Assessing Potential Algal Blooms in a Shallow Fluvial Lake by Combining Hydrodynamic Modelling and Remote-Sensed Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
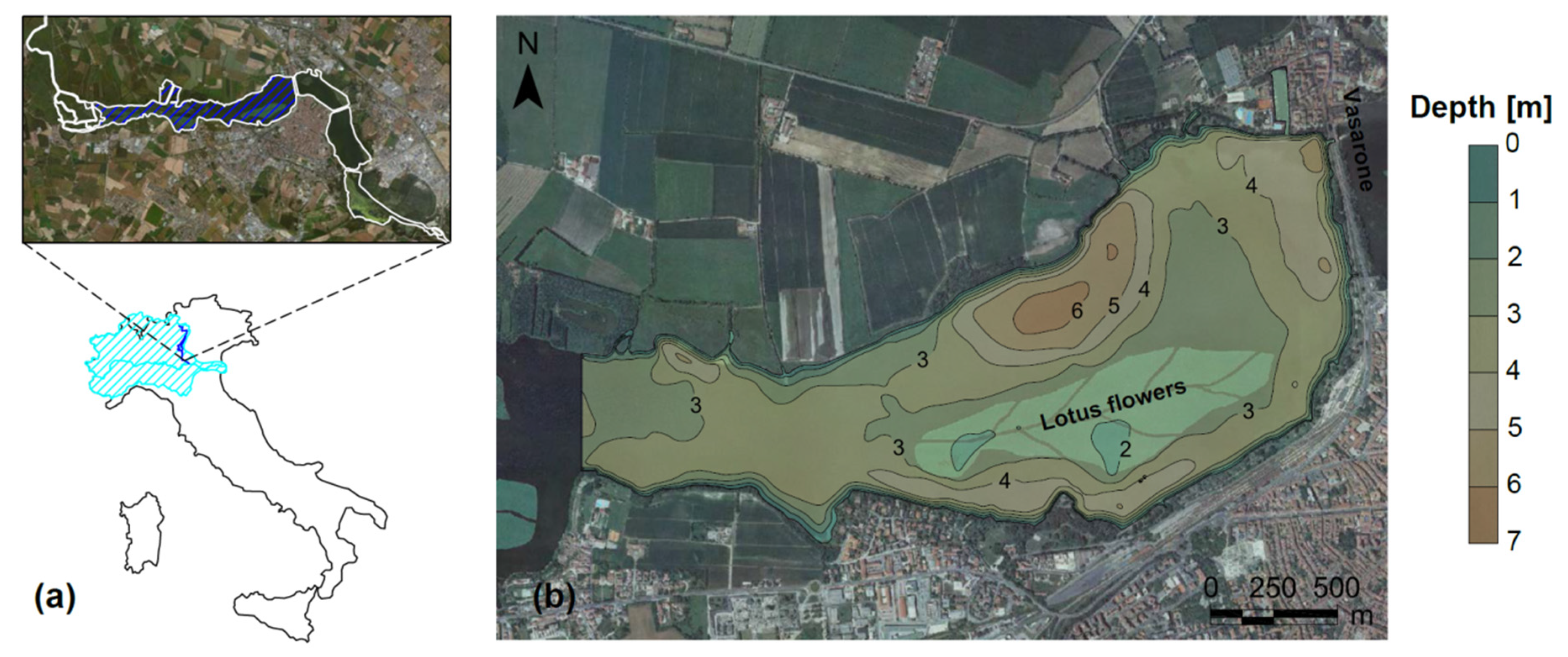
2.2. Remote Sensing Data
2.3. Numerical Hydrodynamic Lake Model
| Sensor | Data | Algorithm | R2 | rRMSE [%] | References |
|---|---|---|---|---|---|
| APEX | 21 September 2011 | 54.647 × [Rw(690,697) − Rw(670,673)] − 52.47 | 0.86 | 27 | [4,21] |
| CHRIS | 29 June 2008 | 81.257 × [Rw(706) − Rw(669)] – 54.5 | 0.74 | 16 | [32] |
