Preliminary Toxicological Evaluation of the River Danube Using in Vitro Bioassays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Samples
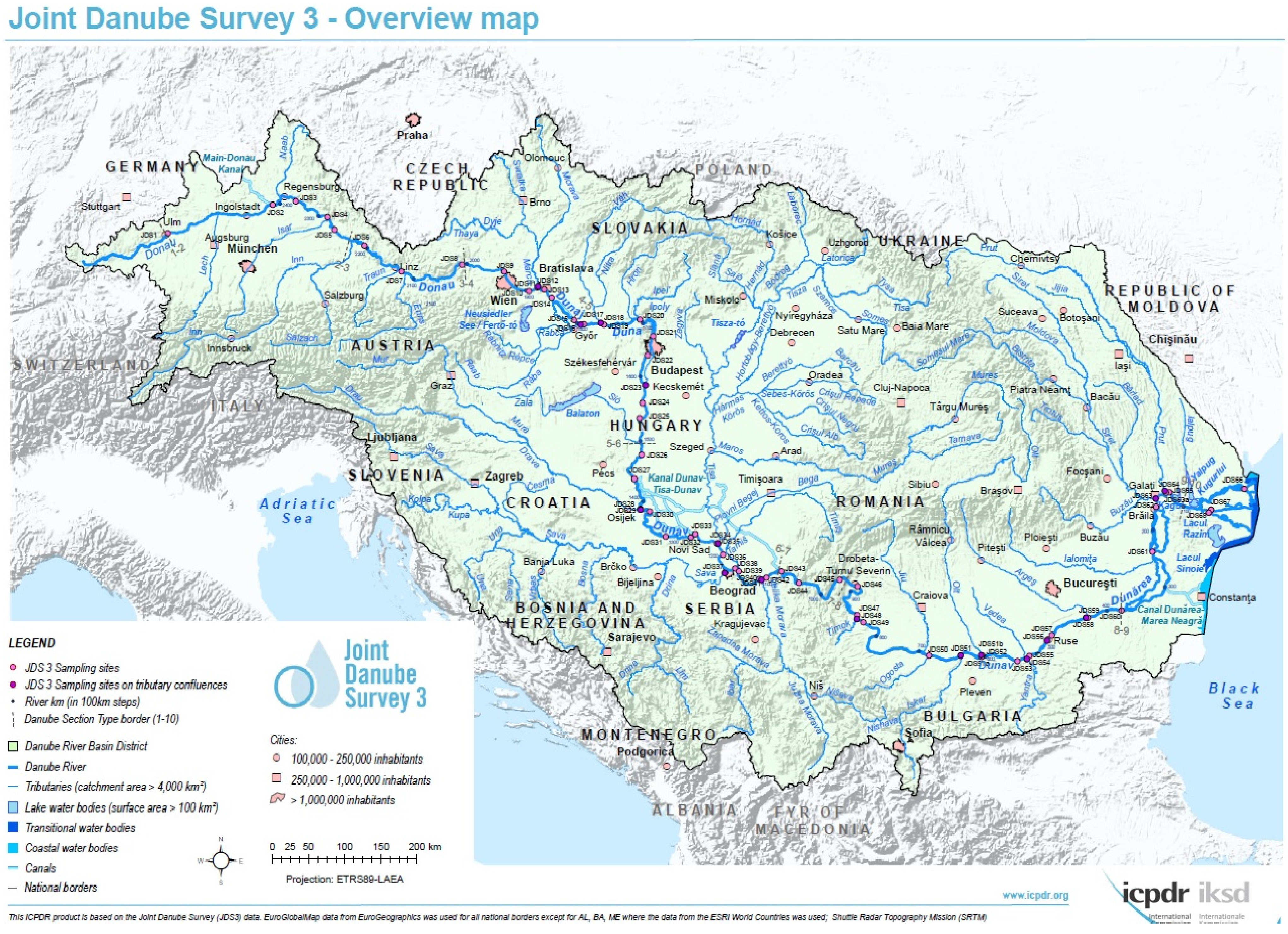
| SP | Name of SP | River km | SP | Name of SP | River km |
|---|---|---|---|---|---|
| JDS1 | Böfinger Halde | 2581 | JDS35 | Tisa | 1215 |
| JDS2 | Kelheim, gauging station | 2415 | JDS36 | DS Tisa/US Sava (Belegis) | 1200 |
| JDS3 | Geisling power plant | 2354 | JDS37 | Sava | 1170 |
| JDS4 | Deggendorf | 2285 | JDS38 | Upstream Pancevo | 1159 |
| JDS5 | Mühlau | 2258 | JDS39 | DownstreamPancevo | 1151 |
| JDS6 | Jochenstein | 2204 | JDS40 | Upstream Vel. Morava | 1107 |
| JDS7 | US dam Abwinden-Asten | 2120 | JDS41 | Velika Morava | 1103 |
| JDS8 | Oberloiben | 2008 | JDS42 | DS Velika Morava | 1097 |
| JDS9 | Klosterneuburg | 1942 | JDS43 | Banatska Palanka | 1071 |
| JDS10 | Wildungsmauer | 1895 | JDS44 | IGR Golubac/Koronin | 1040 |
| JDS11 | US Morava (Hainburg) | 1881 | JDS45 | IGR Tekija/Orsova | 954 |
| JDS12 | Morava | 1880 | JDS46 | Vrbica/Simijan | 926 |
| JDS13 | Bratislava | 1869 | JDS47 | Upstream Timok | 849 |
| JDS14 | Gabcikovo reservoir | 1852 | JDS48 | Timok | 845 |
| JDS15 | Medvedov/Medve | 1806 | JDS49 | Pristol/Novo Salo | 834 |
| JDS16 | Moson Danube | 1794 | JDS50 | Downstream Kozloduy | 685 |
| JDS17 | Klizska Nema | 1790 | JDS51 | Iskar | 637 |
| JDS18 | Vah | 1766 | JDS52 | Downstream Olt | 602 |
| JDS19 | Iza/Szony | 1761 | JDS53 | Downstream Zimnicea/Svistov | 550 |
| JDS20 | Szob | 1707 | JDS54 | Jantra | 537 |
| JDS21 | US Budapest - Megyeri Bridge | 1660 | JDS55 | Downstream Jantra | 532 |
| JDS22 | DS Budapest—M0 | 1632 | JDS56 | Russenski Lom | 498 |
| JDS23 | Rackeve-Soroksar arm-end | 1586 | JDS57 | Downstream Ruse | 488 |
| JDS24 | Dunaföldvar | 1560 | JDS58 | Arges | 432 |
| JDS25 | Paks | 1533 | JDS59 | Downstream Arges | 429 |
| JDS26 | Baja | 1481 | JDS60 | Chiciu/Silistra | 378 |
| JDS27 | Hercegszanto | 1434 | JDS61 | Giurgeni | 235 |
| JDS28 | US Drava | 1384 | JDS62 | Braila | 167 |
| JDS29 | Drava | 1379 | JDS63 | Siret | 154 |
