3 |
| Product Water | <20.0 | <3.0 | <10 | 0.62 | 103.4 | <4.00 | 5.3 |
| ST6d | |||||||
| Feed Water | 77.7 | <3 | <10 | 5.21 | 144.9 | 37.4 | - |
| Product Water | 736.5 | <3 | <10 | 0.41 | 134.6 | 68.2 | - |
| ST8e | |||||||
| Feed Water | 41 | <3 | <10 | 4.89 | 138 | 286.9 | - |
| Product Water | <20 | <3 | <10 | 0.28 | 32.5 | 29 | - |
| MT1b | |||||||
| Feed Water | 52.9 | <3.0 | <10 | 4.92 | 139.9 | 170 | 4.8 |
| Product Water | <20.0 | <3.0 | <10 | 0.37 | 6.7 | 17.6 | 2.5 |
| MT2b | |||||||
| Feed Water | 93.1 | <3 | <10 | 4.67 | 133.3 | 101.1 | - |
| Product Water | <20 | <3 | <10 | 0.06 | 10.2 | 5.1 | - |
| MT3d | |||||||
| Feed Water | 74.9 | <3 | <10 | 4.83 | 134.3 | 342 | - |
| Product Water | <20 | <3 | <10 | 0.12 | 209.1 | <4 | - |
| MT4d | |||||||
| Feed Water | <20 | <3 | <10 | 1.43 | 28.5 | 137.5 | - |
| Product Water | <20 | <3 | <10 | 0.44 | 0.8 | 7.7 | - |
| PS15 | |||||||
| Feed Water | 189.80 | <3 | <10 | 12.73 | 353.9 | 91.36 | - |
| Product Water | 31.80 | <3 | <10 | 0.22 | 86.1 | 205.10 | - |
| PS16 | |||||||
| Feed Water | 179.30 | <3 | <10 | 12.03 | 334.4 | 86.30 | - |
| Product Water | 29.00 | <3 | <10 | 0.35 | 30.6 | 828.50 | - |
| Trial | Water Charge | Feed Water | Eh, mV | pH | EC, mS·cm−1 |
|---|---|---|---|---|---|
| L | NaCl, g·L−1 | ||||
| E143a | 5.4 | 5.74 | 0.2 | 5.66 | 11.15 |
| E143b | 5.4 | 4.44 | 0.193 | 6.61 | 8.63 |
| E143c | 5.4 | 4.81 | 0.199 | 6.11 | 9.35 |
| E143d | 5.4 | 5.37 | 0.214 | 5.59 | 10.43 |
| E143e | 5.4 | 6.48 | 0.22 | 5.78 | 12.59 |
| E143f | 5.4 | 1.85 | 0.166 | 5.65 | 3.59 |
| E143g | 5.4 | 2.59 | 0.188 | 6.46 | 5.04 |
| E143h | 5.4 | 2.63 | 0.176 | 6.46 | 5.04 |
| E143i | 5.4 | 2.96 | 0.185 | 6.18 | 5.75 |
| E143j | 5.4 | 3.33 | 0.17 | 5.18 | 6.63 |
| E143k | 5.4 | 3.35 | 0.219 | 6.19 | 6.63 |
| Trial | Eh, mV | pH | EC, mS·cm−1 | Storage | Stored Water Analysis | pH | EC, mS·cm−1 |
|---|---|---|---|---|---|---|---|
| Weeks | Eh, mV | ||||||
| E143a | 0.214 | 6.80 | 0.725 | 6 | 0.232 | 6.82 | 0.814 |
| E143b | 0.226 | 6.19 | 3.29 | 4 | 0.214 | 7.00 | 3.62 |
| E143c | 0.248 | 5.22 | 4.10 | 4 | 0.227 | 6.86 | 4.25 |
| E143d | 0.258 | 5.31 | 5.73 | 4 | 0.243 | 6.51 | 5.80 |
| E143e | 0.224 | 5.54 | 9.35 | 4 | 0.238 | 6.58 | 9.57 |
| E143f | 0.185 | 6.68 | 6.71 | 4 | 0.220 | 6.96 | 7.01 |
| E143g | 0.197 | 6.76 | 4.70 | 4 | 0.215 | 7.13 | 4.75 |
| E143h | 0.201 | 6.90 | 6.27 | 4 | 0.219 | 7.05 | 6.28 |
| E143i | 0.165 | 5.12 | 9.51 | 4 | 0.261 | 6.13 | 9.52 |
| E143j | 0.212 | 5.31 | 6.15 | 4 | 0.271 | 6.17 | 6.19 |
| E143k | 0.207 | 6.04 | 6.56 | 4 | 0.261 | 6.34 | 6.47 |
| Trial | Water Recovered | Water Consumed | Duration | Air Flow | CO2 Flow | Air Flow Rate | CO2 Flow Rate | Temperature |
|---|---|---|---|---|---|---|---|---|
| L | L | h | h | h | L·h−1 | L·h−1 | ||
| E143a | 4.60 | 0.80 | 9.00 | 9.00 | 0.00 | 13.2 | 3.3 | 5–12 °C |
| E143b | 4.60 | 0.80 | 8.33 | 5.05 | 3.28 | 13.2 | 3.3 | 5–10 °C |
| E143c | 4.70 | 0.70 | 4.37 | 2.56 | 1.81 | 13.2 | 3.3 | 5–10 °C |
| E143d | 4.80 | 0.60 | 4.02 | 1.76 | 2.26 | 13.2 | 3.3 | 5–10 °C |
| E143e | 4.70 | 0.70 | 3.87 | 1.71 | 2.16 | 13.2 | 3.3 | 5–10 °C |
| E143f | 4.65 | 0.75 | 2.70 | 2.70 | 0.00 | 13.2 | 3.3 | 5–10 °C |
| E143g | 4.50 | 0.90 | 3.08 | 3.08 | 0.00 | 13.2 | 3.3 | 5–10 °C |
| E143h | 3.90 | 1.50 | 14.30 | 14.30 | 0.00 | 13.2 | 3.3 | 5–10 °C |
| E143i | 4.00 | 1.40 | 8.90 | 4.50 | 4.40 | 13.2 | 3.3 | 5–10 °C |
| E143j | 3.90 | 1.50 | 7.50 | 2.20 | 5.30 | 13.2 | 3.3 | 5–10 °C |
| E143k | 3.90 | 1.50 | 3.58 | 2.58 | 1.00 | 13.2 | 3.3 | 5–10 °C |
| Trial | Product Water | Apparent NaCl Removed | Actual NaCl Removed | Cumulative NaCl Removed | Cumulative Water Processed | Cumulative |
|---|---|---|---|---|---|---|
| g·L−1 | g·L−1 | g·L−1 | g·L−1 | L | NaCl in Feed g·L−1 | |
| E143a | 0.064 | 5.68 | 5.686 | 5.69 | 5.4 | 5.74 |
| E143b | 1.514 | 2.93 | 3.154 | 8.84 | 10.8 | 10.19 |
| E143c | 1.933 | 2.88 | 3.132 | 11.97 | 16.2 | 15.00 |
| E143d | 2.786 | 2.58 | 2.894 | 14.87 | 21.6 | 20.37 |
| E143e | 4.686 | 1.80 | 2.403 | 17.27 | 27.0 | 26.85 |
| E143f | 3.491 | −1.64 | −1.154 | 16.12 | 32.4 | 28.70 |
| E143g | 2.363 | 0.23 | 0.623 | 16.74 | 37.8 | 31.30 |
| E143h | 3.248 | −0.62 | 0.284 | 17.02 | 43.2 | 33.93 |
| E143i | 4.948 | −1.98 | −0.702 | 16.32 | 48.6 | 36.89 |
| E143j | 3.028 | 0.31 | 1.146 | 17.47 | 54.0 | 40.22 |
| E143k | 3.268 | 0.08 | 0.992 | 18.46 | 59.4 | 43.57 |
| Trial | Water Charge, L | Feed Water | Eh, mV | pH | EC, mS·cm−1 |
|---|---|---|---|---|---|
| NaCl, g·L−1 | |||||
| E144a | 5.4 | 4.07 | 0.203 | 6.50 | 7.91 |
| E144b | 5.4 | 4.07 | 0.192 | 6.35 | 7.94 |
| E144c | 5.4 | 1.00 | 0.181 | 6.31 | 2.16 |
| E144d | 5.4 | 1.10 | 0.191 | 6.16 | 2.34 |
| E144e | 5.4 | 2.05 | 0.218 | 6.37 | 4.04 |
| E144f | 5.4 | 5.83 | 0.192 | 6.34 | 11.25 |
| Trial | Eh, mV | pH | EC, mS·cm−1 | Storage Weeks | Stored Water Analysis | pH | EC, mS·cm−1 |
|---|---|---|---|---|---|---|---|
| Eh, mV | |||||||
| E144a | 0.199 | 5.64 | 7.03 | 3 | 0.282 | 6.51 | 5.8 |
| E144b | 0.222 | 6.64 | 7.32 | 3 | 0.282 | 6.57 | 6.6 |
| E144c | 0.154 | 5.23 | 3.92 | - | - | - | - |
| E144d | 0.169 | 5.38 | 2.74 | - | - | - | - |
| E144e | 0.153 | 5.30 | 4.08 | - | - | - | - |
| E144f | 0.080 | 5.56 | 8.58 | - | - | - | - |
| Trial | Water Recovered | Water Consumed | Duration | Air Flow | CO2 Flow | Air Flow Rate | CO2 Flow Rate | Temperature |
|---|---|---|---|---|---|---|---|---|
| L | L | h | h | h | L·h−1 | L·h−1 | °C | |
| E144a | 4.00 | 1.40 | 5.1 | 3.8 | 1.3 | 13.2 | 3.3 | 5–12 |
| E144b | 4.00 | 1.40 | 104.3 | 70.5 | 33.8 | 13.2 | 3.3 | 8–15 |
| E144c | 4.50 | 0.90 | 690.0 | 0.0 | 690.0 | 13.2 | 3.3 | 11–15 |
| E144d | 4.15 | 1.25 | 291.0 | 0.0 | 291.0 | 13.2 | 3.3 | 12–16 |
| E144e | 4.00 | 1.40 | 537.0 | 0.0 | 537.0 | 13.2 | 3.3 | 16–19 |
| E144f | 3.50 | 1.90 | 1295.0 | 0.0 | 1295.0 | 13.2 | 3.3 | 13–19 |
| Trial | Product Water Salinity Based on EC | Product Water Salinity Based on Spectrometry | Apparent NaCl Removed | Actual NaCl Removed | Cumulative NaCl Removed | Cumulative Water Processed | Cumulative |
|---|---|---|---|---|---|---|---|