| MIVIS | 26 July 2007 | 170.81 × [Rw(677) − Rw(710)] × Rw(747) + 36.105 | 0.77 | 18 | [31] |
| Parameter | MIVIS 26 July 2007 | CHRIS-PROBA 29 June 2008 | CHRIS-PROBA 28 August 2011 | APEX 21 September 2011 |
|---|---|---|---|---|
| Q [m3 s−1] | 2.6 | 10 | 9.7 | 12.7 |
| Wind direction [°] | 74 | 63 | 270 | 269 |
| W10 [m s−1] | 2.18 | 1.26 | 1.49 | 2.35 |
2.4. Data Analyses
3. Results and Discussion
3.1. Primary Producers and Physico-Chemical Characterization
| Year | pH | Conductivity [µS cm−1] | NH4+ [mg L−1] | NO3- [mg L−1] | TN [mg L−1] | SRP [mg L−1] | TP [mg L−1] | Chl-a [mg m−3] | O2 [% Sat] |
|---|---|---|---|---|---|---|---|---|---|
| 2007 | 8.2 | 386 | 0.08 | 0.30 | 1.75 | 0.001 | 0.14 | 90 | 143 |
| 2008 | 8.6 | 300 | 0.05 | 0.62 | 0.57 | 0.003 | 0.02 | 38 | 179 |
| 2011 | 8.2 | 327 | 0.05 | 1.14 | 1.45 | 0.018 | 0.07 | 58 | 165 |
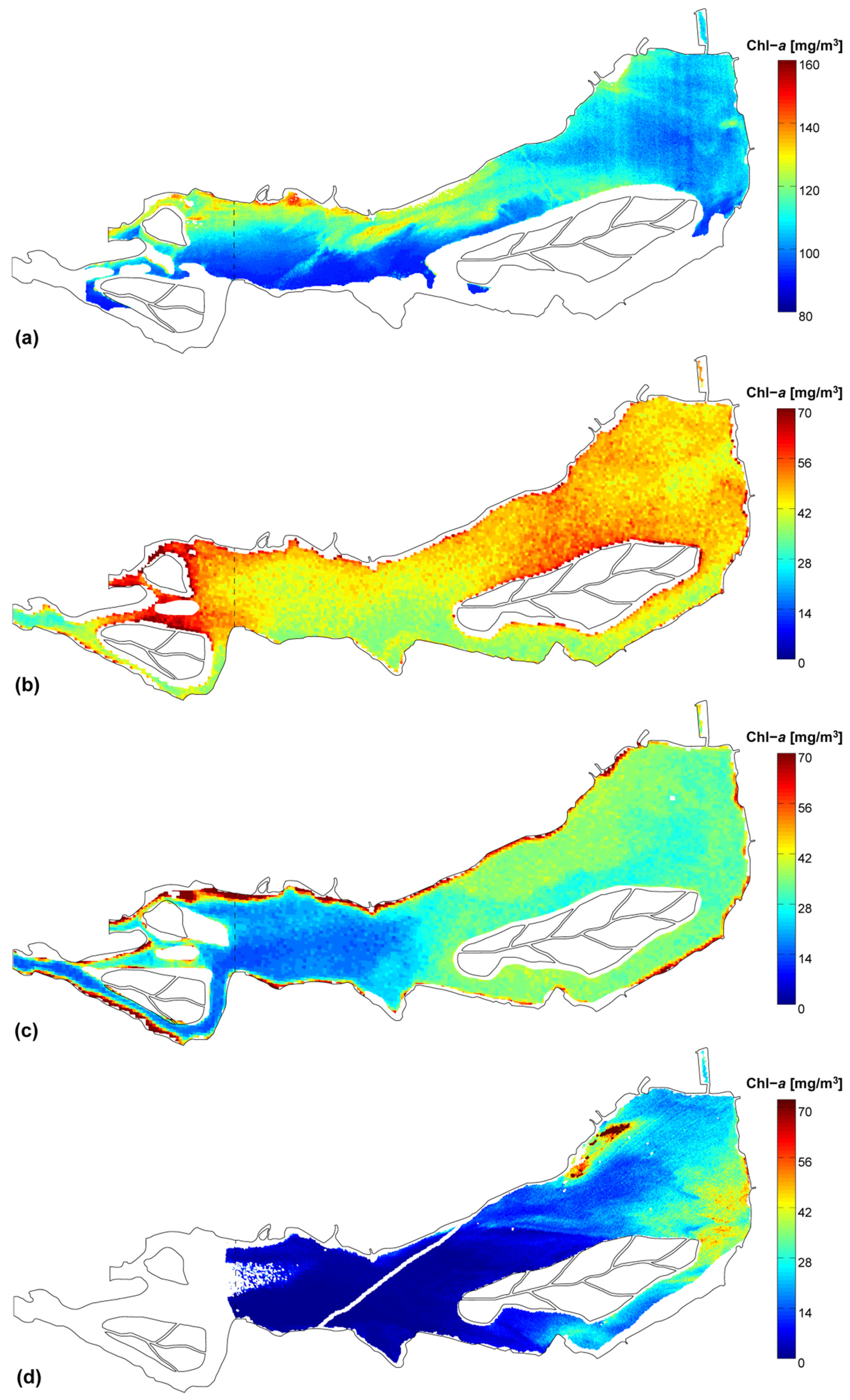
3.2. Chlorophyll-a Distribution Maps
3.3. 3D Hydrodynamic Model
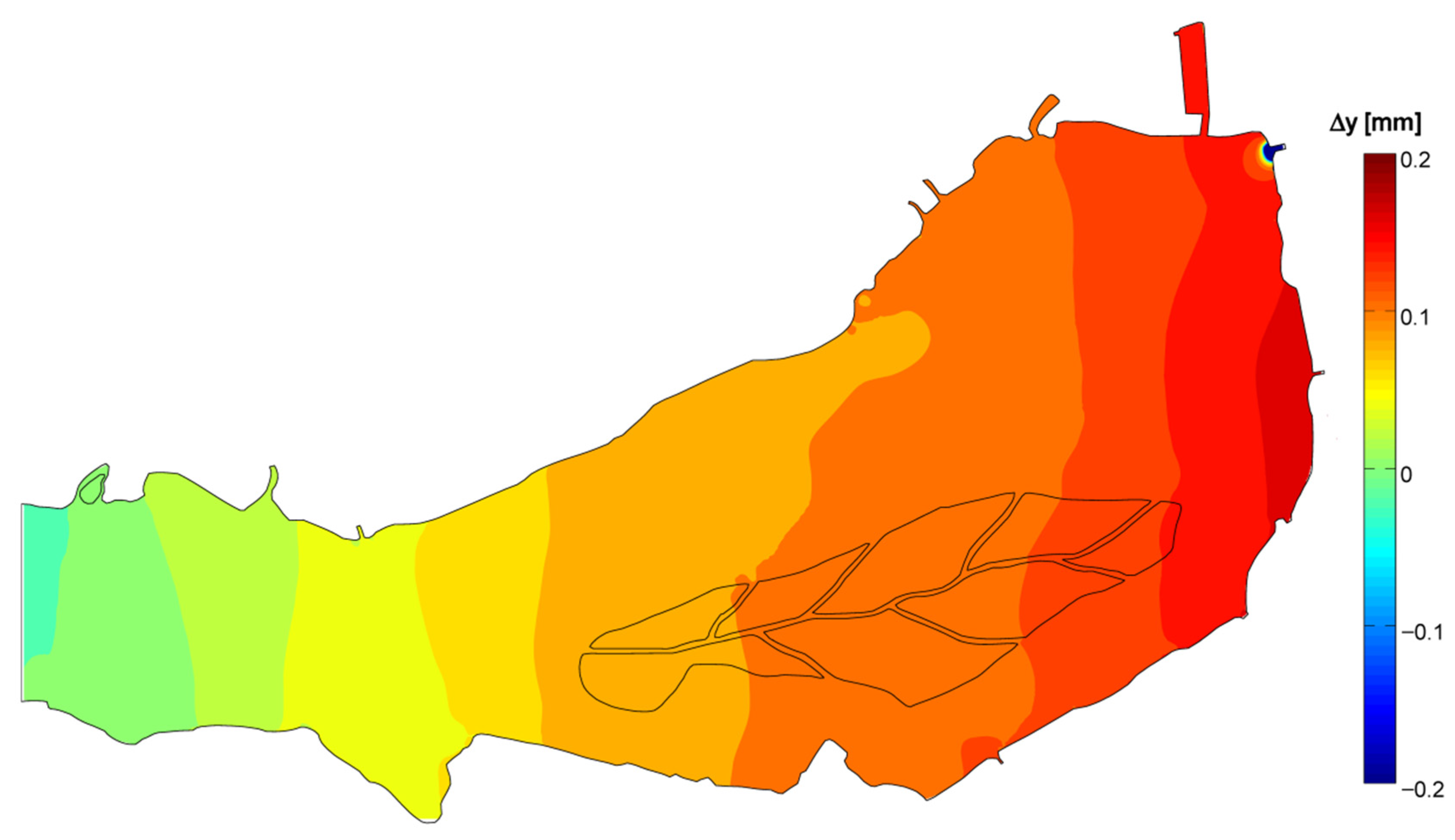
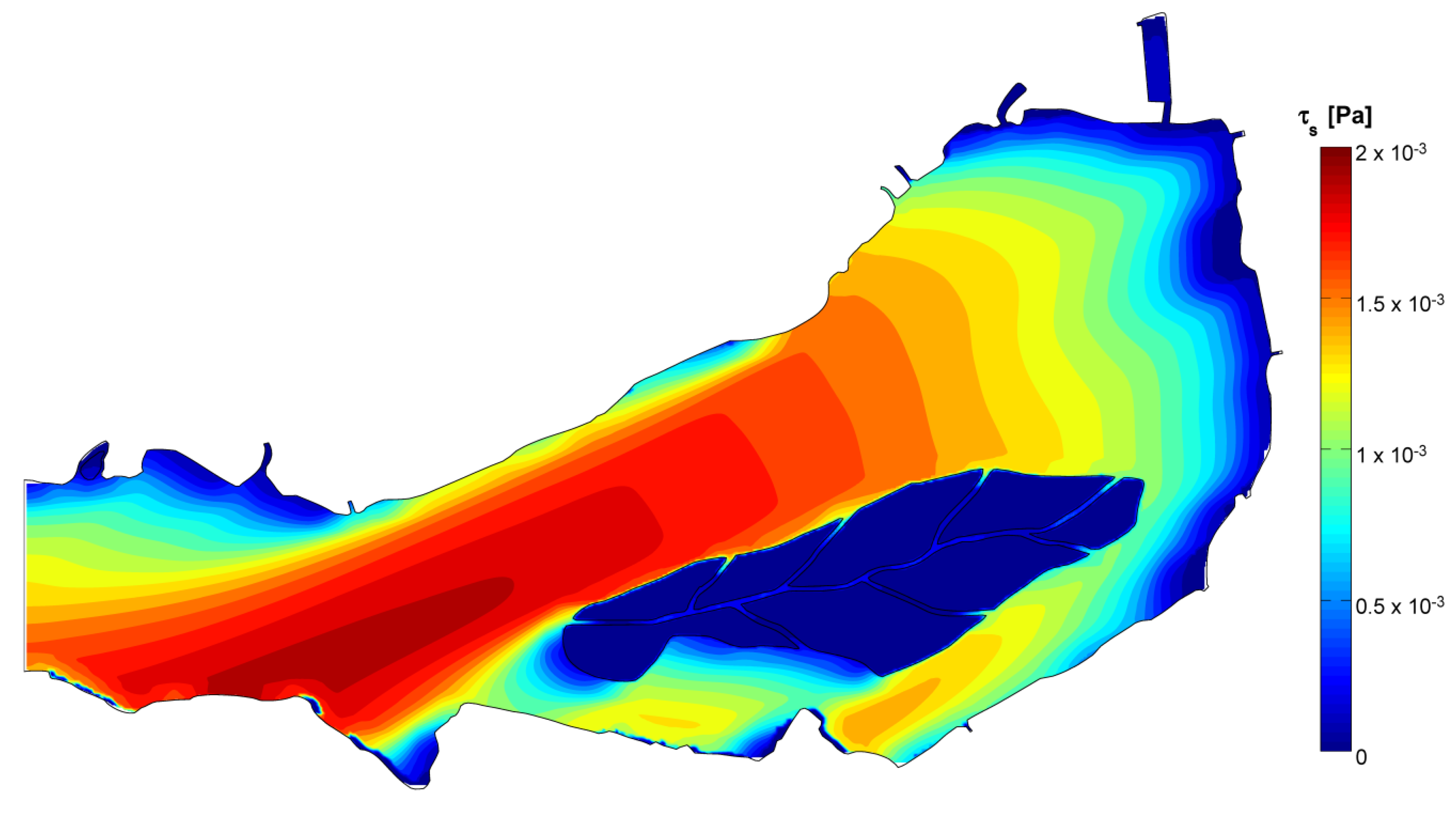

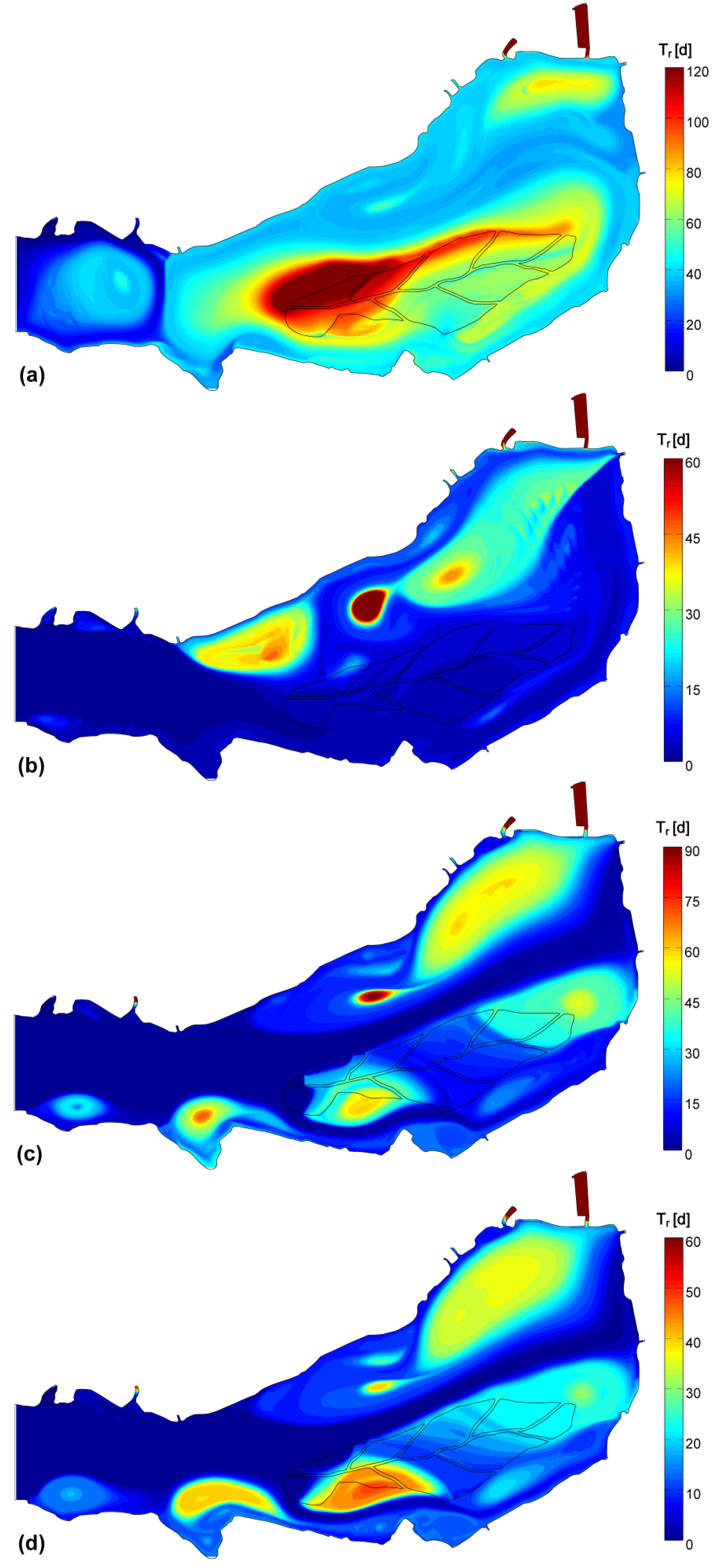
3.4. Data and Simulation Analyses