| JDS30 | DS Drava (Erdut/Bogojevo) | 1367 | JDS64 | Prut | 135 |
| JDS31 | Ilok/Backa Palanka | 1300 | JDS65 | Reni | 130 |
| JDS32 | US Novi Sad | 1262 | JDS66 | Vilova/Kilia Arm | 18 |
| JDS33 | DS Novi Sad | 1252 | JDS67 | Sulina Arm | 26 |
| JDS34 | US Tisa (Stari Slankamen) | 1216 | JDS68 | St.Gheorge Arm | 104 |
2.2. Toxicity Assay: umuC
2.3. Cytotoxicity Assay: MTS
3. Results and Discussion
3.1. UmuC Results without Enzymatic S9 Activation

3.2. UmuC Results with Enzymatic S9 Activation
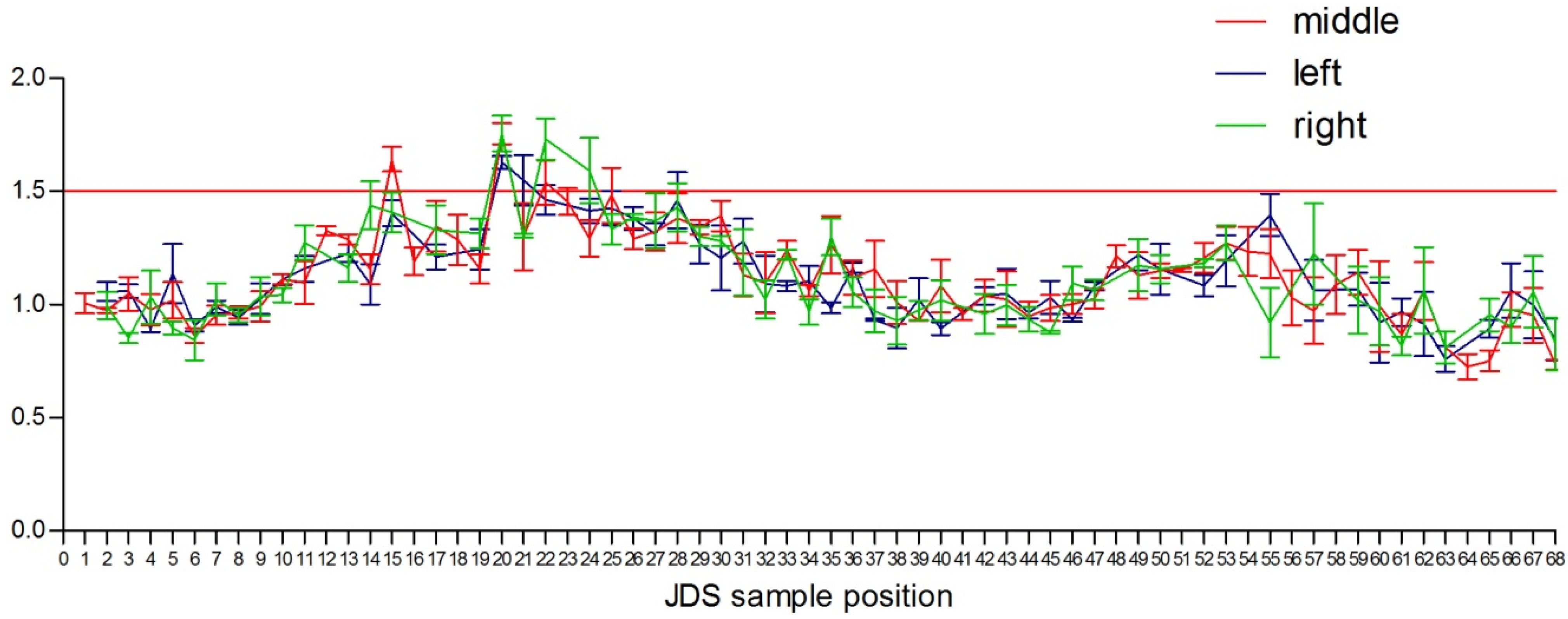
3.3. MTS Testing

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- JDS 3. In Joint Danube Survey 3. A Comprehensive Analysis of Danube Water Quality; ICPDR—International Commission for the Protection of the Danube River: Vienna, Austria, 2015.
- Leusch, F.D.; Khan, S.J.; Gagnon, M.M.; Quayle, P.; Trinh, T.; Coleman, H.; Rawson, C.; Chapman, H.F.; Blair, P.; Nice, H.; et al. Assessment of Wastewater and Recycled Water Quality: A Comparison of Lines of Evidence from in Vitro, in Vivo and Chemical Analyses. Water Res. 2014, 50, 420–431. [Google Scholar] [CrossRef]
- Zegura, B.; Heath, E.; Cernosa, A.; Filipic, M. Combination of in Vitro Bioassays for the Determination of Cytotoxic and Genotoxic Potential of Wastewater, Surface Water and Drinking Water Samples. Chemosphere 2009, 75, 1453–1460. [Google Scholar] [CrossRef]
- Giuliani, F.; Koller, T.; Wurgler, F.E.; Widmer, R.M. Detection of Genotoxic Activity in Native Hospital Waste Water by the umuC Test. Mutat. Res. 1996, 368, 49–57. [Google Scholar] [CrossRef]
- Hamer, B.; Bihari, N.; Reifferscheid, G.; Zahn, R.K.; Muller, W.E.; Batel, R. Evaluation of the SOS/umu-Test Post-Treatment Assay for the Detection of Genotoxic Activities of Pure Compounds and Complex Environmental Mixtures. Mutat. Res. 2000, 466, 161–171. [Google Scholar] [CrossRef]
- Dizer, H.; Wittekindt, E.; Fischer, B.; Hansen, P.D. The Cytotoxic and Genotoxic Potential of Surface Water and Wastewater Effluents as Determined by Bioluminescence, Umu-Assays and Selected Biomarkers. Chemosphere 2002, 46, 225–233. [Google Scholar] [CrossRef]