| g·L−1 | g·L−1 | g·L−1 | g·L−1 | g·L−1 | NaCl in feed g·L−1 | ||
| E144a | 3.55 | - | 0.52 | 1.44 | 1.44 | 5.4 | 4.07 |
| E144b | 3.69 | - | 0.38 | 1.34 | 2.78 | 10.8 | 8.15 |
| E144c | 1.90 | 0.53 | 0.47 | 0.56 | 3.34 | 16.2 | 9.15 |
| E144d | 1.27 | 1.04 | 0.06 | 0.30 | 3.64 | 21.6 | 10.25 |
| E144e | 2.03 | 1.30 | 0.75 | 1.09 | 4.73 | 27.0 | 12.30 |
| E144f | 4.35 | 2.77 | 3.06 | 4.04 | 8.77 | 32.4 | 18.13 |
| Trial | Water Charge, L | Feed Water | Eh, mV | pH | EC, mS·cm−1 | Product Water | pH | EC, mS·cm−1 | NaCl, g·L−1 | Desalination | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl, g·L−1 | Eh, mV | NaCl, g·L−1 | |||||||||
| E146a | 240 | 1.38 | 0.188 | 6.83 | 2.88 | 0.152 | 8.01 | 4.24 | 1.04 | 0.34 | |
| E146b | 240 | 2.73 | 0.164 | 6.67 | 5.4 | 0.151 | 8.31 | 5.35 | 2.09 | 0.64 | |
| E146c | 240 | 3.76 | 0.202 | 6.92 | 7.34 | 0.172 | 8.29 | 10.16 | 0.64 | 3.12 | |
| E146d | 240 | 6.27 | 0.156 | 6.99 | 12.2 | 0.178 | 8.27 | 18.19 | 4.68 | 1.59 | |
| E146e | 240 | 2.92 | 0.196 | 6.96 | 5.76 | 0.156 | 8.10 | 6.51 | 1.96 | 0.96 | |
| E146f | 240 | 2.24 | 0.162 | 6.90 | 4.47 | 0.142 | 8.19 | 5.29 | 1.39 | 0.85 | |
| E146g | 240 | 2.45 | 0.164 | 6.89 | 4.94 | 0.145 | 8.15 | 5.73 | 0.71 | 1.74 | |
| E146h | 240 | 3.73 | 0.164 | 6.97 | 7.39 | 0.157 | 8.37 | 8.62 | 1.87 | 1.86 | |
| E146i | 240 | 2.25 | 0.164 | 7.08 | 4.56 | 0.134 | 8.57 | 4.84 | 0.62 | 1.63 | |
| E146j | 240 | 2.75 | 0.176 | 6.19 | 5.48 | 0.155 | 7.57 | 5.57 | 1.85 | 0.90 | |
| E146k | 240 | 2.07 | 0.176 | 6.34 | 4.25 | 0.174 | 7.50 | 4.59 | 0.69 | 1.38 | |
| E146l | 240 | 1.39 | 0.181 | 6.24 | 2.9 | 0.173 | 7.65 | 3.69 | 2.73 | −1.34 | |
| E146m | 240 | 2.17 | 0.181 | 6.33 | 4.38 | 0.174 | 7.50 | 4.59 | 0.61 | 1.56 | |
| E146n | 240 | 1.00 | 0.181 | 6.31 | 2.26 | 0.117 | 6.86 | 2.86 | 0.5 | 0.50 | |
| E146o | 240 | 1.10 | 0.191 | 6.16 | 2.34 | 0.134 | 7.10 | 3.14 | 0.76 | 0.34 | |
| E146p | 240 | 2.05 | 0.218 | 6.37 | 4.04 | 0.129 | 7.05 | 4.77 | 1.65 | 0.40 | |
| E146q | 240 | 5.83 | 0.192 | 6.35 | 11.25 | 0.099 | 7.40 | 12.27 | 2.15 | 3.68 | |



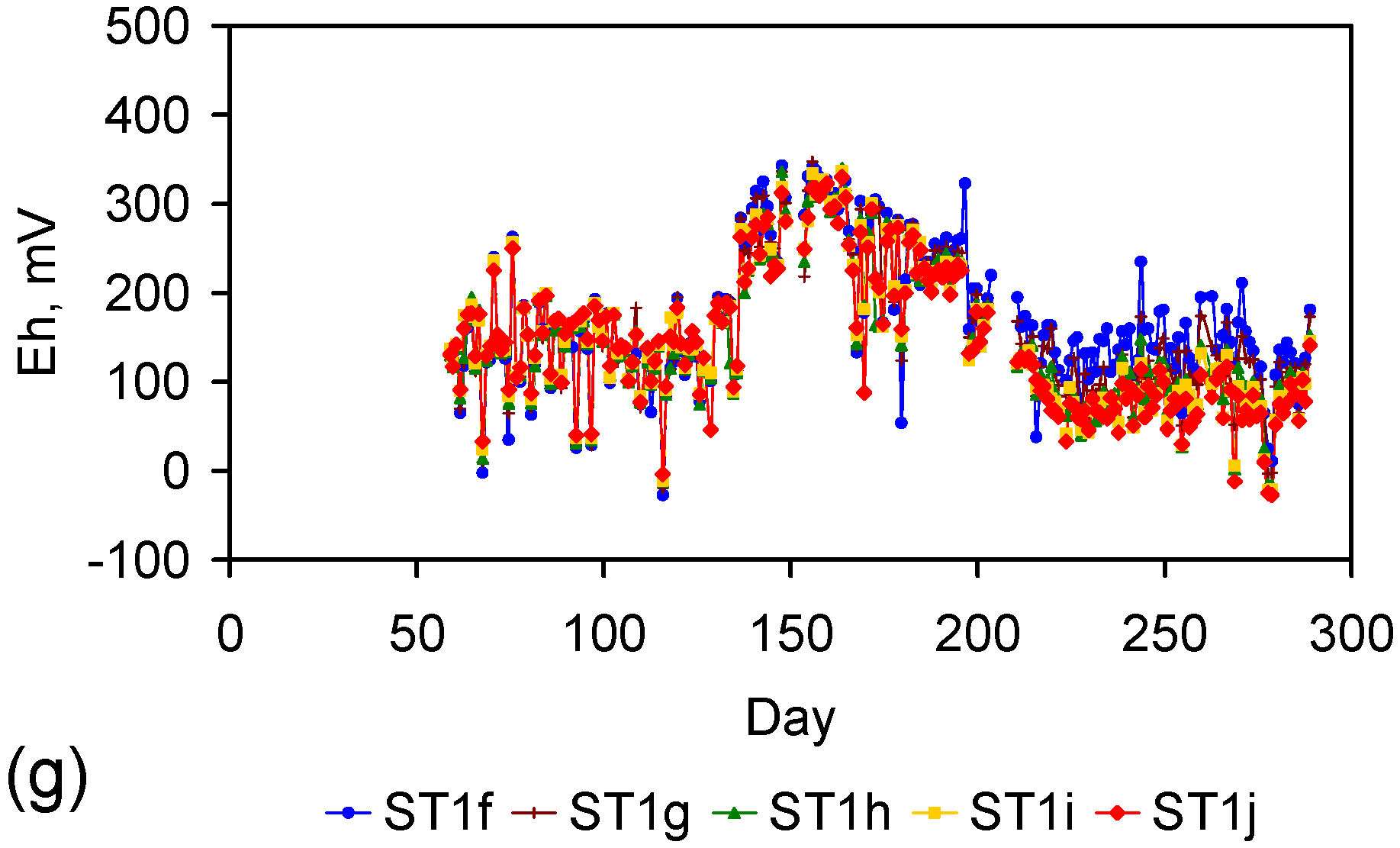

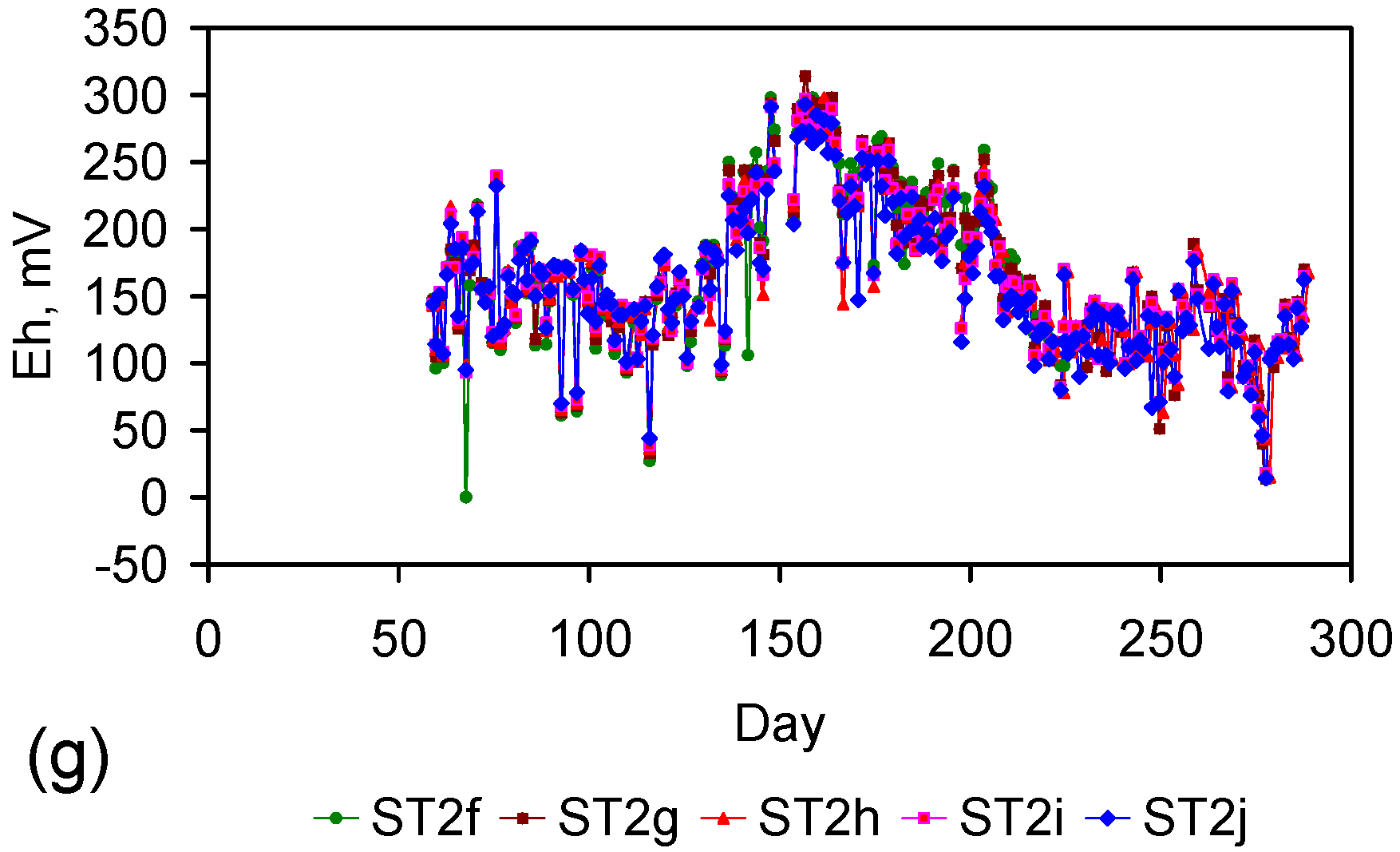

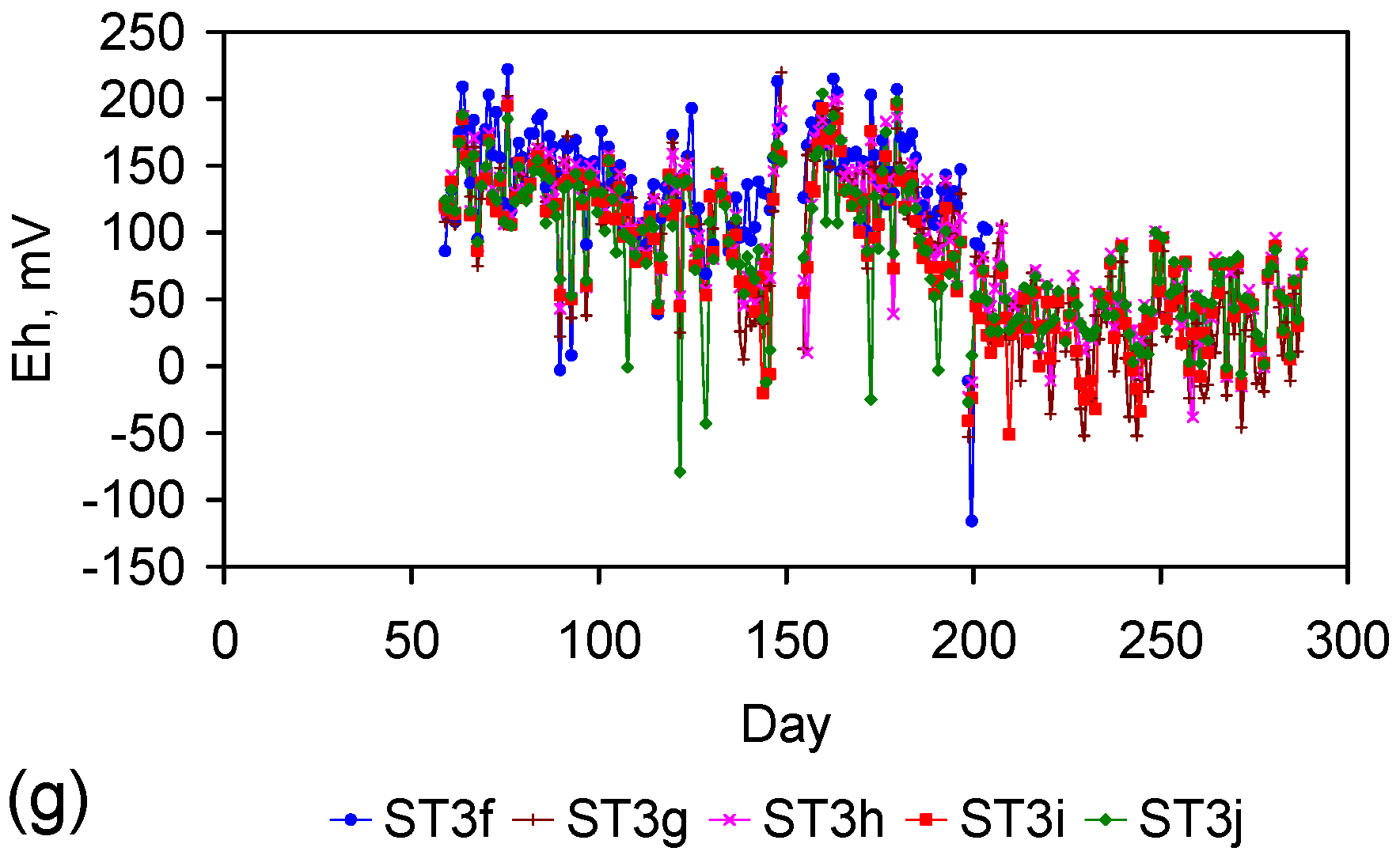






















Appendix D. ZVM TP and ZVM TPA Compositions
| Trial Group | Manufacturing Process Type | ZVM TP Usage | Trials | Trial Results |
|---|---|---|---|---|
| 1 | B | Particles | PS1–PS4, PS16 | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C1 |
| 2 | A | Particles | PS5, PS15 | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C2 |
| 3 | C | Particles | PS8 | Figure C2 |
| 4 | C | Particles | PS9 | Figure C2 |
| 5 | C | Particles | PS10 | Figure C2 |
| 6 | D | Particles | PS11 | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C3 |
| 7 | D | Particles | PS12 | Figure C3 |
| 8 | D | Particles | PS13 | Figure C3 |
| 9 | A (g) | Cu sheathed pellets | PS14 | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C3 |
| 10 | D | Container | CSD1a–CSD1d | Table 5 |
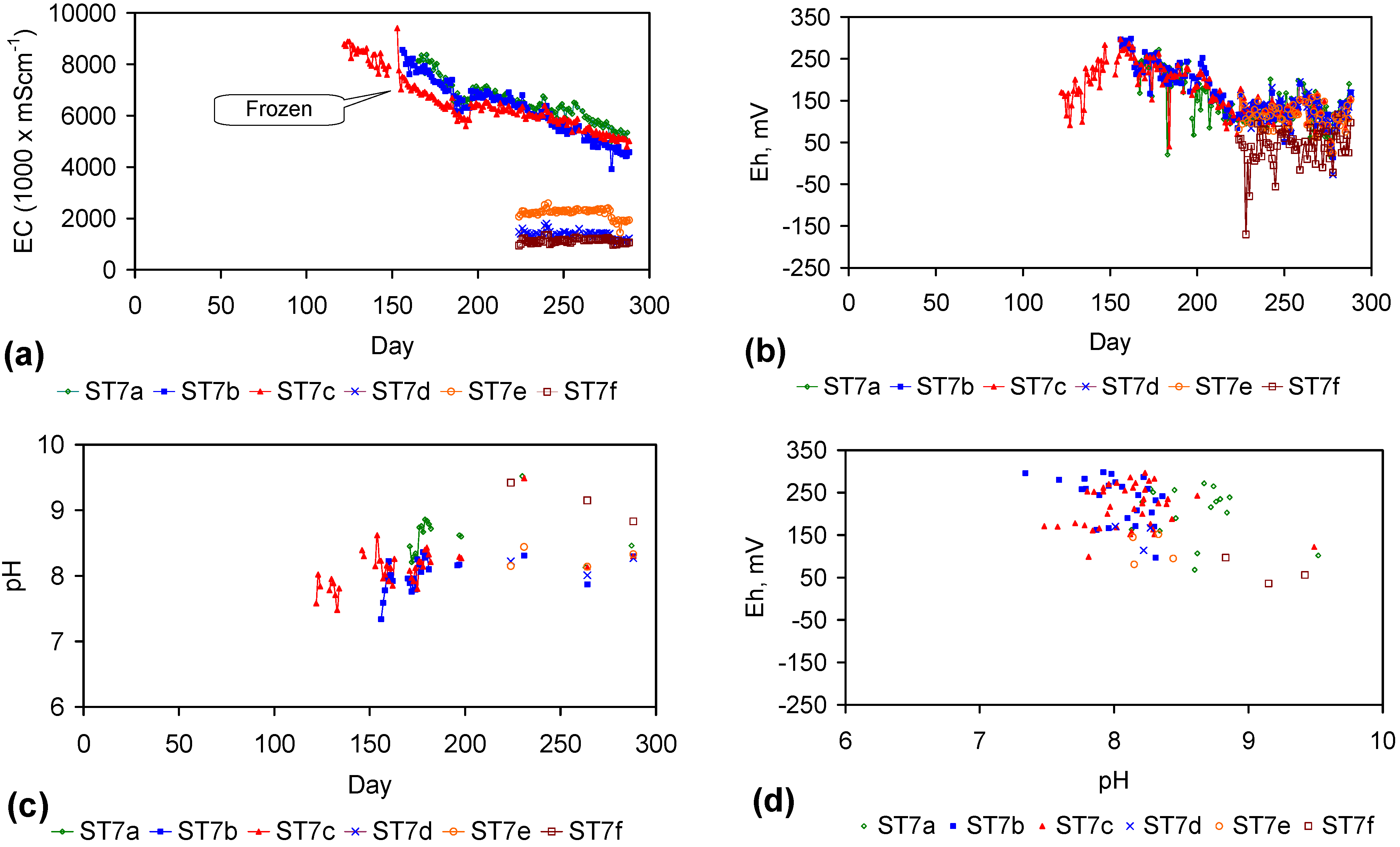
| 11 | B | Cu sheathed pellets | AS4–AS6, ST1a–ST5j, ST6a–ST6d | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C4, Figure C5, Figure C6, Figure C7, Figure C8, Figure C9 and Figure C10 |
| 12 | A | Cu sheathed pellets | AS1–AS3 | Figure C11 |
| 13 | C | Cu sheathed pellets | ST8a | Figure C12 |
| 14 | C | MDPE sheathed pellets | ST8b–ST8e | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C12 |
| 15 | C | MDPE sheathed pellets | MT1a–MT1d | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C13 |
| 16 | C | MDPE sheathed pellets | MT2a–MT2d | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C14 |
| 17 | D | MDPE sheathed pellets | MT3a–MT3d | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C15 |
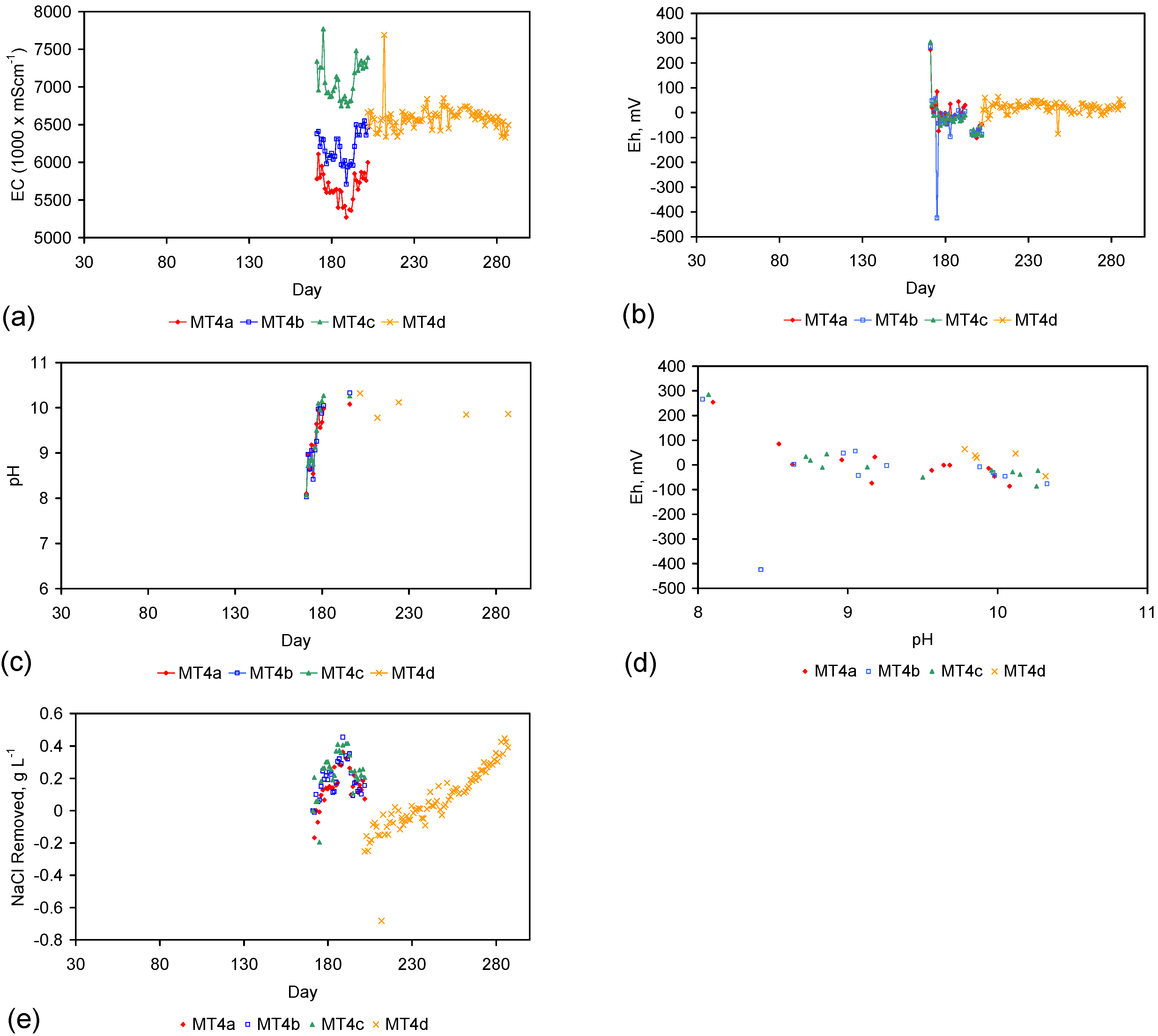
| 18 | D | MDPE sheathed pellets | MT4a–MT4d | Table 1, Table C1, Table C2, Table C3 and Table C4; Figure C16 |
| 19 | B | Reused Cu sheathed pellets | ST7a (originally used as ST2d); ST7b (originally used as ST2g); ST7c (originally used as ST3d); ST7d (originally used as ST2f); ST7e (originally used as ST3b); ST7f (originally used as ST3f); | Figure C17 |
| Trial Group | Fe | Al | Cu | MnO2 | ZnO | CaCO3 | MgCO3 | K2CO3 | C | CaO |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.3950 | 0.040 | 0.006 | - | - | - | - | - | - |
| 2 | 1 | 1.4200 | 0.162 | - | - | - | - | - | - | - |
| 3 | 1 | 0.1100 | - | - | 0.110 | - | - | - | - | - |
| 4 | 1 | 0.1580 | 0.006 | - | - | 0.0900 | 0.060 | 0.017 | 0.16 | - |
| 5 | 1 | 0.5200 | 0.019 | - | - | - | - | - | - | - |
| 6 | 1 | - | - | - | 0.056 | 0.2086 | 0.152 | - | - | - |
| 7 | 1 | 0.8400 | 0.090 | - | 0.068 | 0.0640 | 0.090 | - | - | - |
| 8 | 1 | 0.9700 | 0.040 | - | - | 0.0530 | 0.090 | - | - | 0.07 |
| 9 | 1 | - | - | - | - | - | - | - | - | - |
| 10 | 1 | 0.3200 | - | - | - | - | - | - | - | - |
| 11,19 | 1 | 0.3950 | 0.040 | 0.006 | - | - | - | - | - | - |
| 12 | 1 | 1.4200 | 0.162 | - | - | - | - | - | - | - |
| 13 | 1 | 0.1100 | - | - | 0.110 | - | - | - | - | - |
| 14 | 1 | 0.1100 | - | - | 0.110 | - | - | - | - | - |
| 15 | 1 | 0.1580 | 0.006 | - | - | 0.0900 | 0.060 | 0.017 | 0.16 | - |
| 16 | 1 | 0.5200 | 0.019 | - | - | - | - | - | - | - |
| 17 | 1 | 0.8100 | 0.080 | 0.100 | 0.070 | 0.1500 | 0.100 | - | 0.06 | - |
| 18 | 1 | 0.8200 | 0.040 | - | - | 0.1100 | 0.080 | - | 0.14 | - |
| Trial Group | Manufacturing Process Type | ZVM TP Usage | Trials | Trial Results | Cartridge Size, L | Fe, g | K-Feldspar, g | Al, g |
|---|---|---|---|---|---|---|---|---|
| 20 | E | Cartridge | E143a–E143k | Table C5, Table C6, Table C7 and Table C8 | 0.4 | 135 | 97 | - |
| 21 | E | Cartridge | E144a–E144f | Table C9, Table C10, Table C11 and Table C12 | 0.4 | 51 | 90 | 38 |
| 22 | E | Cartridge | E145 | Figure C18 | 2.0 | 449 | 1000 | - |
| 23 | E | Cartridge | E146a–E146p | Table C13, Figure C19, Figure C20, Figure C21, Figure C22, Figure C23, Figure C24, Figure C25, Figure C26, Figure C27, Figure C28, Figure C29, Figure C30, Figure C31, Figure C32, Figure C33, Figure C34, Figure C35, Figure C36 and Figure C37 | 0.4 | 124 | 276 | - |