| Parameter | MIVIS 26 July 2007 | CHRIS-PROBA 29 June 2008 | CHRIS-PROBA 28 August 2011 | APEX 21 September 2011 | |
|---|---|---|---|---|---|
| Theoretical Tr [d] | 43.6 | 11.2 | 11.6 | 8.8 | |
| Simulated Tr | μ [d] | 45.9 | 10.0 | 20.0 | 16.1 |
| CV | 59% | 150% | 129% | 118% | |
| Remote-sensed Chl-a | μ [mg/m3] | 107.8 | 45.9 | 32.3 | 15.1 |
| CV | 9% | 12% | 30% | 86% | |
- The generalized transport in the wind direction is responsible for major phytoplankton arrangement, as observed, for instance, by George et al. [18]. In the Superior Lake of Mantua, the main wind axis is aligned to the riverine flow, so that, according to the wind direction, the two forces may sum up or oppose one another, resulting either in packing on one of the ends of the lake or in a more uniform distribution.
- The stagnation in correspondence of gyres traps water and its phytoplankton content, favoring species that prefer motionless waters, such as cyanobacteria [52], to proliferate under stable hydrodynamic conditions. Opposed to this phenomenon are the lower concentrations observed where strong currents develop, because of turbulence disturbance and faster water renewal.
