Stable Isotopic Analysis on Water Utilization of Two Xerophytic Shrubs in a Revegetated Desert Area: Tengger Desert, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Methods
2.2.1. Plant and Soil Samples
2.2.2. Isotope Analysis
3. Result
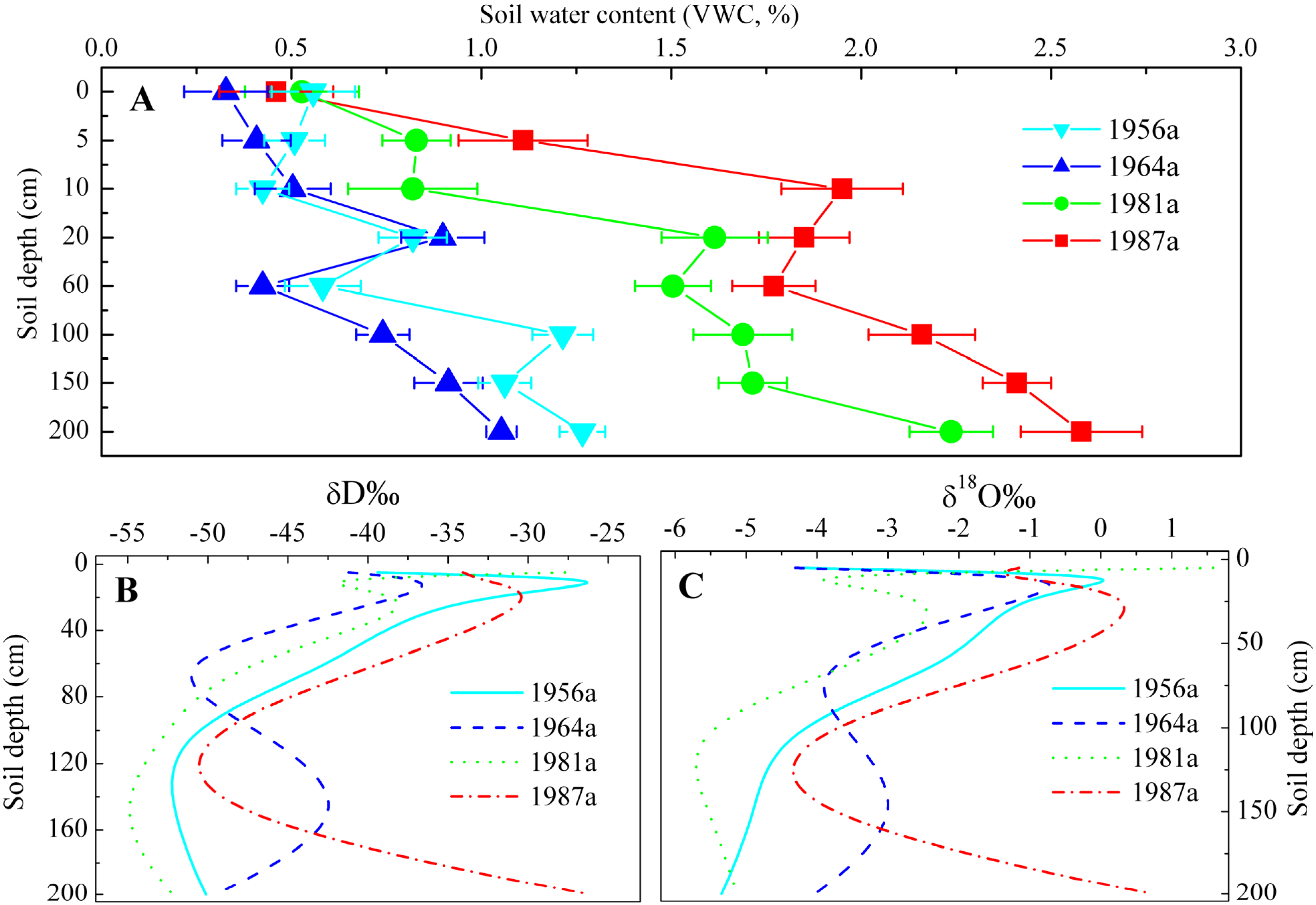