Appendix E. Interpretation of Salinity
- (1)
- Assessment of salinity using UV-visible spectrometry
- (a)
- Determination of control samples and salinity using UV-visible spectrometry, when nano-particles are absent;
- (b)
- Determination of salinity when nano-particle formation results in the product water absorbance being greater than the feed water absorbance, and the nano-particles can be removed by settlement;
- (c)
- Determination of salinity when nano-particle formation results in the product water absorbance being less than the feed water absorbance during desalination;
- (2)
- Assessment of salinity using EC
- (a)
- Determination of control samples and salinity using EC, when nano-particles are absent;
- (b)
- Determination of salinity when EC shows an initial rise due to nano-particle formation followed by a decline during desalination;
- (c)
- Determination of salinity removed when the water volume reduces during desalination;
- (3)
- Assessment of salinity using UV-visible spectrometry and EC
- (a)
- Determination of salinity when nano-particle formation results in absorbance increasing with time, while EC initially rises before remaining stable at an elevated level.
E1. Assessment of Product Water Salinity Using UV-Visible Spectrophotometry
E1.1. Equations Used in the Assessment of Salinity: Control Samples
| Wavelength, nm | Normalized Absorbance | Raw Absorbance |
|---|---|---|
| Salinity, g·L−1 | Salinity, g·L−1 | |
| 200 | Salinity, g·L−1 = 766.28x2 − 3.2388x + 1.8149 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 205 | Salinity, g·L−1 = 636.34x2 − 10.996x + 1.8025 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 210 | Salinity, g·L−1 = 533.61x2 + 6.1084x + 1.8424 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 215 | Salinity, g·L−1 = 784.61x2 + 3.7002x + 1.8436 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 220 | Salinity, g·L−1 = 866.19x2 + 32.111x + 1.8318 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 225 | Salinity, g·L−1 = 450.66x2 + 33.898x + 1.5123 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 230 | Salinity, g·L−1 = 118.7x2 + 27.487x + 1.3347 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 235 | Salinity, g·L−1 = 7.8675x2 + 52.237x + 0.9155 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 240 | Salinity, g·L−1 = 11.138x2 + 49.401x + 0.957 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 245 | Salinity, g·L−1 = 38.641x2 + 42.294x + 0.948 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 250 | Salinity, g·L−1 = 18.317x2 + 47.257x + 1.0025 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 255 | Salinity, g·L−1 = 37.841x2 + 43.73x + 1.0558 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 260 | Salinity, g·L−1 = 10.321x2 + 52.13x + 1.0098 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 265 | Salinity, g·L−1 = 20.714x2 + 49.407x + 0.9941 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 270 | Salinity, g·L−1 = 27.368x2 + 48.753x + 1.0832 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 275 | Salinity, g·L−1 = 27.115x2 + 47.752x + 1.1226 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 280 | Salinity, g·L−1 = 32.059x2 + 52.604x + 0.9819 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 285 | Salinity, g·L−1 = 27.529x2 + 55.578x + 0.9332 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 290 | Salinity, g·L−1 = 19.31x2 + 59.508x + 0.8993 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 295 | Salinity, g·L−1 = 29.723x2 + 62.585x + 0.8414 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 300 | Salinity, g·L−1 = 17.616x2 + 65.145x + 0.8971 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 350 | Salinity, g·L−1 = 56.721x2 + 83.129x + 0.9896 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 400 | Salinity, g·L−1 = 116.87x2 + 77.832x + 1.0311 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 500 | Salinity, g·L−1 = 302.83x2 + 101.92x + 1.2602 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 600 | Salinity, g·L−1 = 1385x2 + 158.9x + 1.3455 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 700 | Salinity, g·L−1 = 7993.4x2 + 187.23x + 1.6814 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 800 | Salinity, g·L−1 = 22,332x2 + 100.29x + 1.6396 | Salinity, g·L−1 = [xraw/xinitial]·R |
| 900 | - | Salinity, g·L−1 = [xraw/xinitial]·R |
E1.2. Assessment of Salinity: Real Time Analyses
n = number of estimates of salinity
E1.3. Salinity Calculation when Product Water Absorbance > Feed Water Absorbance
E1.4. Salinity Estimation from Raw Spectrum vs. Normalized Spectrum

E2. Salinity Estimation from Electrical Conductivity
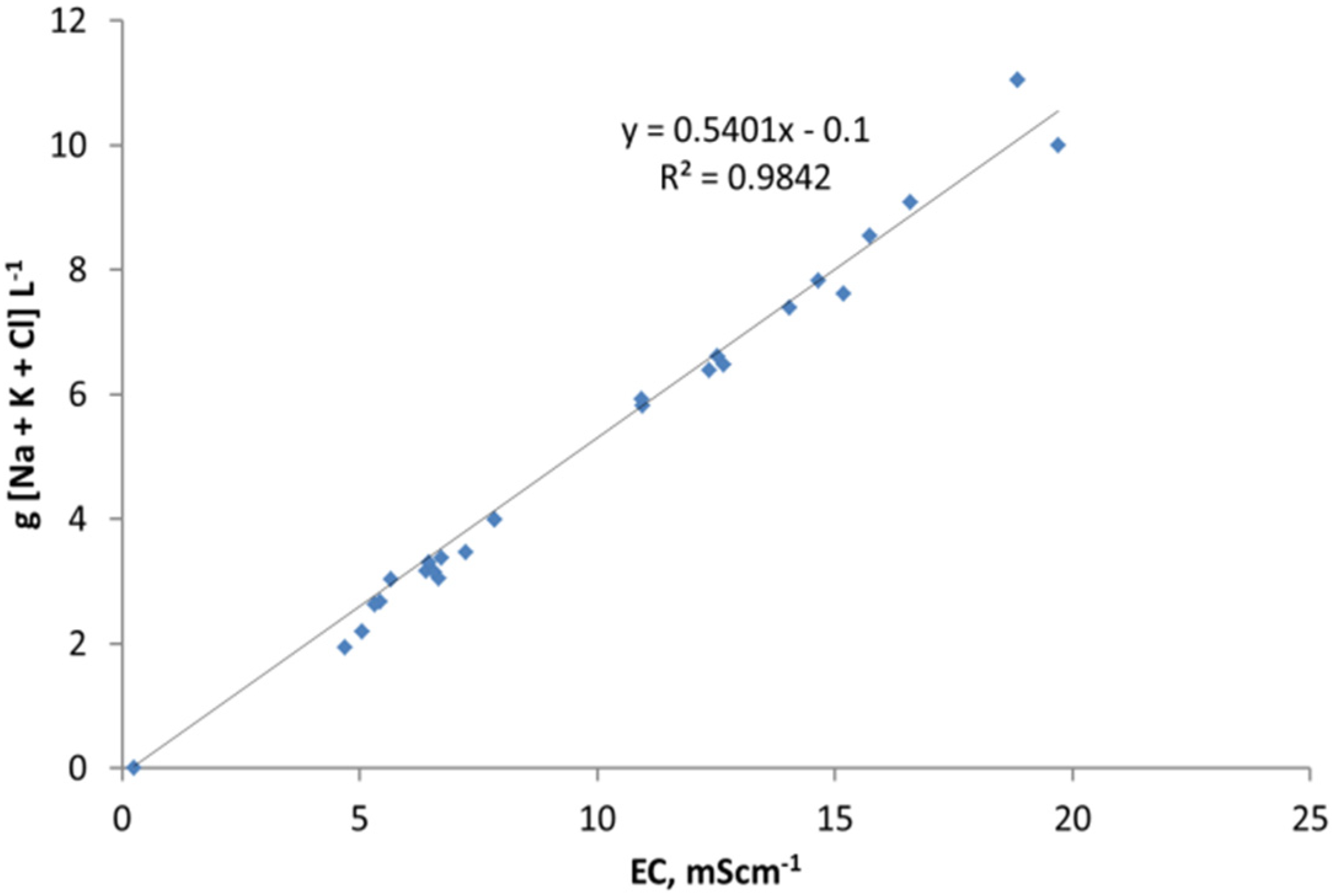
E2.1. Salinity Estimation When EC Shows an Initial Rise Followed by an EC Decline
E2.2. Salinity Estimation When the Volume of Water Reduces with Time
- The residual salt concentration at time, t, in the reduced water body volume is [RSt=t] (g·L−1).
- The initial water volume at time t = 0 is [Wt=0].
- The water volume at time t = t is [Wt=t].
- The residual salt concentration at time t in the water body volume is expressed here in terms of the original feed water volume is [RSa] (g·L−1), where:Residual Salt [RSa], g·L−1 = ([Wt=t]/[Wt=0]) [RSt=t]
e.g., 1.25 g·L−1 = 0.5L/1.0L × 2.5 g·L−1 - The mass balance requires that the amount of NaCl removed isSalt Removed, g·L−1 = [St=0] − [RSa]
where [St=0] = salinity, g·L−1, at time t = 0.
Where, [WZVM] = water removed by ZVM; [WEvap] = water removed by evaporation; [Woutflow] = water removed from the water body, this includes leakage and infiltration; [WPrecip] = water received from rainfall; [WInflow] = water added to the water body.
- EC rises, when water is removed at a rate, which is disproportionately larger than the rate of NaCl removal.
- EC falls, when water is removed at a rate, which is disproportionately less than the rate of NaCl removal.
- EC remains constant, when water is removed at a rate, which is similar to the rate of NaCl removal.