- The small-scale transport by local currents is responsible for the formation of phytoplankton assemblies on top or outside of the generalized wind advection.
| Variable | MIVIS 26 July 2007 | CHRIS-PROBA 29 June 2008 | CHRIS-PROBA 28 August 2011 | APEX 21 September 2011 |
|---|---|---|---|---|
| Δy | 0.071 | −0.368 | 0.455 | 0.792 |
| τs | −0.055 | −0.087 | −0.472 | −0.193 |
| Tr | −0.175 | 0.276 | 0.215 | 0.276 |
| V | 0.170 | −0.159 | −0.183 | 0.008 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teodoru, C.; Wehrli, B. Retention of sediments and nutrients in the Iron Gate I Reservoir on the Danube River. Biogeochemistry 2005, 76, 539–565. [Google Scholar] [CrossRef]
- Humborg, C.; Ittekkot, V.; Cociasu, A.; Bodungen, B.V. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 1997, 386, 385–388. [Google Scholar] [CrossRef]
- McCartney, M. Living with dams: Managing the environmental impacts. Water Policy 2009, 11, 121–139. [Google Scholar] [CrossRef]
- Bresciani, M.; Rossini, M.; Morabito, G.; Matta, E.; Pinardi, M.; Cogliati, S.; Julitta, T.; Colombo, R.; Braga, F.; Giardino, C. Analysis of within- and between-day chlorophyll-a dynamics in Mantua Superior Lake, with a continuous spectroradiometric measurement. Mar. Freshwater Res. 2013, 64, 303–316. [Google Scholar]
- Harris, G.P. Phytoplankton productivity and growth measurements: Past, present and future. J. Plankton Res. 1984, 6, 219–237. [Google Scholar] [CrossRef]
- Reynolds, C.S. Scales of disturbance and their role in plankton ecology. Hydrobiologia 1993, 249, 157–171. [Google Scholar] [CrossRef]
- Verhagen, J.H.G. Modeling phytoplankton patchiness under the influence of wind-driven currents in lakes. Limnol. Oceanogr. 1994, 39, 1551–1565. [Google Scholar] [CrossRef]
- De Souza Cardoso, L.; da Motta Marques, D. Hydrodynamics-driven plankton community in a shallow fluvial lake. Aquat. Ecol. 2009, 43, 73–84. [Google Scholar]
- George, D.G.; Heaney, S.I. Factors Influencing the Spatial Distribution of Phytoplankton in a Small Productive Lake. J. Ecol. 1978, 66, 133–155. [Google Scholar] [CrossRef]
- Jones, R.I.; Fulcher, A.S.; Jayakody, J.K.U.; Laybourn-Parry, J.; Shine, A.J.; Walton, M.C.; Young, J.M. The horizontal distribution of plankton in a deep, oligotrophic lake—Loch Ness, Scotland. Freshwater Biol. 1995, 33, 161–170. [Google Scholar] [CrossRef]
- Ciraolo, G.; Lipari, G.; Napoli, E.; Józsa, J.; Krámer, T. Three-dimensional numerical analysis of turbulent wind-induced flows in the lake Balaton (Hungary). In Shallow Flows—Research Presented at the International Symposium on Shallow Flows, Delft, Netherlands, 16–18 June 2003; Uijttewaal, W.S.J., Jirka, G.H., Eds.; CRC Press/Balkema: Leiden, The Netherlands, 2004. [Google Scholar]
- Wiens, J.A. Spatial Scaling in Ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Pinel-Alloul, B. Spatial heterogeneity as a multiscale characteristics of zooplankton community. Hydrobiologia 1995, 104, 17–42. [Google Scholar] [CrossRef]
- Legendre, L.; Demers, S. Towards Dynamic Biological Oceanography and Limnology. Can. J. Fish. Aquat. Sci. 1984, 41, 2–19. [Google Scholar] [CrossRef]
- Thackeray, S.J.; George, D.G.; Jones, R.I.; Winfield, I.J. Quantitative analysis of the importance of wind-induced circulation for the spatial structuring of planktonic populations. Freshwater Biol. 2004, 49, 1091–1102. [Google Scholar] [CrossRef]
- George, D.G. Wind-induced water movements in the South Basin of Windermere. Freshwater Biol. 1981, 11, 37–60. [Google Scholar] [CrossRef]
- Livingstone, D.A. On the orientation of lake basins. Am. J. Sci. 1954, 252, 547–554. [Google Scholar] [CrossRef]
- George, D.G.; Edwards, R.W. The Effect of Wind on the Distribution of Chlorophyll A and Crustacean Plankton in a Shallow Eutrophic Reservoir. J. Appl. Ecol. 1976, 13, 667–690. [Google Scholar] [CrossRef]
- George, D.G.; Winfield, I.J. Factors influencing the spatial distribution of zooplankton and fish in Loch Ness, UK. Freshwater Biol. 2000, 43. [Google Scholar] [CrossRef]
- Pinardi, M.; Bartoli, M.; Longhi, D.; Viaroli, P. Net autotrophy in a fluvial lake: the relative role of phytoplankton and floating-leaved macrophytes. Aquat. Sci. 2011, 73, 389–403. [Google Scholar] [CrossRef]