3.1. Variations in Stable Isotope Composition of Precipitation, Soil Water and Xylem Water
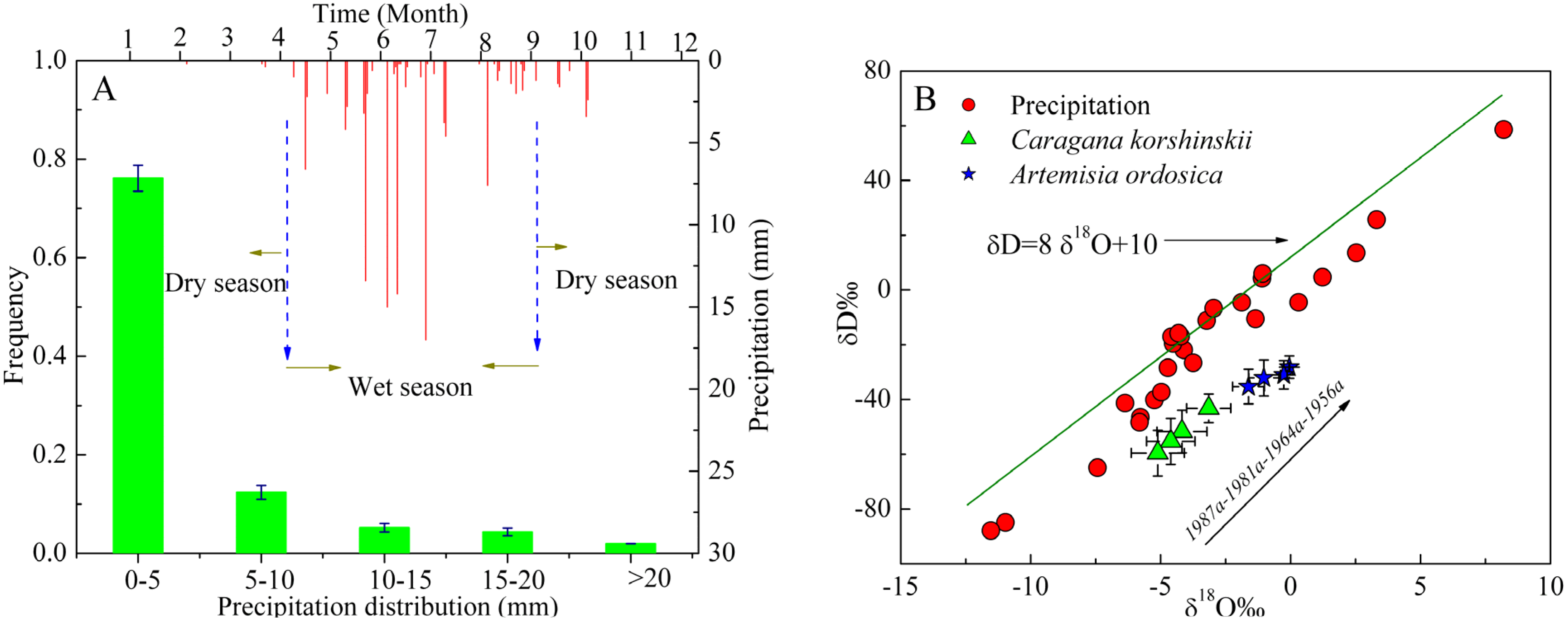