E3. Salinity Assessment When Nano-Particles Are Released from the ZVM TP/TPA
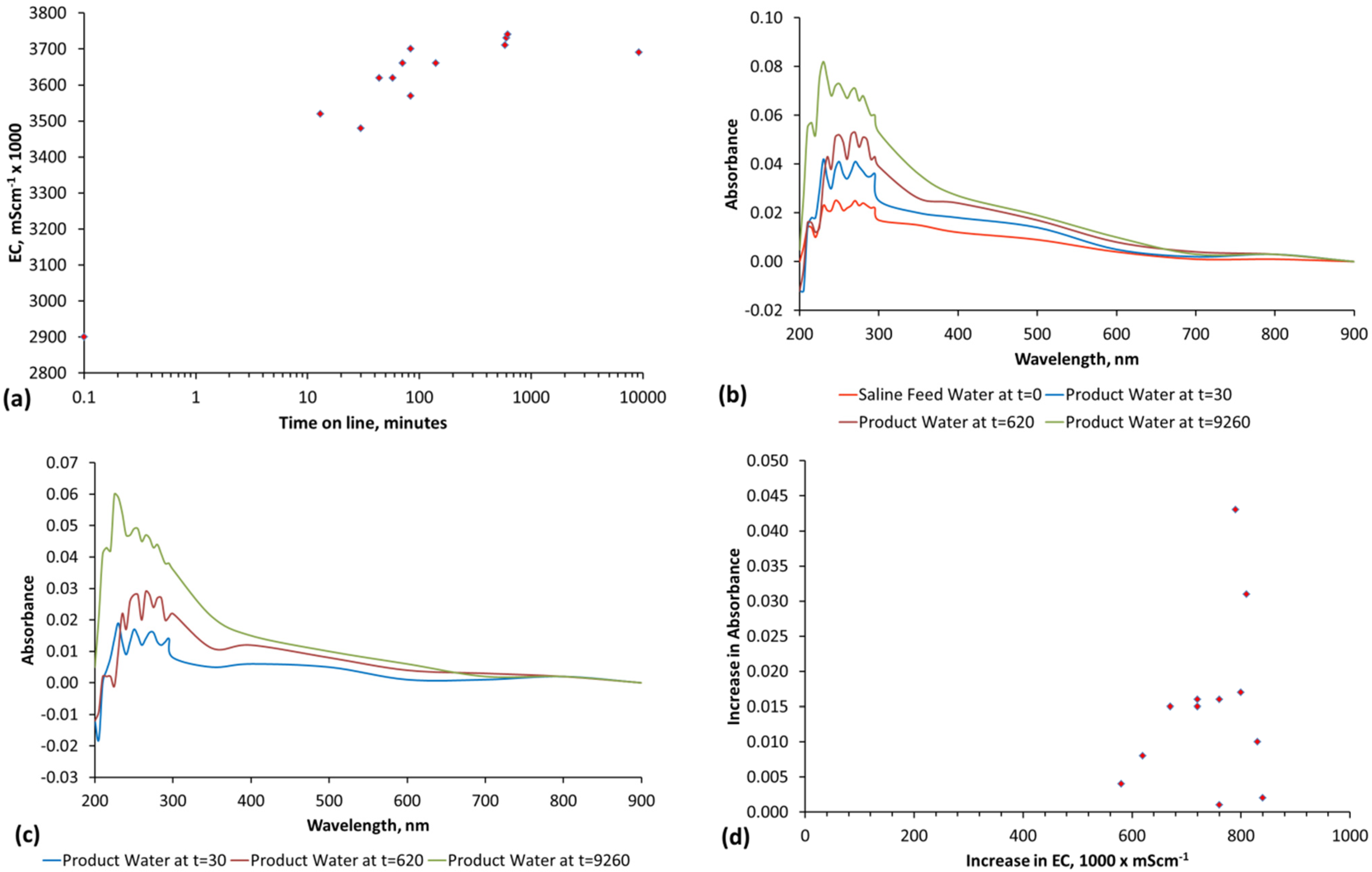

3.69 = 0.249 + 2.651 + [C]
[C] = 3.69 − [0.249 + 3.651] = 0.79
Identification of EC Due to Salinity Decline, when EC Rises Due to Nano-Particle Addition
EC9260 min = [0.249] + [2.651] + [1.27 to 2.19] − [0] − [0]
EC9260 min = 4.17 to 5.09 mS·cm−1
EC9260 min = [0.249] + [2.651] + [1.27 to 2.19] − [0.729 to 1.649] − [0.249]
EC9260 min = 3.69 mS·cm−1
EC9260 min = [2.651] − [0.729 to 1.649]
Salinty9260 min = 1.002 − 1.922 mS·cm−1
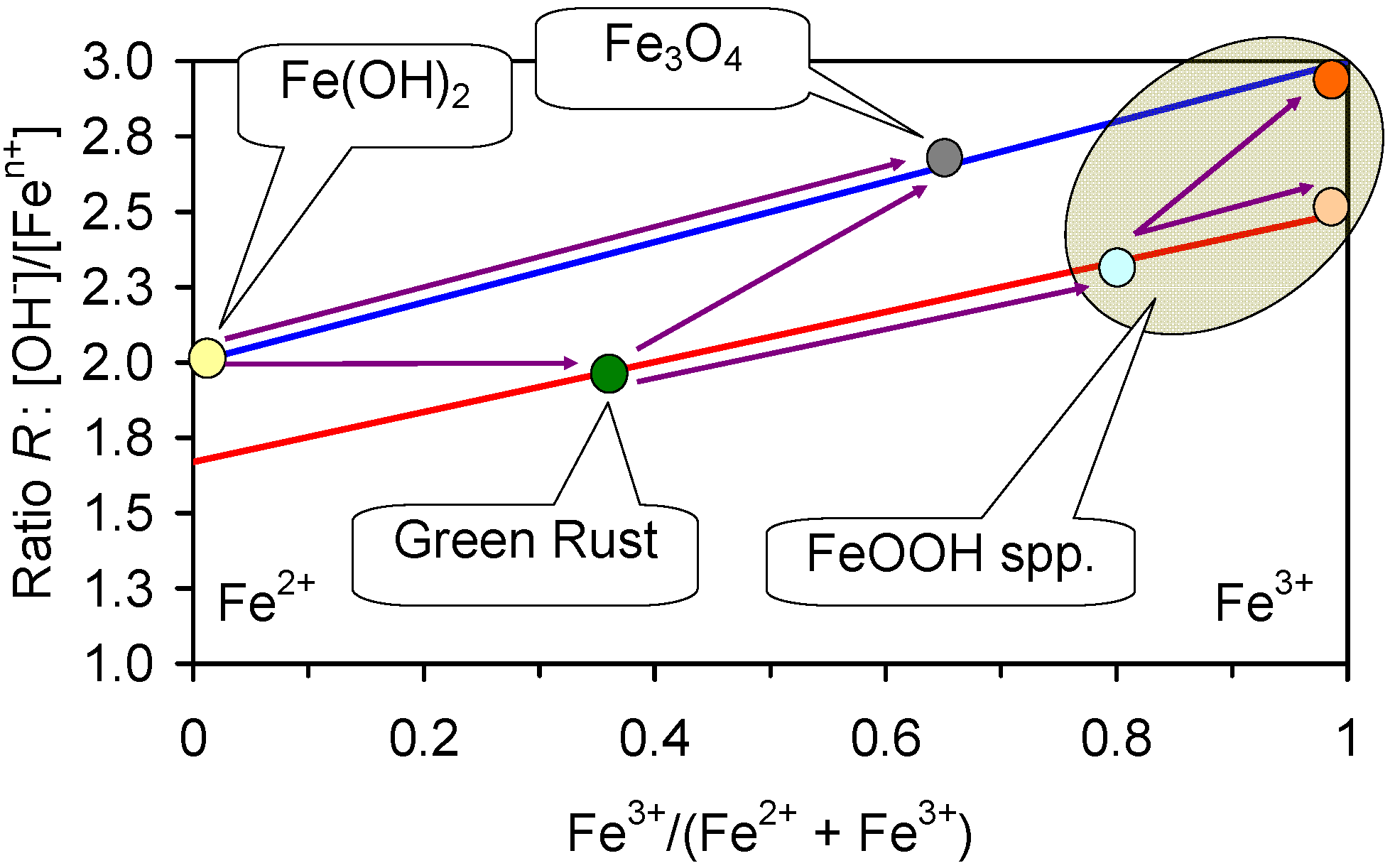
Appendix F. Summary of the Interaction between Fe Corrosion, Eh, pH, EC and Salinity Removal
F1. Oxidation of Fe0
- During corrosion the Fe0 core is initially oxidized to Fe(OH)2.
- The Fe(OH)2 acts as a template for FeOOH growth (rusty purple) around the particle (Figure F1). i.e., a Fe0 particle will corrode to have an Fe0 core, an Fe(OH)2 inner layer (or Fe3O4 inner layer) and an FeOOH outer layer.
- The FeOOH templates the formation of green rust (NaI(a=1−c−b)FeII(b=1−c−a)FeIIIc(OH)2]x−·[(x/n)Cln−·mH2O]x+) sheets growing into the water body from the corroded Fe particle. With increased oxidation these plates gradually transform to FeOOH. In the presence of Cl− ions, any α-FeOOH (goethite) or γ-FeOOH (lepidocrocite) which may initially form, transforms to β-FeOOH (akaganeite) over time. In the presence of HCO3−, CO32− ions, Cl− is replaced in the green rust by CO32−. In the presence of SO42− ions, Cl− (and CO32− ions) are replaced by SO42−. In the presence of SO42− ions, goethite is the dominant FeOOH species and any akagneite formed will transform to goethite. In the presence of high concentrations of HCO3−, CO32− ions the green rust (and some of the FeOOH) may be replaced by FeCO3 [236]. In freshwater, lepidiocrocite and goethite are the dominant FeOOH species. A more detailed analysis of the interaction of HCO3−, CO32−, Cl−, and SO42− ions is provided in Section 6.
- The dissolved Fen+ ions in the water are oxidized to form spherulites of FeOOH which are entrained in the water.

- Entrapment in the growing FeOOH and (NaI(a=1−c−b)FeII(b=1−c−a)FeIIIc(OH)2]x− [(x/n)Cln−·mH2O]x+) sheets;
- Concentration in the pore water and hydration shells surrounding the FeOOH and (NaI(a=1−c−b)FeII(b=1−c−a)FeIIIc(OH)2]x−·[(x/n)Cln−·mH2O]x+) sheets.
F2. Relationship between Eh, pH, Ion Concentration, and Equilibrium Constant, K
ΔG0 = −RT × ln K
ΔE0= RT/nF × ln K
Eh = ΔE0 + 0.0591/n × log(MP) or for Eh independent reactions
log(MP) = −d − pH
F2.1. Measurement of Eh
F2.2. Removal of NaCl in a Redox Environment
F3. Relationship between EC and Reaction Kinetics
- The magnitude of [D] is a function of the reaction rate, k, where [18]:kobserved = kactual × [Pw] × [as]
Where [Pw] = amount of ZVM, g·L−1; [as] = surface area of ZVM, m2·g−1.
kobserved = observed reaction rate; kactual = actual reaction rate. - For a constant [Pw], [D] will decrease as [as] decreases (i.e., the length of time required to reduce the salinity of water from x g·L−1 to y g·L−1 will increase as [as] decreases).
- kobserved is therefore a function of [Pw] and [as].
- The cost of desalination reduces as: (i) [Pw] decreases; (ii) [as] decreases; and (iii) kactual increases.
F3.1. Assessment of the Impact of Changing [as] and [aa] on Salinity
[Wst=1] = 1.1[Wst=0]0.98
Corrected 24 h salinity change for c = ([Pw] × [as] × [aa])actual/([Pw] × [as] × [aa])reference = 0.5
[CWt=1] = [Wst=0] − (c([Wst=0] − (1.1[Wst=0]0.98))
[Wt=1] = 1.1[Wt=0]0.98
Corrected 24 h salinity change for [as] = 1.0; [aa] = 0.9
[CWt=t+1] = [Wt=t] − ([at+1]([Wt=t+1] − (1.1[Wt=t]0.98))
[at+1] = ([as]actual/[as]reference) × ([aa]actual/[aa]reference) × ([Pw]actual/[Pw]reference) × ([k]actual/[k]reference)
[Pw] = Reference ZVM concentration, g·L−1 = 8 g·L−1
[aa] = Reference % of ZVM surface area available for adsorption = 100%

G. Fe Corrosion Species Involved in Desalination
- Reduced Fe corrosion products, e.g., magnetite (Fe3O4) and related species.
- White and green rusts, i.e., Fe(OH)2 and layered hydroxides (green rusts).
- Brown rusts, i.e., Fe(OH)3, FeOOH spp.
- (1)
- In freshwater containing no Cl−, HCO3−, CO32−, S2−, HS−, SO42− ions the reaction sequence is as follows:
- (a)
- Stage 1: Fe0 corrodes to Fe(OH)2
- (b)
- Stage 2: Fe(OH)2 corrodes to Fe(OH)3 and FeOOH (lepiocrocite and goethite). H+(ads) ions may reduce Fe(OH)2 which is adjacent to the Fe0 to Fe3O4 (magnetite).
- (2)
- In freshwater containing no Cl−, S2−, HS−, SO42− and containing HCO3−, CO32− ions the reaction sequence is as follows:
- (a)
- Stage 1: Fe0 corrodes to Fe(OH)2
- (b)
- Stage 2: Fe(OH)2 corrodes to GR1(CO32−) which corrodes to Fe(OH)3 and FeOOH (lepiocrocite and goethite). H+(ads) ions may reduce Fe(OH)2 which is adjacent to the Fe0 to Fe3O4 (magnetite). In the presence of SO42− ions GR1 is replaced by GR2(SO42−) and the CO32− ions are expelled into the water.
- (3)
- In saline water containing no HCO3−, CO32−, S2−, HS−, SO42− ions the reaction sequence is as follows:
- (a)
- Stage 1: Fe0 corrodes to Fe(OH)2
- (b)
- Stage 2: Fe(OH)2 corrodes to GR1(Cl−). The GR1(Cl−) corrodes to form FeOOH. The dominant FeOOH sp. is akaganeite. Any lepiocrocite and goethite which form as an intermediate stage are transformed over the longer term to akaganeite. H+ (ads) ions may reduce Fe(OH)2 which is adjacent to the Fe0 to Fe3O4 (magnetite).
- (4)
- In saline water containing HCO3−, CO32− ions and no S2−, HS−, SO42− ions the reaction sequence is as follows:
- Stage 1: Fe0 corrodes to Fe(OH)2
- Stage 2: Fe(OH)2 corrodes to GR1(CO32−). The GR1(CO32−) corrodes to form FeOOH. The dominant FeOOH sp is akaganeite. Any lepiocrocite and goethite which form as an intermediate stage are transformed over the longer term to akaganeite. H+(ads) ions may reduce Fe(OH)2 which is adjacent to the Fe0 to Fe3O4 (magnetite). In the presence of SO42− ions GR1 is replaced by GR2(SO42−) and the Cl− or CO32− ions are expelled into the water and the final FeOOH product is goethite. In the presence of S2−, HS− ions, Fe(OH)2 and FeOOH corrode to FexSy and S precipitates.
Appendix H. Identification of the Radicals Involved in NaCl Removal
- (1)
- Fe0 [227]: 216 nm, 268 nm (minor);
- (2)
- (3)
- Fe-Ion exchange material [226]: 500–550 nm;
- (4)
- (5)
- ClO2 [217]: 292 nm;
- (6)
- ClO− [228]: 290 nm;
- (7)
- NaClO [228]: 291.4 nm;
- (8)
- HClO [217]: 360 nm;
- (9)
- ClOOCl [219]: 250 nm;
- (10)
- OH–H2O• Radical Structures [221,222,223,224];
- (a)
- AX transition (adsorption of OH radical from the gas phase):
- (i)
- Free OH: 263 nm; 277–288 nm; 299–306 nm;
- (ii)
- OH–H2O• Structures—AX transition: H-bond donor structure C1: 244 nm; 289 nm; 310–334 nm;
- (iii)
- OH–H2O• Structures—AX transition: H-bond acceptor structure C3: 263 nm; 278–290 nm; 299–300 nm; 327 nm;
- (iv)
- OH–H2O• Structures—AX transition: hemi-bonded structure C2: 220 nm; 252 nm; 266–282 nm;
- (b)
- CT transition (adsorption of OH radical from the water):
- (i)
- H-bond acceptor structure C3: 223–229 nm; 245–249 nm; 258–265 nm; 676 nm;
- (ii)
- OH–H2O Structures—CT transition: hemi-bonded structure C2: 211–217 nm; 228–231 nm;
- (11)
- HO2− [225]: 225 nm;
- (12)
- O2− [225]: 245 nm;
- (13)
- Cl− [238]: 210–220 nm;
- (14)
- NaO− [249]: 262/8 nm, 275/6 nm, 301 nm, 324/8 nm, 337 nm, 351 nm, 365 nm, 395 nm.