- Kittinger, C.; Marth, E.; Reinthaler, F.F.; Zarfel, G.; Pichler-Semmelrock, F.; Mascher, W.; Mascher, G.; Mascher, F. Water Quality Assessment of a Central European River—Does the Directive 2000/60/EC Cover all the Needs for a Comprehensive Classification? Sci. Total Environ. 2013, 447, 424–429. [Google Scholar] [CrossRef]
- Hernando, M.D.; Heath, E.; Petrovic, M.; Barcelo, D. Trace-Level Determination of Pharmaceutical Residues by LC-MS/MS in Natural and Treated Waters. A Pilot-Survey Study. Anal. Bioanal Chem. 2006, 385, 985–991. [Google Scholar] [CrossRef]
- Macova, M.; Toze, S.; Hodgers, L.; Mueller, J.F.; Bartkow, M.; Escher, B.I. Bioanalytical Tools for the Evaluation of Organic Micropollutants during Sewage Treatment, Water Recycling and Drinking Water Generation. Water Res. 2011, 45, 4238–4247. [Google Scholar]
- Armishaw, P.; Millar, R. A Natural Matrix (Pureed Tomato) Candidate Reference Material Containing Residue Concentrations of Pesticide Chemicals. Fresenius J. Anal. Chem. 2001, 370, 291–296. [Google Scholar] [CrossRef]
- Reifferscheid, G.; Heil, J.; Oda, Y.; Zahn, R.K. A Microplate Version of the SOS/umu-Test for Rapid Detection of Genotoxins and Genotoxic Potentials of Environmental Samples. Mutat. Res. 1991, 253, 215–222. [Google Scholar] [CrossRef]
- Knasmuller, S.; Mersch-Sundermann, V.; Kevekordes, S.; Darroudi, F.; Huber, W.W.; Hoelzl, C.; Bichler, J.; Majer, B.J. Use of Human-Derived Liver Cell Lines for the Detection of Environmental and Dietary Genotoxicants; Current State of Knowledge. Toxicology 2004, 198, 315–328. [Google Scholar] [CrossRef]
- Reifferscheid, G.; Heil, J. Validation of the SOS/umu Test using Test Results of 486 Chemicals and Comparison with the Ames Test and Carcinogenicity Data. Mutat. Res. 1996, 369, 129–145. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A Exposure, Effects, and Policy: A Wildlife Perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Haarstad, K.; Bavor, H.J.; Maehlum, T. Organic and Metallic Pollutants in Water Treatment and Natural Wetlands: A Review. Water Sci. Technol. 2012, 65, 76–99. [Google Scholar] [CrossRef]