- Bolpagni, R.; Bresciani, M.; Laini, A.; Pinardi, M.; Matta, E.; Ampe, E.M.; Giardino, C.; Viaroli, P.; Bartoli, M. Remote sensing of phytoplankton-macrophyte coexistence in shallow hypereutrophic fluvial lakes. Hydrobiologia 2014, 737, 67–76. [Google Scholar] [CrossRef]
- Søballe, D.M.; Bachmann, R.W. Influence of reservoir transit on riverine algal transport and abundance. Can. J. Fish. Aquat. Sci. 1984, 41, 1803–1813. [Google Scholar] [CrossRef]
- Walz, N.; Welker, M. Plankton development in a rapidly flushed lake in the River Spree system (Neuendorfer See, Northeast Germany). J. Plankton Res. 1998, 20, 2071–2087. [Google Scholar] [CrossRef]
- Welker, M.; Walz, N. Plankton dynamics in a river-lake system—On continuity and discontinuity. Hydrobiologia 1999, 408, 233–239. [Google Scholar] [CrossRef]
- Rennella, A.M.; Quiros, R. The effects of hydrology on plankton biomass in shallow lakes of the Pampa Plain. Hydrobiologia 2006, 556, 181–191. [Google Scholar] [CrossRef]
- Gower, J.F.R.; Denman, K.L.; Holyer, R.J. Phytoplankton patchiness indicates the fluctuation spectrum of mesoscale oceanic structure. Nature 1980, 288, 157–159. [Google Scholar] [CrossRef]
- Blauw, A.N.; Los, H.F.J.; Bokhorst, M.; Erftemeijer, P.L.A. GEM: A generic ecological model for estuaries and coastal waters. Hydrobiologia 2009, 618, 175–198. [Google Scholar] [CrossRef]
- Salacinska, K.; El Serafy, G.Y.; Los, H.F.J.; Blauw, A. Sensitivity analysis of the two dimensional application of the Generic Ecological Model (GEM) to algal bloom prediction in the North Sea. Ecol. Model. 2010, 221, 178–190. [Google Scholar] [CrossRef]
- Mooij, W.M.; Trolle, D.; Jeppesen, E.; Arhonditsis, G.; Belolipetsky, P.V.; Chitamwebwa, D.B.R.; Degermendzhy, A.G.; DeAngelis, D.L.; de Senerpont Domis, L.N.; Downing, A.S.; et al. Challenges and opportunities for integrating lake ecosystem modelling approaches. Aquat. Ecol. 2010, 44, 633–667. [Google Scholar] [CrossRef] [Green Version]
- Los, H.F.J.; Villars, M.T.; Van der Tol, M.W.M. A 3-dimensional primary production model (BLOOM/GEM) and its applications to the (southern) North Sea (coupled physical-chemical-ecological model). J. Mar. Syst. 2008, 74, 259–294. [Google Scholar] [CrossRef]
- Bresciani, M.; Giardino, C.; Longhi, D.; Pinardi, M.; Bartoli, M.; Vascellari, M. Imaging spectrometry of productive inland waters. Application to the lakes of Mantua. Ital. J. Remote Sens. 2009, 41, 147–156. [Google Scholar] [CrossRef]
- Bresciani, M.; Giardino, C.; Bartoli, M.; Longhi, D.; Pinardi, M. Assessment of chlorophyll-a and algal blooms in inland waters from hyperspectral data. In Proceedings of the Hyperspectral 2010 Workshop, ESA SP-683, Frascati, Italy, 17–19 March 2010.
- Cutter, M.A. A low cost hyperspectral mission. Acta Astron. 2004, 55, 631–636. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Schalles, J.F.; Hladik, C.M. Remote chlorophyll-a retrieval in turbid, productive estuaries: Chesapeake Bay case study. Remote Sens. Environ. 2007, 109, 464–472. [Google Scholar] [CrossRef]
- Levasseur, M.; Therriault, J.-C.; Legendre, L. Tidal currents, winds and the morphology of phytoplankton spatial structures. J. Mar. Res. 1983, 41, 655–672. [Google Scholar] [CrossRef]
- Józsa, J. On the internal boundary layer related wind stress curl and its role in generating shallow lake circulations. J. Hydrol. Hydromech. 2014, 62, 16–23. [Google Scholar]
- Ottesen Hansen, N.-E. Effects of Boundary layers on mixing in small Lakes. Dev. Water Sci. 1979, 11, 341–356. [Google Scholar]
- Krámer, T. Solution-Adaptive 2D Modelling of Wind-Induced Lake Circulation. Ph.D. Thesis, Budapest University of Technology and Economics, Budapest, Hungary, 2006. [Google Scholar]
- Coastal Engineering Research Center (CERC). Shore Protection Manual, 4th ed.; Waterways Experiment Station: Vicksburg, MS, USA, 1984. [Google Scholar]
- Józsa, J. Shallow Lake Hydrodynamics—Theory, Measurement and Numerical Model Applications; Mundus-Euroaquae lecture notes; Budapest University of Technology and Economics: Budapest, Hungary, 2006. [Google Scholar]
- CD-adapco. STAR-CCM+ 9.02 User Guide; online manual; CD-adapco: Melville, NY, USA, 2014. [Google Scholar]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A New k-ε Eddy Viscosity Model for High Reynolds Number: Model Development and Validation; NASA Technical Memorandum 106721; NASA Lewis Research Center: Cleveland, OH, USA, 1994.