ClO− + 0.5H2 = HClO + e−
E1: Cl− + OH− = ClO− + H+ + 2e−
ΔH0 = 122.9 kJ·M−1; ΔG0 = 122.9 kJ·M−1; ΔE0 = −0.623 V
E2: Cl− + OH− = ClO− + 0.5H2 + e−
ΔH0 = 122.9 kJ·M−1; ΔG0 = 122.9 kJ·M−1; ΔE0 = −1.24 V
Eh = ΔE0 − 0.0591m/n × pH + 0.0591/n × log[Cl−/ClO−]
Eh (E1), V = −0.623 − 0.02955 × pH + 0.02955 × log[Cl−/ClO−]
Eh (E2), V = −1.24 − 0.0591 × pH + 0.0591 × log[Cl−/ClO−]
E3: 2Cl− + O2− = 2ClO− + 2e−
ΔH0 = −107.1 kJ·M−1; ΔG0 = −36.8 kJ·M−1; ΔE0 = 0.381 V
Eh (E3), V, = 0.381 − 0.02955 × pH + 0.02955 × log[Cl−/ClO−]
m = number of H+ ions; n = number of electrons transferred
ΔH0 = −107.1 kJ·M−1; ΔG0 = −36.8 kJ·M−1; ΔE0 = 0.381 V
Eh (E3), V, = 0.381 − 0.02955 × pH + 0.02955 × log[Cl−/ClO−]
ΔH0 = 33.9 kJ·M−1; ΔG0 = 48.0 kJ·M−1; ΔE0 = −0.497 V
Eh (E4), V = −0.497 − 0.0591 × pH + 0.0591 × log[FeOH+/FeOH2+]
ΔH0 = 40.6 kJ·M−1; ΔG0 = 74.2 kJ·M−1; ΔE0 = −0.768 V
Eh (E5), V = −0.768 − 0.0591 × pH + 0.0591 × log[Fe2+/Fe3+]
Fe2+ = Fe3+ + e−
O2 + e− = O2−
ΔH0 = 0 kJ·M−1; ΔG0 = 0 kJ·M−1
ΔH0 = −4.6 kJ·M−1; ΔG0 = 83.7 kJ·M−1
2ClO = ClO–OCl
ΔH0 = 0 kJ·M−1; ΔG0 = 0 kJ·M−1
Reaction Demonstrated by Figure C37 at wavelength 245 nm
O2 + e− = O2−
Reaction demonstrated by pH changes with time (Figures C19–C36)
0.5O2− + H2O = HO-OH + 2e− = 2OH−
Reaction Demonstrated by Figure C37 at wavelength 225 nm
HO–OH + OH− = HO2− + H2O
Reactions Demonstrated by Figure C37 at wavelength 290 nm
2Cl− + O2− = 2ClO− + 2e−
Reactions Demonstrated by Figure C37 at wavelength 250 nm
ClO− = ClO + e−
Cl− + H2O + O2 = OH− + HO-OCl
ClO− + H2O2 = ClO + OH + OH−
ClO− + HO2− = ClO + 0.5O2− + OH−
2ClO = ClO–OCl
ClO + OH = HO–OCl
Reaction [238] demonstrated by Figure C37 at 240 nm
O2− + Fe2+ = Fe3+ + O22−
Reactions demonstrated by Figure C37 at 265 nm and 275 nm
2Na+ + O22− = 2NaO−

References
- Zang, Y.; Liu, Y.; Jing, Y.; Zhao, Z.; Quan, X. Steady performance of a zero valent iron packed anaerobic reactor for azo dye waste water treatment under variable influent quality. J. Environ. Sci. 2012, 24, 720–727. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Yan, W.; Zhang, W.X. Nanoscale zero valent iron (nZVI) for the treatment of concentrated Cu(II) wastewater: A field demonstration. Environ. Sci. Process Impacts 2014, 16, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, W.-X. Enhanced biological treatment of industrial waste water with bi-metallic zero-valent iron. Environ. Sci. Technol. 2008, 42, 5384–5389. [Google Scholar] [CrossRef] [PubMed]
- Rima, J.; Rahme, K.; Assaker, K. Advanced oxidation of olive mill waste water OMW by an oxidative free-radical process induced with zero valent iron. J. Food Res. 2014, 3, 70–82. [Google Scholar] [CrossRef]
- Antia, D.D.J. Sustainable zero-valent metal (ZVM) water treatment associated with diffusion, infiltration, abstraction and recirculation. Sustainability 2010, 2, 2988–3073. [Google Scholar] [CrossRef]
- Antia, D.D.J. Modification of aquifer pore water by static diffusion using nano-zero-valent metals. Water 2011, 3, 79–112. [Google Scholar] [CrossRef]
- Gavaskar, A.; Tatar, L.; Condit, W. Cost and Performance Report: Nanoscale Zero-Valent Iron Technologies for Source Remediation; Contract Report CR-05-007-ENV; Naval Facilities Engineering Command, Engineering Service Center (NAVFAC): Port Hueneme, CA, USA, 2005; p. 44. [Google Scholar]
- Cunningham, J.A.; Reinhard, M. Injection-extraction treatment well pairs: An alternative to permeable reactive barriers. Ground Water 2002, 40, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K.; Schierz, A.; Georgi, A.; Kopinke, F.-D. Colloidal activated carbon and carbo-iron-novel materials for in-situ groundwater treatment. Glob. NEST J. 2008, 10, 54–61. [Google Scholar]
- Cundy, A.B.; Hopkinson, L.; Whitby, R.L.D. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Care, S.; Crane, R. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. Mar. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matlochova, A.; Placha, D.; Rapantova, N. The application of nanoscale materials in groundwater remediation. Pol. J. Environ. Stud. 2013, 22, 1401–1410. [Google Scholar]
- Zhang, W.-X. Dispersed Zero-Valent Iron Colloids. U.S. Patent US7128841 B2, 11 March 2004. [Google Scholar]
- Truex, M.J.; Vermeul, V.R.; Mendoza, D.P.; Fitz, B.G.; Mackley, R.D.; Oostrom, M.; Wietsma, T.W.; Macbeth, T.W. Injection of zero valent iron into an unconfined aquifer using shear thinning fluids. Groundw. Monit. Remediat. 2011, 31, 50–58. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Puls, R.W.; Sewell, G.W. Long-term performance of permeable reactive barriers using zero valent iron: Geochemical and microbiological effects. Ground Water 2003, 41, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abbas, T.R.; Jassam, S.H. Removal of zinc from contaminated groundwater by zero-valent iron permeable reactive barrier. Desalin. Water Treat. 2014. [Google Scholar] [CrossRef]
- Antia, D.D.J. Groundwater water remediation by static diffusion using nano-zero valent metals [ZVM](Fe0, Cu0, Al0), n-FeHn+, n-Fe(OH)x, n-FeOOH, n-Fe-[OxHy](n+/−). In Nanomaterials for Environmental Protection, 1st ed.; Kharisov, B.I., Kharissova, O.V., Dias, H.V.R., Eds.; Wiley Inc.: Hoboken, NJ, USA, 2014; Chapter 1; pp. 3–25. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 1st ed.; NACE International, Cebelcor: Houston, TX, USA, 1974. [Google Scholar]
- Fronczyk, J.; Pawluk, K.; Michniak, M. Application of permeable reactive barriers near roads for chloride ions removal. Ann. Wars. Univ. Life Sci. SGGW Land Reclaim. 2010, 42, 249–259. [Google Scholar] [CrossRef]
- Fronczyk, J.; Pawluk, K.; Garbulewski, K. Multilayer PRBs—Effective technology for protection of the groundwater environment in traffic infrastructures. Chem. Eng. Trans. 2012, 28, 67–72. [Google Scholar]
- Kozin, P.A.; Bolly, J.-F. Proton binding and ion exchange at the akaganeite/water interface. J. Phys. Chem. C 2013, 117, 6409–6419. [Google Scholar] [CrossRef]
- Ahn, H.; Jo, H.Y.; Kim, G.-Y.; Koh, Y.-K. Effect of NaCl on Cr(VI) reduction by granular zero valent iron (ZVI) in aqueous solutions. Mater. Trans. 2012, 53, 1324–1329. [Google Scholar] [CrossRef]
- Gatcha-Bandjun, N.; Noubactep, C.; Mbenguela, B.L. Water treatment with Fe0/H2O systems: Learning from internal electrolysis. Fresenius Environ. Bull. 2014, 23, 2663–2669. [Google Scholar]
- Hwang, Y.; Kim, D.; Shin, H.-S. Inhibition of nitrate reduction by NaCl adsorption on a nano-zero valent iron surface during concentrate treatment for water reuse. Environ. Technol. 2015, 36, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.G.; Garbatov, Y.; Zayed, A.; Wang, G. Non linear corrosion model for immersed steel plates accounting for environmental factors. Trans. Soc. Naval Arch. Mar. Eng. 2005, 113, 306–322. [Google Scholar]
- Misstear, B.; Banks, D.; Clark, L. Water Wells and Boreholes, 1st ed.; Wiley Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Egal, M.M.; Ramamurthy, A.S. Nanofer ZVI: Morphology, particle characteristics, kinetics and applications. J. Nanomater. 2014. [Google Scholar] [CrossRef]
- Xie, K.; Li, J.; Lai, Y.; Lu, W.; Zhang, Z.; Liu, Y.; Zhou, L.; Huang, H. Highly ordered iron oxide nanotube arrays as electrode for electrochemical energy storage. Electrochem. Commun. 2011, 13, 657–660. [Google Scholar] [CrossRef]
- Wada, Y.; Bierkens, M.F.P. Sustainability of global water use: Past reconstruction and future projections. Environ. Res. Lett. 2014, 9. [Google Scholar] [CrossRef]
- Amarasinghe, U.A.; Smakhtin, V. Global Water Demand Projections: Past, Present and Future; Report 156; International Water Management Institute (IWMI): Columbo, Sri Lanka, 2014. [Google Scholar]
- Knapp, K.C.; Baerenklau, K.A. Ground water quantity and quality management: Agricultural production and aquifer salinization over long time scales. J. Agric. Resour. Econ. 2006, 31, 616–641. [Google Scholar]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shaala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Food and Agricultural Organization (FAO). The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW)—Managing Systems at Risk; Food and Agricultural Organization of the United Nations and Earth Scan: Abingdon, UK, 2011. [Google Scholar]
- Payen, S.; Basset-Mens, C.; Follain, S.; Grunberger, O.; Marlet, S.; Nunez, M.; Perret, S. Pass the salt please! From a review to a theoretical framework for integrating salinization impacts in food LCA. In Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Foods Sector, San Francisco, CA, USA, 8–10 October 2014; Available online: http://lcafood2014.org/papers/140.pdf (accessed on 26 June 2015).
- Al Hashemi, R.; Zarreen, S.; Al Raisi, A.; Al Marzooqi, F.A.; Hasan, S.W. A review of desalination trends in Gulf Cooperation Council Countries. Int. Interdiscip. J. Sci. Res. 2014, 1, 72–96. [Google Scholar]
- Sorour, M.; Hani, H.A.; Shaalan, H.F.; Al-Bazedi, G.A. Preliminary techno-economics assessment of developed desalination/salt recovery facility based on membrane and thermal techniques. Desalin. Water Treat. 2014. [Google Scholar] [CrossRef]
- Venkatesan, R. Comparison between LTTD and RO process of sea-water desalination: An integrated economic, environmental and ecological framework. Curr. Sci. 2014, 106, 378–386. [Google Scholar]
- Shanghai CNPC Powder Material Co. Ltd. Pure Electrolytic Carbonyl Atomized Sponge with High Purity Reduced Iron Powder. Available online: http://uk.alibaba.com/product/234481077-pure-electrolytic-carbonyl-atomized-sponge-reduced.html (accessed on 3 July 2015).
- Chengdu Punda Metal Material Co. Ltd. Reduced Iron Powder. Available online: http://uk.alibaba.com/product/60277383280-Reduced-Iron-Powder.html (accessed on 3 July 2015).
- Guizhou Dade Zhaoshen Building Materials Co. Ltd. Iron Powder/Nano Iron Powder/Reduced Iron Powder. Available online: http://uk.alibaba.com/product/60076279979-Iron-Powder-nano-iron-powder-reduced.html (accessed on 3 July 2015).
- Shanghai Xingyue Energy Technology Co. Ltd. High Purity Competitive Iron Powder Price Ton. Available online: http://uk.alibaba.com/product/60029958942-High-purity-competitive-iron-powder-price.html (accessed on 3 July 2015).
- Gansu Winshine Metallurgy Chemicals Co. Ltd. Micron Carbonyl Iron Powder. Available online: http://uk.alibaba.com/product/1815265627-Micron-carbonyl-iron-powder.html?s=p (accessed on 3 July 2015).
- Chinese Standards, Carbon Structural Steels, GB/T 700-2006. Available online: http://www.chinesestandard.net/PDF-English-Translation/GBT700-2006.html (accessed on 3 July 2015).
- Anyang Jinxiang Goods and Materials Co. Ltd. Carbon Structural Steel Used as Building_Materials Q235C U12358. Available online: http://jinxiangcn.en.alibaba.com/product/60085795767-800580517/Carbon_structural_steel_used_as_building_materials_Q235C_U12358.html (accessed on 26 June 2015).
- Chengdu Nuclear 857 New Materials Co. Ltd. China Factory Outlet Price Water-Atomized Iron Powder. Available online: http://www.alibaba.com/product-detail/China-factory-outlet-price-water-atomized_1320161433.html?spm=a2700.7724838.35.1.bpBwCt (accessed on 3 July 2015).
- Zheng Zhou Dongyao Nano Materials Co. Ltd. Nano Fe Powder, Iron Powder Price. Available online: http://uk.alibaba.com/product/60233340038-nano-Fe-powder-iron-powder-price.html (accessed on 3 July 2015).
- Zheng Zhou Dongyao Nano Materials Co. Ltd. Iron Powder Nano Ferrum Powder Price/Iron Fe Nano Powder. Available online: http://uk.alibaba.com/product/60262863989-nano-iron-powder-price-iron-Fe.html (accessed on 3 July 2015).
- Chengdu Best New Materials Co. Ltd. Best10N Iron Gate Door Prices/Nano Iron Powder. Available online: http://www.alibaba.com/product-detail/Best10N-iron-gate-door-prices-nano_60244110679.html (accessed on 5 July 2015).
- Chengdu Best New Materials Co. Ltd. Best10N CIF Price of Nano Iron Powder Price. Available online: http://www.alibaba.com/product-detail/Best10N-CIF-price-of-nano-iron_60243197709.html?spm=a2700.7724838.35.1.mqK1kf (accessed on 3 July 2015).
- Shanghai Ruizheng Chemical Technology Co. Ltd. Nano-Iron Powder. Available online: http://www.alibaba.com/product-detail/nano-iron-powder_60181455696.html?spm=a2700.7724838.35.1.XSGeDs (accessed on 3 July 2015).
- Shanghai CNPC Powder Material Co. Ltd. Nano Low Oxide Content Carbonyl Iron Powder Price. Available online: http://uk.alibaba.com/product/60217902707-nano-low-oxide-content-carbonyl-iron.html (accessed on 3 July 2015).
- Chengdu Nuclear 857 New Materials Co. Ltd. Competitive Price Iron Carbonyl Powder from Nuclear CDH857 Factory. Available online: http://uk.alibaba.com/product/60026003051-competitive-price-iron-carbonyl-powder-from.html (accessed on 3 July 2015).