- Baderna, D.; Colombo, A.; Romeo, M.; Cambria, F.; Teoldi, F.; Lodi, M.; Diomede, L.; Benfenati, E. Soil Quality in the Lomellina Area using in Vitro Models and Ecotoxicological Assays. Environ. Res. 2014, 133, 220–231. [Google Scholar] [CrossRef]
- Walum, E.; Hedander, J.; Garberg, P. Research Perspectives for Pre-Screening Alternatives to Animal Experimentation: On the Relevance of Cytotoxicity Measurements, Barrier Passage Determinations and High Throughput Screening in Vitro to Select Potentially Hazardous Compounds in Large Sets of Chemicals. Toxicol. Appl. Pharmacol. 2005, 207, 393–397. [Google Scholar] [CrossRef]
- Baderna, D.; Colombo, A.; Amodei, G.; Cantu, S.; Teoldi, F.; Cambria, F.; Rotella, G.; Natolino, F.; Lodi, M.; Benfenati, E. Chemical-Based Risk Assessment and in Vitro Models of Human Health Effects Induced by Organic Pollutants in Soils from the Olona Valley. Sci. Total Environ. 2013, 463–464, 790–801. [Google Scholar] [CrossRef]
- Kallweit, A.R.; Baird, C.H.; Stutzman, D.K.; Wischmeyer, P.E. Glutamine Prevents Apoptosis in Intestinal Epithelial Cells and Induces Differential Protective Pathways in Heat and Oxidant Injury Models. JPEN J. Parenter. Enteral Nutr. 2012, 36, 551–555. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittinger, C.; Baumert, R.; Folli, B.; Lipp, M.; Liebmann, A.; Kirschner, A.; Farnleitner, A.H.; Grisold, A.J.; Zarfel, G.E. Preliminary Toxicological Evaluation of the River Danube Using in Vitro Bioassays. Water 2015, 7, 1959-1968. https://doi.org/10.3390/w7051959
Kittinger C, Baumert R, Folli B, Lipp M, Liebmann A, Kirschner A, Farnleitner AH, Grisold AJ, Zarfel GE. Preliminary Toxicological Evaluation of the River Danube Using in Vitro Bioassays. Water. 2015; 7(5):1959-1968. https://doi.org/10.3390/w7051959
Chicago/Turabian StyleKittinger, Clemens, Rita Baumert, Bettina Folli, Michaela Lipp, Astrid Liebmann, Alexander Kirschner, Andreas H. Farnleitner, Andrea J. Grisold, and Gernot E. Zarfel. 2015. "Preliminary Toxicological Evaluation of the River Danube Using in Vitro Bioassays" Water 7, no. 5: 1959-1968. https://doi.org/10.3390/w7051959