- Teeter, A.M.; Johnson, B.H.; Berger, C.; Stelling, G.; Scheffner, N.W.; Garcia, M.H.; Parchure, T.M. Hydrodynamic and sediment transport modeling with emphasis on shallow-water, vegetated areas (lakes, reservoirs, estuaries and lagoons). Hydrobiologia 2001, 444, 1–23. [Google Scholar] [CrossRef]
- Zinke, P. Application of a porous media approach for vegetation flow resistance. In River Flow 2012, San José, Costa Rica, 5–7 September 2012; Murillo Muñoz, R., Ed.; CRC Press/Balkema: Leiden, The Netherlands, 2012. [Google Scholar]
- Grant, W.D.; Madsen, O.S. Combined wave and current interaction with a rough bottom. J. Geophys. Res. 1979, 84, 1797–1808. [Google Scholar] [CrossRef]
- Dickman, M. Some effects of lake renewal on phytoplankton productivity and species composition. Limnol. Oceanogr 1969, 14, 660–666. [Google Scholar] [CrossRef]
- Krivtsov, V.; Sigee, C. Importance of biological and abiotic factors for geochemical cycling in a freshwater eutrophic lake. Biogeochemistry 2005, 74, 205–230. [Google Scholar] [CrossRef]
- Yamamuro, M.; Hiratsuka, J.; Ishitobi, Y.; Hosokawa, S.; Nakamura, Y. Ecosystem shift resulting from loss of eelgrass and other submerged aquatic vegetation in two estuarine lagoons, Lake Nakaumi and Lake Shinji, Japan. J. Oceanogr. 2006, 62, 551–558. [Google Scholar] [CrossRef]
- Ekholm, P.; Malve, O.; Kirkkala, T. Internal and external loading as regulators of nutrient concentrations in the agriculturally loaded Lake Pyhajarvi (southwest Finland). Hydrobiologia 1997, 345, 3–14. [Google Scholar] [CrossRef]
- Burger, D.F.; Hamilton, D.P.; Pilditch, C.A.; Gibbs, M.M. Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiologia 2007, 584, 13–25. [Google Scholar] [CrossRef]
- Luettich, R.A.; Harleman, D.R.F.; Somlyody, L. Dynamic Behavior of Suspended Sediment Concentrations in a Shallow Lake Perturbed by Episodic Wind Events. Limnol. Oceanogr. 1990, 35, 1050–1067. [Google Scholar] [CrossRef]
- Becker, V.; de Souza Cardoso, L.; da Motta Marques, D. Development of Anabaena Bory ex Bornet & Flahault (Cyanobacteria) blooms in a shallow, subtropical lake in southern Brazil. Acta Limnol. Bras. 2004, 16, 306–317. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinardi, M.; Fenocchi, A.; Giardino, C.; Sibilla, S.; Bartoli, M.; Bresciani, M. Assessing Potential Algal Blooms in a Shallow Fluvial Lake by Combining Hydrodynamic Modelling and Remote-Sensed Images. Water 2015, 7, 1921-1942. https://doi.org/10.3390/w7051921
Pinardi M, Fenocchi A, Giardino C, Sibilla S, Bartoli M, Bresciani M. Assessing Potential Algal Blooms in a Shallow Fluvial Lake by Combining Hydrodynamic Modelling and Remote-Sensed Images. Water. 2015; 7(5):1921-1942. https://doi.org/10.3390/w7051921
Chicago/Turabian StylePinardi, Monica, Andrea Fenocchi, Claudia Giardino, Stefano Sibilla, Marco Bartoli, and Mariano Bresciani. 2015. "Assessing Potential Algal Blooms in a Shallow Fluvial Lake by Combining Hydrodynamic Modelling and Remote-Sensed Images" Water 7, no. 5: 1921-1942. https://doi.org/10.3390/w7051921