- Chengdu Huarui Industrial Co. Ltd. Carbonyl Iron Powder for Powder Metallurgy. Available online: http://www.alibaba.com/product-detail/Carbonyl-Iron-powder-for-powder-metallurgy_1808258920.html?spm=a2700.”7724838.35.1.ZJ9KG2&s=p (accessed on 3 July 2015).
- Shanghai CNPC Powder Material Co. Ltd. Low Price 99% Hyperpure Superfine Carbonyl UItrafine Iron Powder Iron Alloy Powder. Available online: http://www.alibaba.com/product-detail/Low-Price-99-Hyperpure-Superfine-Carbonyl_60117436046.html?spm=a2700.7724838.35.1.ZJ9KG2 (accessed on 3 July 2015).
- Guizhou Dade Zhaoshen Building Materials Co. Ltd. Supply Nano Iron Powder/Reduced Iron Powder. Available online: http://uk.alibaba.com/product/60227762429-Supply-nano-iron-powder-reduced-iron.html (accessed on 3 July 2015).
- Chengdu Best New Materials Co. Ltd. Best10H Iron Powder Price Ton. Available online: http://uk.alibaba.com/product/60238493033-Best10H-iron-powder-price-ton.html (accessed on 3 July 2015).
- Zouping Changshan Town Zefeng Fertilizer Factory. Iron (Fe) Powder Competitive Price. Available online: http://www.alibaba.com/product-detail/Iron-Fe-Powder-Competitive-price_1421082390.html?spm=a2700.7724838.35.1.p24b5G (accessed on 3 July 2015).
- Shanghai Xingyue Energy Technology Co. Ltd. High Purity Competitive Reduced Iron Powder Price. Available online: http://www.alibaba.com/product-detail/High-purity-competitive-reduced-iron-powder_60031676891.html?spm=a2700.7724838.35.1.1EU2qr (accessed on 3 July 2015).
- Lingshou County Jiaqi Mineral Processing Factory. Manufacture Iron Powder Price. Available online: http://www.alibaba.com/product-detail/manufacture-iron-powder-price_553077006.html?spm=a2700.7724838.35.1.1EU2qr (accessed on 3 July 2015).
- Soochow Hengqiu Graphene Technology Co. Ltd. 99.99% Purity 40nm Iron Metal Nanoparticles Nano Ferrous Powders. Available online: http://www.alibaba.com/product-detail/99-99-Purity-40nm-Iron-Metal_60136367986.html?spm=a2700.7724838.35.1.4CKA6f (accessed on 3 July 2015).
- International Chamber of Commerce. The New Incoterms® 2010 Rules. Available online: http://www.iccwbo.org/products-and-services/trade-facilitation/incoterms-2010/ (accessed on 29 June 2015).
- Ramberg, J. ICC Guide to INCOTERMS® 2010; No.720E; International Chamber of Commerce (ICC) Publication: Paris, France.
- DHL Express. Basic Overview of The Incoterms® 2010 Rules. Available online: http://www.dhl.co.uk/content/dam/downloads/g0/logistics/conditions/Incoterms_2010_EN_v2.pdf (accessed on 3 July 2015).
- Owen, T. Fundamentals of UV-Visible Spectroscopy; Hewlett-Packcard Co.: Palo Alto, CA, USA, 1996. [Google Scholar]
- NanoComposix. UV/Vis/IR Spectroscopy Analysis of Nanoparticles; NanoComposix: San Diego, CA, USA, 2012. [Google Scholar]
- Taylor, R.M. Influence of chloride on the formation of iron oxides from Fe(II) chloride I: Effect of [Cl]/[Fe] on the formation of magnetite. Clays Clay Miner. 1984, 32, 167–174. [Google Scholar] [CrossRef]
- Perez, F.R.; Barrero, C.A.; Walker, A.R.H.; Garcia, K.E.; Nomura, K. Effects of chloride concentration, immersion time and steel composition on the spinel phase formation. Mater. Chem. Phys. 2009, 117, 214–223. [Google Scholar] [CrossRef]
- Remazeilles, C.; Refait, P. Formation, fast oxidation and thermodynamic data of Fe(II) hydroxychlorides. Corros. Sci. 2008, 50, 856–864. [Google Scholar] [CrossRef]
- Refait, P.; Remazeilles, C. On the formation of beta-FeOOH (akaganeite) in chloride containing environments. Corros. Sci. 2007, 50, 844–857. [Google Scholar]
- Refait, P.; Genin, J.M.R. The mechanism of oxidation of ferrous hydroxychloride beta-Fe2(OH)3Cl in chloride containing aqueous solution: The formation of beta-FeOOH akaganeite, an X-ray diffraction, Mossbauer spectroscopy and electrochemical study. Corros. Sci. 1997, 39, 539–553. [Google Scholar] [CrossRef]
- Drissi, S.H.; Refait, P.; Abdelmoula, M.; Genin, J.M.R. The preparation and thermodynamic properties of Fe(II)-Fe(III) hydroxide-carbonate (green rust a): Pourbaix diagram of iron in carbonate containing aqueous media. Corros. Sci. 1995, 37, 2025–2041. [Google Scholar] [CrossRef]
- Abdelmoula, M.; Refait, P.; Drissi, S.H.; Mihe, J.P.; Genin, J.M.R. Conversion electron Mossbauer spectroscopy and X-Ray diffraction studies of the formation of carbonate containing green rust one by corrosion of metallic iron in NaHCO3 and (NaHCO3 + NaCl) solution. Corros. Sci. 1996, 38, 623–633. [Google Scholar] [CrossRef]
- Refait, P.; Drissi, S.H.; Pytkiewicz, J.; Genin, J.M.R. The anodic species competition in iron aqueous corrosion: Role of various green rust compounds. Corros. Sci. 1997, 39, 1699–1710. [Google Scholar] [CrossRef]
- Refait, P.; Abdelmoula, M.; Genin, J.M.R. Mechanisms of formation and structure of green rust one in aqueous corrosion of iron in the presence of chloride ions. Corros. Sci. 1998, 40, 1547–1560. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Al-Judaibi, A.; Asiri, M. Corrosion of C-steel in the Red Sea: Effect of immersion time and inhibitor concentration. Int. J. Electrochem. Sci. 2015, 10, 4252–4278. [Google Scholar]
- Ruby, C.; Aissa, R.; Gehin, A.; Cortot, J.; Adelmoula, M.; Genin, J.-M. Green rusts synthesis by coprecipitation of FeII-FeIII ions and mass balance diagram. C. R. Geosci. 2006, 338, 420–443. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Peleka, E.N.; Deliyanni, E.A. Nanocrystalline akaganeite as adsorbent for surfactant removal from aqueous solutions. Materials 2013, 6, 184–197. [Google Scholar] [CrossRef]
- He, J.; Hong, S.; Zhang, L.; Gan, F.; Ho, Y.-S. Equilibrium and thermodynamic parameters of adsorption of methylene blue onto rectorite. Fresnius Environ. Bull. 2010, 19, 2651–2656. [Google Scholar]
- Zeng, Y.; Zeng, Z.; Ju, T.; Zhang, F. Adsorption-performance and mechanism of perchloroethylene on a novel nano composite β-FeOOH-AC. Microporous Mesoporoous Mater. 2015, 210, 60–68. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; He, H.; Pei, C.; He, P. A novel reusable nanocomposite: FeOOH/CBC and its adsorptive property for methyl orange. Appl. Surf. Sci. 2015, 332, 456–462. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, J.; Zhou, L. Template—Free hydrothermal synthesis of β-FeOOH nanorods and ther catalytic activity in the degradation of methyl orange by a photo-fenton-like process. Open J. Inorg. Non-Met. Mater. 2013, 3, 58–65. [Google Scholar]
- Zarzycki, P.; Kerisit, S.; Rosso, K.M. Molecular dynamics study of Fe(II) adsorption, electronic exchange, and mobility at goethite (α-FeOOH) surfaces. J. Phys. Chem. 2015, 119, 3111–3123. [Google Scholar]
- Kersten, M.; Karabacheva, S.; Vlasova, N.; Branscheid, R.; Schurk, K.; Stanjek, H. Surface complexation modelling of arsenate adsorption by akaganeite (β-FeOOH)—Dominant granular ferric hydroxide. Colloids Surf. A Physiochem. Eng. Asp. 2014, 448, 73–80. [Google Scholar] [CrossRef]
- Kozin, P.A.; Boily, J.-F. Mineral surface charge development in mixed electrolyte solutions. J. Colloid Interface Sci. 2014, 418, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Jia, Y. A facile solution approach for the synthesis of akaganeite (β-FeOOH) nanorods and their ion exchange mechanism towards As(V) ions. Appl. Surf. Sci. 2014, 290, 102–106. [Google Scholar] [CrossRef]
- Mackay, A.L. Beta-Ferric oxyhydroxide. Mineral. Mag. 1960, 32, 545–557. [Google Scholar] [CrossRef]
- Mackay, A.L. Beta-Ferric oxyhydroxide—Akaganeite. Mineral. Mag. 1962, 33, 270–280. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Komaba, S. Recent research progress on iron- and manganese-based positive electrode materials for rechargeable sodium batteries. Sci. Technol. Adv. Mater. 2014, 15. [Google Scholar] [CrossRef]
- Dante, S.; Hou, Z.; Risbud, S.; Stroeve, P. Nucleation of iron oxy-hydroxide nanoparticles by layer-by-layer polyionic assemblies. Langmuir 1999, 15, 2176–2182. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, K. Crystal splitting in the growth of β-FeO(OH). J. Cryst. Growth 2007, 308, 185–188. [Google Scholar] [CrossRef]
- Song, H.-J.; Liu, L.; Jia, X.-H. Synthesis of multiwalled carbon nanotubes/β-FeOOH nanocomposites with high adsorption capacity. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Parameshwari, R.; Priyadarshini, P.; Chandrasekaran, G. Optimization, structural, spectroscopic and magnetic studies on stable akaganeite nanoparticles via co-precipitation method. Am. J. Mater. Sci. 2011, 1, 18–25. [Google Scholar]
- Konishi, H.; Yamashita, M.; Uchida, H.; Mizuki, J. Characterisation of rust layer formed on Fe, Fe–Ni and Fe–Cr alloys exposed to Cl-rich environment by Cl and Fe K-edge XANES measurements. Mater. Trans. 2005, 46, 329–336. [Google Scholar] [CrossRef]
- Post, J.E.; Heany, P.J.; von Dreele, R.B.; Hanson, J.C. Neutron and temperature resolved synchrotron X-ray powder diffraction study of akaganeite. Am. Mineral. 2003, 88, 782–788. [Google Scholar]
- Yue, J.; Jiang, X.; Yu, A. Experimental and theoretical study on the β-FeOOH nanorods: Growth and conversion. J. Nanopart. Res. 2011, 13, 3961–3974. [Google Scholar] [CrossRef]
- Singh, S.S.; Kodama, H. Effect of the presence of aluminum ions in iron solutions on the formation of iron oxyhydroxides (FeOOH) at room temperature under acidic environment. Clays Clay Miner. 1994, 42, 606–616. [Google Scholar] [CrossRef]
- Garcia, K.E.; Barrero, C.A.; Morales, A.L.; Greneche, J.M. Characterization of akaganeite synthesed in the presence of Al3+, Cr3+ and Cu2+ ions and urea. Mater. Chem. Phy. 2008, 112, 120–126. [Google Scholar] [CrossRef]
- Ishikawa, T.; Katoh, R.; Yasukawa, A.; Kandori, K.; Nakayama, T.; Yuse, F. Influence of metals on the formation of β-FeOOH particles. Corros. Sci. 2001, 43, 1727–1738. [Google Scholar] [CrossRef]
- Nesterova, M.; Moreau, J.; Banfield, J.F. Model biomimetic study of templated growth and assembly of nanocrystalline FeOOH. Geochim. Cosmochim. Acta 2003, 67, 1177–1187. [Google Scholar] [CrossRef]
- Peulon, S.; Baraize, Q.; Chausse, A. Iron compounds electrodeposited onto a transparent semiconductor: Synthesis and characterization by UV-vis spectroscopy. Electrochim. Acta 2007, 52, 7681–7688. [Google Scholar] [CrossRef]
- Tiwari, D.; Lee, S.-M. Ferrate (VI) in the treatment of waste waters: A new generation green chemical. In Waste Water Treatment and Reutilization, 1st ed.; Einschlag, F.S.G., Ed.; Intech: Rijeka, Croatia, 2011; Chapter 12; pp. 241–276. [Google Scholar]
- Liu, Y.-C.; Xie, J.-L. Electrochemical preparation of sodium ferrate (VI) using silicon iron as anode in non-diaphragm electrobath. J. Sichuan Univ. (Nat. Sci. Ed.) 2004, 41, 373–378. [Google Scholar]
- Geng, F.; Zhao, Z.; Geng, J.; Cong, H.; Cheng, H.-M. A simple and low temperature hydrothermal route for the synthesis of tubular alpha FeOOH. Mater. Lett. 2007, 61, 4794–4796. [Google Scholar] [CrossRef]
- Sherman, D.M.; Waite, T.D. Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Mineral. 1985, 70, 1262–1269. [Google Scholar]
- Villalba, J.C.; Constantino, V.R.I.; Anaissi, F.J. Iron oxyhydroxide nanostructured in montmorillonite clays: Preparation and characteristics. J. Colloid Interface Sci. 2010, 349, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Szali, Z.; Kiss, K.; Jakab, G.; Sipos, P.; Belucz, B.; Nemeth, T. The use of UV-VIS-NIR reflectance spectroscopy to identify iron minerals. Astron. Nachr. 2013, 334, 940–943. [Google Scholar] [CrossRef]
- Castellan, G.W. Physical Chemistry, 3rd ed.; Addison Wesley: Reading, MA, USA, 1983. [Google Scholar]
- Janacek, K.; Sigler, K. Osmotic pressure: Thermodynamic basis and units of measurement. Folio Microbiol. 1996, 44, 2–9. [Google Scholar] [CrossRef]
- Combes, J.M.; Manceau, A.; Calas, G.; Bottero, J.Y. Formation of ferric oxides from aqueous solutions: A polyhedral approach by X-ray absorption spectroscopy: I. Hydrolysis and formation of ferric gels. Geochim. Cosmochim. Acta 1989, 53, 583–594. [Google Scholar] [CrossRef]
- Rustad, J.R.; Felmy, A.R.; Hay, B.P. Molecular statics calculations for iron oxide and oxyhydroxide minerals: Towards a flexible model of the reactive mineral-water interface. Geochim. Cosmochim. Acta 1996, 60, 1553–1562. [Google Scholar] [CrossRef]
- Hiemstra, T.; van Riemsdijk, W.H. Adsorption and surface oxidation of Fe(II) on metal (hydr)oxides. Geochim. Cosmochim. Acta 2007, 71, 5913–5933. [Google Scholar] [CrossRef]
- Ghose, S.K.; Waychunas, G.A.; Trainor, T.P.; Eng, P.J. Hydrated goethite (α-FeOOH) (100) interface structure: Ordered water and surface functional groups. Geochim. Cosmochim. Acta 2010, 74, 1943–1953. [Google Scholar] [CrossRef]
- Majzian, J.; Mazeina, L.; Navrotsky, A. Enthalpy of water adsorption and surface enthalpy of lepidocrocite (γ-FeOOH). Geochim. Cosmochim. Acta 2007, 71, 615–623. [Google Scholar] [CrossRef]
- Keller, A.A.; Garner, K.; Miller, R.J.; Lenihan, H.S. Toxicity of nano-zero valent iron to freshwater and marine organisms. PLoS ONE 2012, 7, e43983. [Google Scholar] [CrossRef] [PubMed]
- Kozin, P.A. Charge Development at Iron Oxyhydroxide Surfaces. Ph.D. Thesis, Umea University, Umea, Sweden, 2014. [Google Scholar]
- Sverjensky, D.A. Prediction of surface charge on oxides in salt solutions: Revisions for 1:1 (M+L−) electrolytes. Geochim. Cosmochim. Acta 2005, 69, 225–257. [Google Scholar] [CrossRef]
- Kozin, P.A.; Shchukarev, A.; Bolly, J.-F. Electrolyte ion binding at iron oxyhydroxide mineral surfaces. Langmuir 2013, 29, 12129–12137. [Google Scholar] [CrossRef] [PubMed]
- Tourky, A.R.; Azim, A.A.A.; Anwar, M.M. Effect of carbon content on the corrosion and passivity of iron. Corros. Sci. 1965, 5, 301–317. [Google Scholar] [CrossRef]
- Zhao, X.; Johnston, C.; Grant, P.S. A novel hybrid supercapacitor with a carbon nanotube cathode and an iron oxide/carbon nanotube composite anode. J. Mater. Chem. 2009, 46, 8755–8760. [Google Scholar] [CrossRef]
- Li, Y.; Kang, L.; Bai, G.; Li, P.; Deng, J.; Liu, X.; Yang, Y.; Gao, F.; Liang, W. Solvothermal synthesis of Fe2O3 loaded activated carbon as electrode materials for high performance electrochemical capacitors. Electrochem. Acta 2014, 134, 67–75. [Google Scholar] [CrossRef]
- Xu, L.; Xia, J.; Xu, H.; Yin, S.; Wang, K.; Huang, L.; Wang, L.; Li, H. Reactable ionic liquid assisted solvothermal synthesis of graphite like C3N4 hybridized alpha Fe2O3 hollow microspheres with enhanced supercapacitive performance. J. Power Sources 2014, 245, 866–874. [Google Scholar] [CrossRef]
- Nasibi, M.; Golozar, M.A.; Rashed, G. Nano iron oxide (Fe2O3)/carbon black electrodes as electrode material for electrochemical capacitors: Effect of the nano-particles dispersion quality. Mater. Chem. Phys. 2013, 139, 12–16. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Chaudhari, S.; Yu, J.-S. Cube like alpha Fe2O3 supported on ordered multimodal porous carbon as high performance electrode material for supercapacitors. ChemSusChem 2014, 7, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Qian, G.; Watkins, J.J. Additive driven self assembly of well ordered mesoporous carbon/iron oxide nanoparticle composites for supercapacitors. Chem. Mater. 2014, 26, 2128–2137. [Google Scholar] [CrossRef]
- Sethuraman, B.; Purushothaman, K.K.; Muralidharan, G. Synthesis of mesh-like Fe2O3/C nano-composite via greener route for high performance supercapacitors. RSC Adv. 2014, 4, 4631–4637. [Google Scholar] [CrossRef]
- He, X.; Zhao, N.; Qiu, J.; Xiao, N.; Yu, M.; Yu, C.; Zhang, Z.; Zheng, M. Synthesis of hierarchial porous carbons for supercapacitors from coal tar pitch with nano-Fe2O3 as template and activation agent coupled with KOH activation. J. Mater. Chem. A 2013, 1, 9440–9448. [Google Scholar] [CrossRef]
- Chen, L.-F.; You, Z.Y.; Wang, J.J.; Li, Q.-X.; Tan, Z.-Q.; Zhu, Y.-W.; Yu, S.-H. Metal like fluoride doped beta FeOOH nanorods grown on carbon cloth for scalable high performance supercapacitors. Nano Energy 2015, 11, 119–128. [Google Scholar] [CrossRef]
- Xiao, T.; Yang, C.; Lu, Y.; Zeng, F. One-Pot hydrothermal synthesis of rod-like FeOOH/reduced grapheme oxide composites for supercapacitor. J. Mater. Sci. Mater. Electron. 2014, 25, 3364–3374. [Google Scholar] [CrossRef]
- Barik, R.; Jena, B.K.; Dash, A.; Mohapatra, M. In situ synthesis of flowery shaped alpha FeOOH/Fe2O3 nanoparticles and their phase dependent supercapacitive behaviour. RSC Adv. 2014, 4, 18827–18834. [Google Scholar] [CrossRef]
- Lee, K.K.; Ng, R.W.Y.; She, K.K.; Chin, W.S.; Sow, C.H. Vertically aligned iron (III) oxyhydroxide/oxide nanosheets grown on iron substrates for electrochemical charge storage. Mater. Lett. 2014, 118, 150–163. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Solution combustion synthesis of Fe2O3/C, Fe2O3–SnO2/C, Fe2O3-ZnO/C composites and their electrochemical characterization in non-aqueous electrolyte for supercapacitor application. Int. J. Electrochem. Sci. 2009, 4, 878–886. [Google Scholar]
- Chen, L.-F.; Yu, Z.-Y.; Ma, X.; Li, Z.-Y.; Yu, S.H. In situ hydrothermal growth of ferric oxides on carbon cloth for low cost and scalable high-energy-density supercapacitors. Nano Energy 2014, 9, 345–354. [Google Scholar] [CrossRef]
- Tang, O.; Wang, W.; Wang, G. The perfect matching between the low cost Fe2O3 nanowire anode and NiO nanoflake cathode significantly enhances the energy density of asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 6662–6670. [Google Scholar] [CrossRef]
- Kang, L.; Xie, L.; Li, P.; Liu, T.; Zhang, X.; Luo, J.; Liang, W. One-Step combustion synthesis of CNTs doped Fe2O3/C nanocomposites as electrode materials for supercapacitors. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 715–720. [Google Scholar] [CrossRef]
- Kulal, P.M.; Dubal, D.P.; Lokhande, C.D.; Fulari, V.J. Chemical synthesis of Fe2O3 thin films for supercapacitor applications. J. Alloys Compd. 2011, 509, 2567–2571. [Google Scholar] [CrossRef]
- Sun, S.; Lang, J.; Wang, R.; Kong, L.; Li, X.; Yan, X. Identifying pseudocapacitance of Fe2O3 in an ionic liquid and its application in asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 14550–14556. [Google Scholar] [CrossRef]
- Tan, H.T.; Ru, X.; Yu, H.; Liu, W.; Xu, C.; Zu, Z.; Hng, H.H.; Yan, Q. Aqueous based chemical route towards ambient preparation of multicomponent core-shell nanotubes. ACS Nano 2014, 8, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Q.; Zan, G. Facile synthesis and high electrochemical performance of porous carbon composites for supercapacitors. RSC Adv. 2014, 4, 35186–35192. [Google Scholar] [CrossRef]
- Luo, P.-W.; Yu, J.-G.; Shi, Z.-Q.; Huang, H.; Liu, L.; Zhao, Y.-N.; Li, G.-D.; Zou, Y.-C. Preparation and supercapacitive properties of Fe2O3/active carbon nanocomposites. Chem. Res. Chin. Univ. 2012, 28, 780–783. [Google Scholar]
- Zhu, C.; Saito, G.; Akiyama, T. A facile solution combustion synthesis of nanosized amorphous iron oxide incorporated in a carbon matrix for use as a high-performance lithium ion battery anode material. J. Alloys Compd. 2015, 633, 424–429. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Wang, D.; Li, X.; Dou, Y.; Liu, Y.; Pan, H. Chemical vapor deposition prepared bi-morphological carbon coated Fe3O4 composites as anode materials for lithium ion batteries. J. Power Sources 2015, 282, 257–264. [Google Scholar] [CrossRef]
- Valvo, M.; Lindgren, F.; Lafont, U.; Bjorefors, F.; Edstrom, K. Towards more sustainable negative electrodes in Na-ion batteries via nanostructured iron oxide. J. Power Sources 2014, 245, 967–978. [Google Scholar] [CrossRef]
- Huang, B.; Tai, K.; Zhang, M.; Xiao, Y.; Dillon, S.J. Comparative study of Li and Na electrochemical reactions with iron oxide nanowires. Electrochim. Acta 2014, 118, 143–149. [Google Scholar] [CrossRef]
- Shimizu, K.; Boily, J.F. Electrochemical signatures of crystallographic orientation and counterion binding at the hematite water interface. J. Phys. Chem. C 2015, 119, 5988–5994. [Google Scholar] [CrossRef]
- Perry, J.H. Chemical Engineers Handbook, 2nd ed.; McGraw Hill: New York, NY, USA, 1941. [Google Scholar]
- Gil-Lalaguna, N.; Sanchez, J.L.; Murillo, M.B.; Gea, G. Use of sewage sludge combustion ash and gasification ash for high temperature desulphurization of different gas streams. Fuel 2015, 141, 99–106. [Google Scholar] [CrossRef]
- McManus, D.; Martell, A.E. The evolution, chemistry, and applications of cheleted iron hydrogen sulphide removal and oxidation process. J. Mol. Catal. A Chem. 1997, 117, 289–297. [Google Scholar] [CrossRef]
- Kotanigawa, T.; Takahashi, H.; Yokoyama, S.; Yanamoto, M.; Maekawa, Y. Mechanism for formation of sulphate in S-promoted iron oxide catalysts for coal liquefaction. Fuel 1988, 67, 927–931. [Google Scholar] [CrossRef]
- Ebbing, D.D.; Gammon, S.D. General Chemistry, 6th ed.; Houghton Mifflin Co.: Boston, MA, USA, 1999. [Google Scholar]
- EU. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Available online: http://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:31998L0083 (accessed on 26 June 2015).
- World Health Organization (WHO). Guidelines for Drinking Water Quality; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Karthika, I.N.; Dheenadayalan, M.S. Study of groundwater quality at selected locations in Dindigul District, India. J. Adv. Chem. Sci. 2015, 1, 67–69. [Google Scholar]
- Bureau of Indian Standards (BIS). In Indian Standard Drinking Water—Specification, (Second Revision); IS 10500; BIS: New Delhi, India, 2012.
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Han, B.; Choi, J.H.; Dantzig, J.A.; Bischof, J.C. A quantitative analysis on latent heat of an aqueous binary mixture. Cryobiology 2006, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K. Modeling of dissolved oxygen concentration recovery in water bodies and application to hypoxic water bodies. World Environ. 2013, 3, 52–59. [Google Scholar]
- Lougee, L.A.; Bollens, S.M.; Avent, S.R. The effects of haloclines on the vertical distribution and migration of zooplankton. J. Exp. Mar. Biol. Ecol. 2002, 278, 111–134. [Google Scholar] [CrossRef]
- Tianjin Yandong Mining Co. Ltd. Low Price Chinese Natural Potassium Feldspar with high K2O. Available online: http://www.alibaba.com/product-detail/Low-Price-Chinese-Natural-Potassium-Feldspar_1940165894.html (accessed on 3 July 2015).
- Mantra Minerals Private Ltd. High Grade Potash Feldspar for Ceramic Competitive Price India. Available online: http://www.alibaba.com/product-detail/High-grade-Potash-Feldspar-for-ceramic_116467809.html (accessed on 3 July 2015).
- Jiangxi Jiali Biochemical High Tech Co. Ltd. High Quality Potash Feldspar in Hot Sale. Available online: http://www.alibaba.com/product-detail/High-Quality-Potash-Feldspar-In-Hot_60137109155.html?s=p (accessed on 3 July 2015).
- Antia, D.D.J. Water remediation—Water remediation using nano-zero-valent metals (n-ZVM). In CRC Concise Encyclopedia of Nanotechnology, 1st ed.; Kharisov, B.I., Kharissova, O.V., Ortiz-Mendez, U., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; Chapter 84; ISBN 9781466580343. (In Press) [Google Scholar]
- Olowe, A.A.; Genin, J.M.R. The mechanism of oxidation of ferrous hydroxide in sulphated aqueous media: Importance of the initial ratio of the reactants. Corros. Sci. 1991, 32, 965–984. [Google Scholar] [CrossRef]
- Refait, P.; Genin, J.M.R. The transformation of chloride containing green rust one into sulphated green rust two by oxidation in mixed Cl− and SO42− aqueous media. Corros. Sci. 1994, 36, 55–65. [Google Scholar] [CrossRef]
- Simon, L.; Refait, P.; Genin, J.M.R. Transformation of Fe(II)–Fe(III) hydroxysulphite into hydroxysulphate green rusts. Hyperfine Interact. 1998, 112, 217–222. [Google Scholar] [CrossRef]
- Genin, J.M.R.; Olowe, A.A.; Refait, P.; Simon, L. On the stoichiometry and Pourbaix diagram of Fe(II)-Fe(III) hydroxyl-sulphate or sulphate containing green rust 2: An electrochemical and Mossbauer spectroscopy study. Corros. Sci. 1996, 38, 1751–1762. [Google Scholar] [CrossRef]
- Oh, S.J.; Kwon, S.J.; Lee, J.-Y.; Yoo, J.-Y.; Choo, W.-Y. Oxidation of Fe2+ ions in sulphate and chloride containing aqueous media. Corrosion 2002, 58, 498–504. [Google Scholar] [CrossRef]
- Davesne, E.; Dideriksen, K.; Christiansen, B.C.; Sonne, M.; Ayala-Luis, K.B.; Koch, C.B.; Hansen, H.C.B.; Stipp, S.L.S. Free energy of formation of green rust sodium sulphate (NaFeII6FeIII3(OH)18(SO4)2(s)). Geochim. Cosmochim. Acta 2010, 74, 6451–6467. [Google Scholar] [CrossRef]
- Ning, J.; Zheng, Y.; Young, D.; Brown, B.; Nesic, S. A thermodynamic study of hydrogen sulphide corrosion of mild steel. Corrosion 2014, 70, 375–389. [Google Scholar] [CrossRef]
- Descostes, M.; Vitorge, P.; Beaucaire, C. Pyrite dissolution in acidic media. Geochim. Cosmochim. Acta 2004, 68, 4559–4569. [Google Scholar] [CrossRef]
- Wearing, C.L. Changes in Fluxes of Dissolved Organic Carbon (DOC) from Small Catchments in Central Scotland. Ph.D. Thesis, University of Stirling, Stirling, UK, 2008. [Google Scholar]
- Refait, P.; Nguyen, D.D.; Jeannin, M.; Sable, S.; Langumier, M.; Sabot, R. Electrochemical formation of green rusts in deaerated seawater like solutions. Electrochim. Acta 2011, 56, 6481–6488. [Google Scholar] [CrossRef]
- Reffass, M.; Sabot, R.; Saavall, C.; Jeannin, M.; Creus, J.; Refait, P. Localised corrosion of carbon steel in NaHCO3/NaCl electrolytes: Role of Fe(II) containing compounds. Corros. Sci. 2006, 48, 709–726. [Google Scholar] [CrossRef]
- Lee, C.T.; Qin, Z.; Odziemkowski, M.; Shoesmith, D.W. The influence of groundwater anions on the impedence behaviour of carbon steel corroding under anoxic conditions. Electrochem. Acta 2006, 51, 1558–1568. [Google Scholar] [CrossRef]
- Xin, J.; Zheng, X.; Han, J.; Shao, H.; Kolditz, O. remediation of trichloroethylene in xanthan gum coated microscale zero valent iron (XG-mZVI) in groundwater: Effects of geochemical constituents. Chem. Eng. J. 2015, 271, 164–172. [Google Scholar] [CrossRef]
- Genin, J.M.R.; Ruby, C.; Gehin, A.; Refait, P. Synthesis of green rusts by oxidation of Fe(OH)2, their products of oxidation and reduction of ferric oxyhydroxides: Eh-pH Pourbaix diagrams. C. R. Geosci. 2006, 338, 433–446. [Google Scholar] [CrossRef]
- United Nations Educational Scientific and Cultural Organization (UNESCO). The United Nations World Water Development Report; UNESCO: Paris, France, 2014. [Google Scholar]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane technology for water production in agriculture: Desalination and waste water reuse. Desalination 2015, 364, 17–32. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Tang, C.Y.; le Clech, P. Opportunities to reach economic sustainability in forward osmosis-reverse osmosis hybrids for seawater desalination. Desalination 2015, 363, 26–36. [Google Scholar] [CrossRef]
- Bundschuh, J.; Ghaffour, N.; Mahmoudi, H.; Goosen, M.; Mushtaq, S.; Hoinkis, J. Low-cost low enthalpy geothermal heat for freshwater production: Innovative applications using thermal desalination process. Renew. Sustain. Energy Rev. 2015, 43, 196–206. [Google Scholar] [CrossRef]
- Burn, S.; Hoang, M.; Zarzo, D.; Olewiak, F.; Campos, E.; Bolto, B.; Barron, O. Desalination techniques—A review of the opportunities in agriculture. Desalination 2015, 364, 2–26. [Google Scholar] [CrossRef]
- Avrin, A.-P.; He, G.; Kammen, D.M. Assessing the impacts of nuclear desalination and geoengineering to address China’s water shortages. Desalination 2015, 360, 1–7. [Google Scholar] [CrossRef]
- Zotalis, K.; Dialynas, E.G.; Mamassis, N.; Angelakis, A.N. Desalination technologies: Hellenic Experience. Water 2014, 6, 1134–1150. [Google Scholar] [CrossRef]
- Dubai Electricty and Water Authority 2015 Slab Tariff. 2 March 2015; Dubai Electricty and Water Authority, Government of Dubai. Available online: http://www.dewa.gov.ae/tariff/tariffdetails.aspx (accessed on 1 May 2015).
- Barron, O.; Ali, R.; Hodgson, G.; Smith, D.; Qureshi, E.; McFarlane, D.; Campos, E.; Zarzo, D. Feasibility assessment of desalination application in Australian traditional agriculture. Desalination 2015, 364, 33–45. [Google Scholar] [CrossRef]
- Suresh, M.; Gurugnanam, B.; Vasudevan, S.; Rao, S.V.L.; Kumaravel, S. Groundwater quality assessment for irrigation uses in Upper Thirumanimuthar sub basin, Cauvery river, Tamil Nadu, India. J. Appl. Geochem. 2010, 12, 95–105. [Google Scholar]
- Varade, A.M.; Yenkie, R.O.; Kodate, J. Assessment of water quality in and around Hingna area of Nagpur District, Maharashtra for irrigational purposes. J. Appl. Geochem. 2013, 15, 488–505. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Irrigation and Drainage Paper No. 29, Rev. 1, Reprinted 1989, 1994; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Terjung, W.H.; Hayes, J.T.; Ji, H.-Y.; O-Rourke, P.A.; Todhunter, P.E. Crop water requirements for rainfed and irrigated rice (Paddy) in China. Arch. Meteorl. Geophys. Bioclimatol. Ser. B 1984, 34, 181–202. [Google Scholar] [CrossRef]
- Antia, D.D.J. Desalination of groundwater and impoundments using nano-zero valent iron, n-Fe0. Meterol. Hydrol. Water Manag. 2015, 3, 21–38. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding counter measures: The development in zero valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, Y. In Situ Remediation of Inorganic Contaminants Using Stabilized Zero-Valent Iron Nano-Particles. U.S. Patent 7,635,236 B2, 30 March 2006. [Google Scholar]
- Tepong-Tsinde, R.; Phukan, M.; Nassi, A.; Noubactep, C.; Ruppert, H. Validating the efficiency of the MB discoloration method for the characterization of Fe0/H2O systems ussing accelerated corrosion by chloride ions. Chem. Eng. J. 2015, 279, 353–362. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Wang, H.; Liu, L.; Xiao, C.; Ma, D. The effects of ionic additives on the aqueous phase Fischer Tropsch synthesis with a ruthenium nonparticle catalyst. Catal. Today 2012, 183, 143–153. [Google Scholar] [CrossRef]
- Antia, D.D.J. Low temperature oil polymerisation and hydrocarbon expulsion from continental shelf and continental slope sediments. Indian J. Pet. Geol. 2009, 16, 1–30. [Google Scholar]
- Antia, D.D.J. Hydrocarbon Formation in Immature Sediments. Adv. Pet. Explor. Dev. 2011, 1, 1–13. [Google Scholar]
- Antia, D.D.J. Polymerisation theory—Formation of hydrocarbons in sedimentary strata (hydrates, clays, sandstones, carbonates, evaporites, volcanoclastics) from CH4 and CO2: Part I. Polymerisation concept, kinetics, sources of hydrogen and redox environment. Indian J. Pet. Geol. 2009, 17, 49–86. [Google Scholar]
- Antia, D.D.J. Polymerisation theory—Formation of hydrocarbons in sedimentary strata (hydrates, clays, sandstones, carbonates, evaporites, volcanoclastics) from CH4 and CO2: Part II: Formation and interpretation of stage 1 to stage 5 oils. Indian J. Pet. Geol. 2010, 17, 11–70. [Google Scholar]
- Antia, D.D.J. Polymerisation theory—Formation of hydrocarbons in sedimentary strata (hydrates, clays, sandstones, carbonates, evaporites, volcanoclastics) from CH4 and CO2: Part III: Hydrocarbon expulsion from the hydrodynamic flow regimes contained within a generating pressure mound. Indian J. Pet. Geol. 2010, 18, 1–50. [Google Scholar]
- Antia, D.D.J. Polymerisation theory—Formation of hydrocarbons in sedimentary strata (hydrates, clays, sandstones, carbonates, evaporites, volcanoclastics) from CH4 and CO2: Part IV: Polymerisation modelling of sequestered carbon dioxide and waste organic liquids to hydrocarbons. Indian J. Pet. Geol. 2011, 18, 1–45. [Google Scholar]
- Li, M.; Shi, Z.-M.; Liu, Y.-B. Crevice corrosion of K60 in dry desertification saline soil. J. Iron Steel Res. Int. 2013, 20, 76–80. [Google Scholar] [CrossRef]
- Stefansson, A.; Lemke, K.H.; Seward, T.M. Iron (III) complexation in hydrothermal solutions—An experimental and theoretical study. In Proceedings of the 15th International Conference on the Properties of Water and Steam, Berlin, Germany, 7–11 September 2008; pp. 1–7.
- Nesic, S. Key issues related to the internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Perez, F.R.; Barrero, C.A.; Amache, O.; Sanchez, L.C.; Garcia, K.E.; Walker, A.R.H. Structural properties of iron phases formed on low alloy steels immersed in sodium chloride rich solutions. Physica B 2009, 404, 1347–1353. [Google Scholar] [CrossRef]
- Hasan, B.O.; Sadek, S.A. Corrosion behaviour of carbon steel in oxygenated sodium sulphate solution under different operating conditions. Adv. Chem. Eng. Res. 2013, 2, 61–71. [Google Scholar]
- Ismail, A.; Adan, N.H. Effect of oxygen concentration on corrosion rate of carbon steel in seawater. Am. J. Eng. Res. 2014, 3, 64–67. [Google Scholar]
- Xu, X.; Cao, C.; Zhou, Y. Facile synthesis of single crystalline mesoporous hematite nano-rods with enhanced supercapacitive performance. Electrochim. Acta 2015, 156, 257–262. [Google Scholar] [CrossRef]
- Branan, C. Rules of Thumb for Chemical Engineers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Curran, G. Water for Livestock: Interpreting Water Quality Tests; Primefact No. 533; New South Wales Department of Primary Industry: Orange, New South Wales, Australia, 2007. [Google Scholar]
- Masters, D.G.; Benes, S.E.; Norman, H.C. Biosaline agriculture for forage and livestock production. Agric. Ecosyst. Environ. 2007, 119, 234–248. [Google Scholar] [CrossRef]
- PIRSA. Livestock Industries Livestock Water Intakes and Salinity Tolerances; Fact Sheet, No. 03/07; Government of South Australia, Primary Industries and Resources SA: Adelaide, Australia, 2006.
- Glauert, S. Livestock and Water Salinity; Farm Note: 249; Government of Western Australia, Department of Agriculture and Food: Perth, Australia, 2007.
- New South Wales Government, Department of Primary Industries (NSW DPI). Water Requirements for Sheep and Cattle; Prime Facts 326; NSW DPI: Sydney, Australia, 2014.
- Di Noto, V.; Mecozzi, M. Determination of seawater salinity by ultraviolet spectroscopic measurement. Appl. Spectrosc. 1997, 61, 1294–1302. [Google Scholar] [CrossRef]
- Chai, B.-H.; Zheng, J.-M.; Zhao, Q.; Pollack, G.H. Spectroscopic studies of solutes in aqueous solution. J. Phys. Chem. A 2008, 112, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, K.; Li, J.; Xu, J.; Liu, S. Simultaneous determination of chlorine dioxide and hypochlorous acid in bleaching system. Bioresources 2011, 6, 1868–1879. [Google Scholar]
- Toniolo, A.; Persico, M.; Pitea, D. Theoretical photoabsorption spectra of ClOOCl and Cl2O. J. Phys. Chem. A 2000, 104, 7278–7283. [Google Scholar] [CrossRef]
- Young, I.A.K.; Jones, R.L.; Pope, F.D. The UV and visible spectra of chlorine peroxide: Constraining the atmospheric photolysis rate. Geophys. Res. Lett. 2014, 41, 1781–1788. [Google Scholar] [CrossRef]
- Neta, P.; Hule, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1039. [Google Scholar] [CrossRef]
- Chipman, D.M. Absorption spectrum of OH radical in water. J. Phys. Chem. A 2008, 112, 13372–13381. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, S. Alkyl radicals as hydrogen bond acceptors: Computational evidence. J. Am. Chem. Soc. 2009, 131, 8627–8635. [Google Scholar] [CrossRef] [PubMed]
- Bill, A.; Lakajka, Z. The hydroperoxy radical as a hydrogen bond acceptor: HOO–HCl complexes—Ab initio study. J. Comput. Chem. 2006, 27, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Beseley, N.A. Proton transfer or hemibonding? The structure and stability of radical cation clusters. Phys. Chem. Chem. Phys. 2013, 15, 16214–16219. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L. Reactivity of HO2/O2− Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Bosinceanu, R.; Sulitanu, N. Synthesis and characterization of FeO(OH)/Fe3O4 nanoparticles encapsulated in zeolite matrix. J. Optoelectron. Adv. Mater. 2008, 10, 3482–3486. [Google Scholar]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. Preparation and characterization of zero valent iron nanoparticles. Dig. J. Nanomater. Biostruct. 2011, 6, 1771–1776. [Google Scholar]
- Thomas, O.; Burgess, C. UV-Visible spectrophotometry of water and waste water. Tech. Instrum. Anal. Chem. 2007, 27, 1–360. [Google Scholar]
- Gimenez, P.; Rameau, J.J.; Reboul, M.C. Experimental pH potential diagram of aluminium for seawater. Corrosion 1981, 37, 673–682. [Google Scholar] [CrossRef]
- Younis, A. Protection of Aluminium Alloy (AA7075) from Corrosion by Sol Gel Technique. Ph.D. Thesis, Chemnitz University of Technology, Chemnitz, Germany, 2012. [Google Scholar]
- Nestoridi, M. The Study of Aluminium Anodes for High Power Density Al-Air Batteries with Brine Electrolytes. Ph.D. Thesis, University of Southampton, Southampton, UK, 2008. [Google Scholar]
- Arjmand, F.; Adrianens, A. Influence of pH and chloride concentration on the corrosion behaviour of unalloyed copper in NaCl solution: A comparative study between the micro and macro scales. Materials 2012, 5, 2439–2464. [Google Scholar] [CrossRef] [Green Version]
- Bojinov, M.; Makela, K. Corrosion of Copper in Anoxic 1 M NaCl Solution; Report 2003-45; VTT Technical Research Centre of Finland, Posiva OY: Eurajoki, Finland, 2003. [Google Scholar]
- Alfantazi, A.M.; Ahmed, T.M.; Tromans, D. Corrosion behaviour of copper alloys in chloride media. Mater. Des. 2009, 30, 2425–2430. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagram for iron at 25–300 °C. Corros. Sci. 1996, 38, 2121–2135. [Google Scholar] [CrossRef]
- Azoulay, I.; Remazeilles, C.; Refait, P. Determination of standard Gibbs Free energy of formation of chukanovite and Pourbaix diagrams of iron in carbonated media. Corros. Sci. 2012, 58, 229–236. [Google Scholar] [CrossRef]
- Bottero, J.-Y.; Manceau, A.; Villieras, F.; Tchoubar, D. Structure and mechanisms of formation of FeOOH (Cl) polymer. Langmuir 1994, 10, 316–319. [Google Scholar] [CrossRef]
- Bagheri, S.; Chandrappa, K.G.; Hamid, S.B.A. Generation of hematite nanoparticles via sol-gel method. Res. J. Chem. Sci. 2013, 3, 62–68. [Google Scholar]
- Ristic, M.; Music, S.; Godec, M. Properties of gamma-FeOOH, alpha-FeOOH and alpha-Fe2O3 particles precipitated by hydrolysis of Fe3+ ions in perchlorate containing aqueous solution. J. Alloys Compd. 2006, 417, 292–299. [Google Scholar] [CrossRef]
- Bouniol, P. Influence of iron on water radiolysis in cement based materials. J. Nucl. Mater. 2010, 403, 167–183. [Google Scholar] [CrossRef]
- BS ISO 11271:2002, BS 7755-3.14:2002, Soil Quality. Determination of Redox Potential. Field Method. November 2002. Available online: http://shop.bsigroup.com/ProductDetail/?pid=000000000030040608 (accessed on 3 July 2015).
- ISO 11271:2002, Soil Quality—Determination of Redox potential—Field Method. Available online: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=34002 (accessed on 3 July 2015).
- Jardim, W.F. Medicao e interpretacao de valores do potencial redox (EH) em matrizes ambientais. Quim. Nova 2014, 37, 1233–1235. [Google Scholar]
- Ricca. In Oxidation-Reduction Potential (ORP); Technical Reference Document #19; Ricca Chemical Company: Arlington, TX, USA, 2005; Available online: http://www.riccachemical.com/Documents/TRD19.pdf (accessed on 26 June 2015).
- Striggow, B. Field Measurement of Oxidation-Reduction Potential (ORP); SESDPROC-113-R1; US Environmental Protection Agency: Athens, GA, USA, 2013.
- Thermo. Water Analysis Instruments Online Library ORP – mV, RmV, and Eh. Available online: https://static.fishersci.com/cmsassets/downloads/segment/Scientific/pdf/WaterAnalysis/Log121TipORPmVRmVandEh.pdf (accessed on 26 June 2015).
- Unisense. Redox and Reference Electrode Manual; Unisense a/s: Aarhus, Denmark, 2012; Available online: http://www.unisense.com/files/PDF/Manualer/Redox%20Sensor%20Manual.pdf (accessed on 26 June 2015).
- Van Walt. Reporting Redox (ORP Reporting); Van Walt Ltd.: Surrey, UK, 2012; Available online: http://www.vanwalt.com/pdf/information-sheets/RedoxReporting.pdf (accessed on 26 June 2015).
- Nazaruk, J.; Gudej, J. Flavonoid compounds from the flowers of Cisium Rivulare (Jacq.) All. Acta Pol. Pharm. Drug Res. 2003, 63, 87–89. [Google Scholar]
- Hsu, P.H. Appearance and stability of hydrolyzed Fe(ClO4)3 solutions. Clays Clay Miner. 1973, 21, 267–277. [Google Scholar] [CrossRef]
- Ockermann, L.T.; Schreyer, J.M. Preparation of sodium ferrate. J. Am. Chem. Soc. 1951, 73, 5478. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Lloyd, B. Progress in the development and use of ferrate (VI) salt as an oxidant and coagulant forwater and waste water treatment. Water Res. 2002, 36, 1397–1408. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Zerouale, A.; Ijjaali, M.; Mohamed, S. Synthesis and characterization of ferrate (VI) alkali metal by wet method. Int. J. Mod. Eng. Res. 2012, 2, 4521–4523. [Google Scholar]
- Deininger, J.P.; Dotson, R.L. Process for the Electrochemical Production of Sodium Ferrate (Fe(VI)). U.S. Patent 4,535,257, 1 July 1983. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antia, D.D.J. Desalination of Water Using ZVI (Fe0). Water 2015, 7, 3671-3831. https://doi.org/10.3390/w7073671
Antia DDJ. Desalination of Water Using ZVI (Fe0). Water. 2015; 7(7):3671-3831. https://doi.org/10.3390/w7073671
Chicago/Turabian StyleAntia, David D. J. 2015. "Desalination of Water Using ZVI (Fe0)" Water 7, no. 7: 3671-3831. https://doi.org/10.3390/w7073671