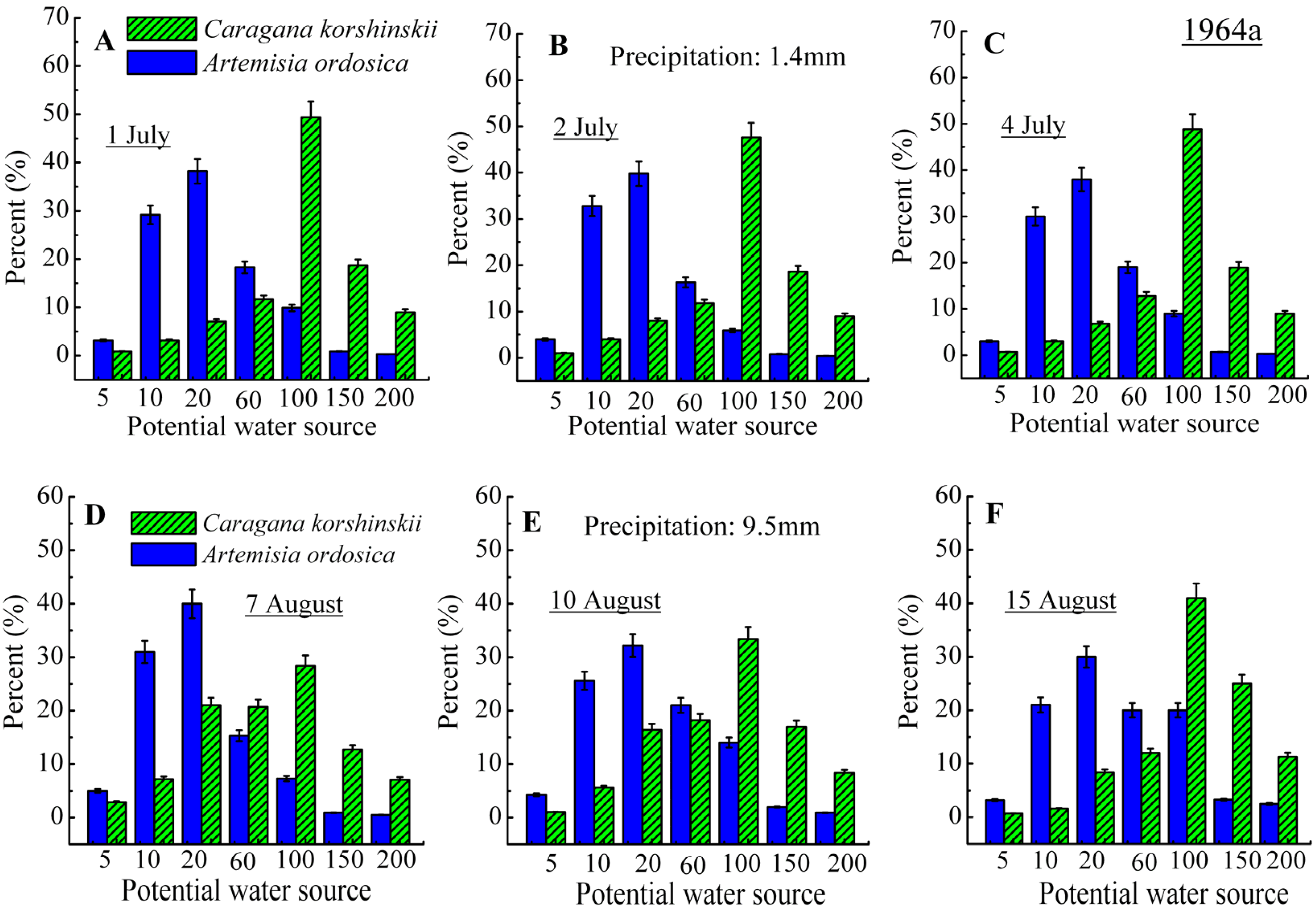
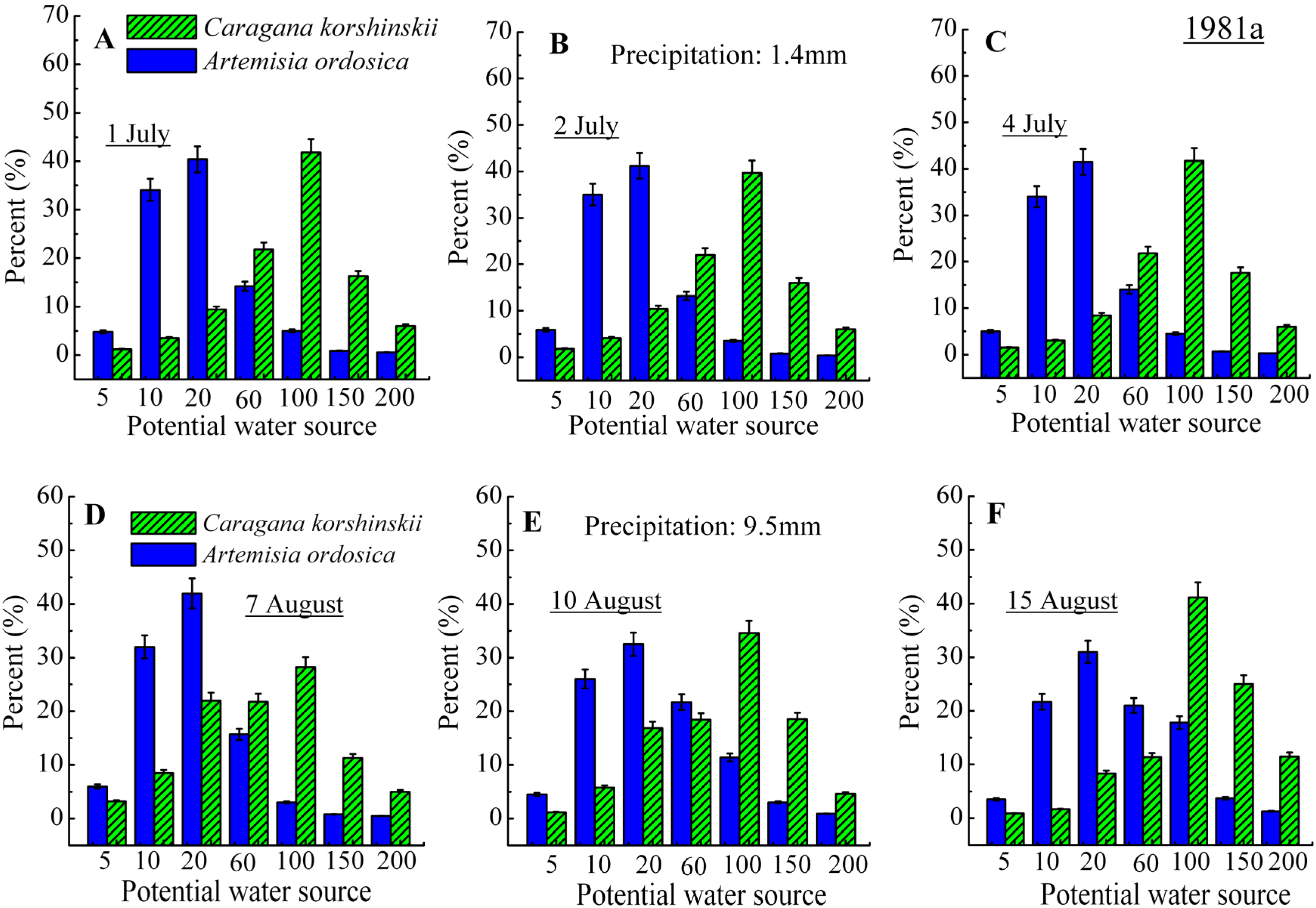
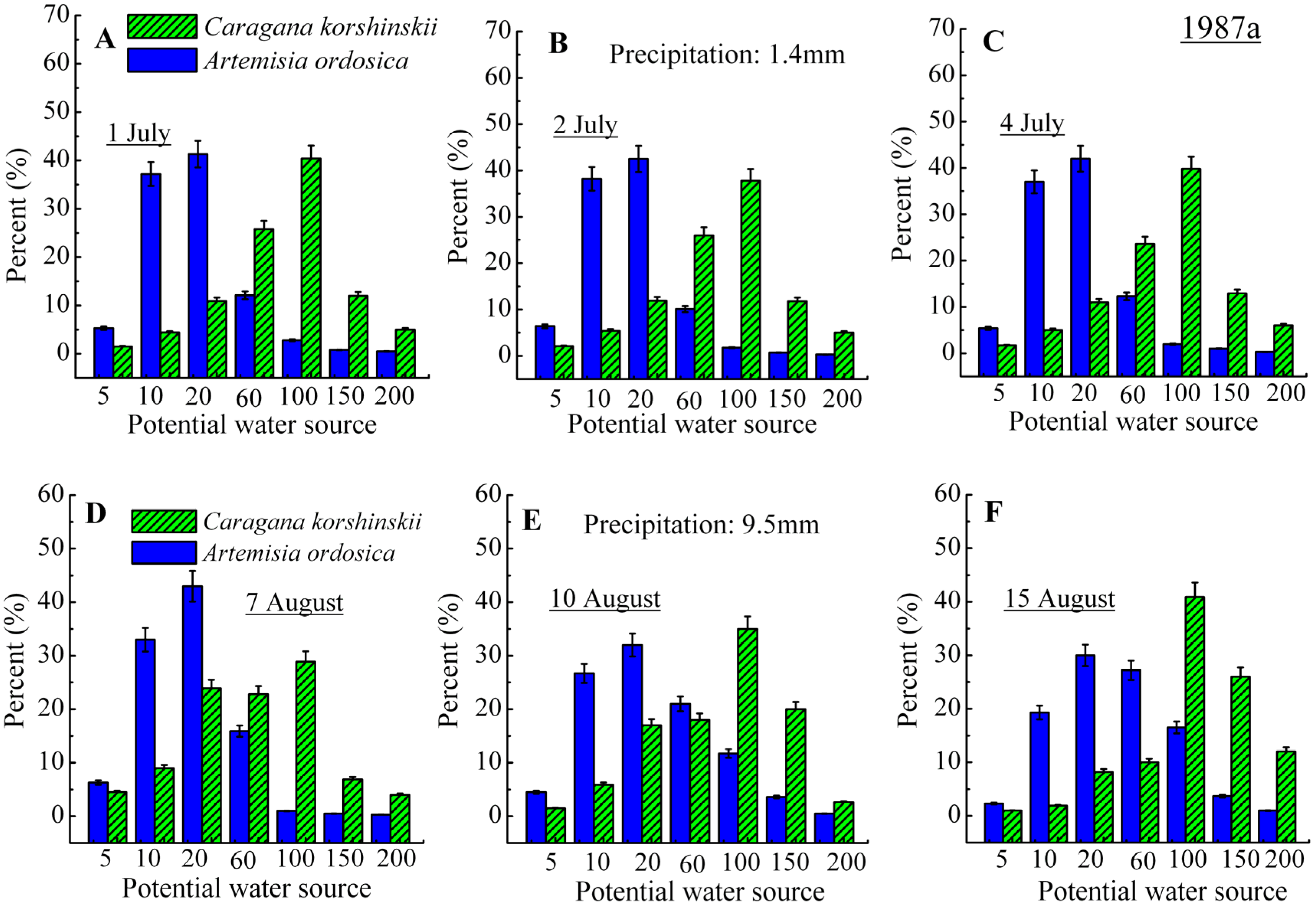
3.2. Identification of Water Source

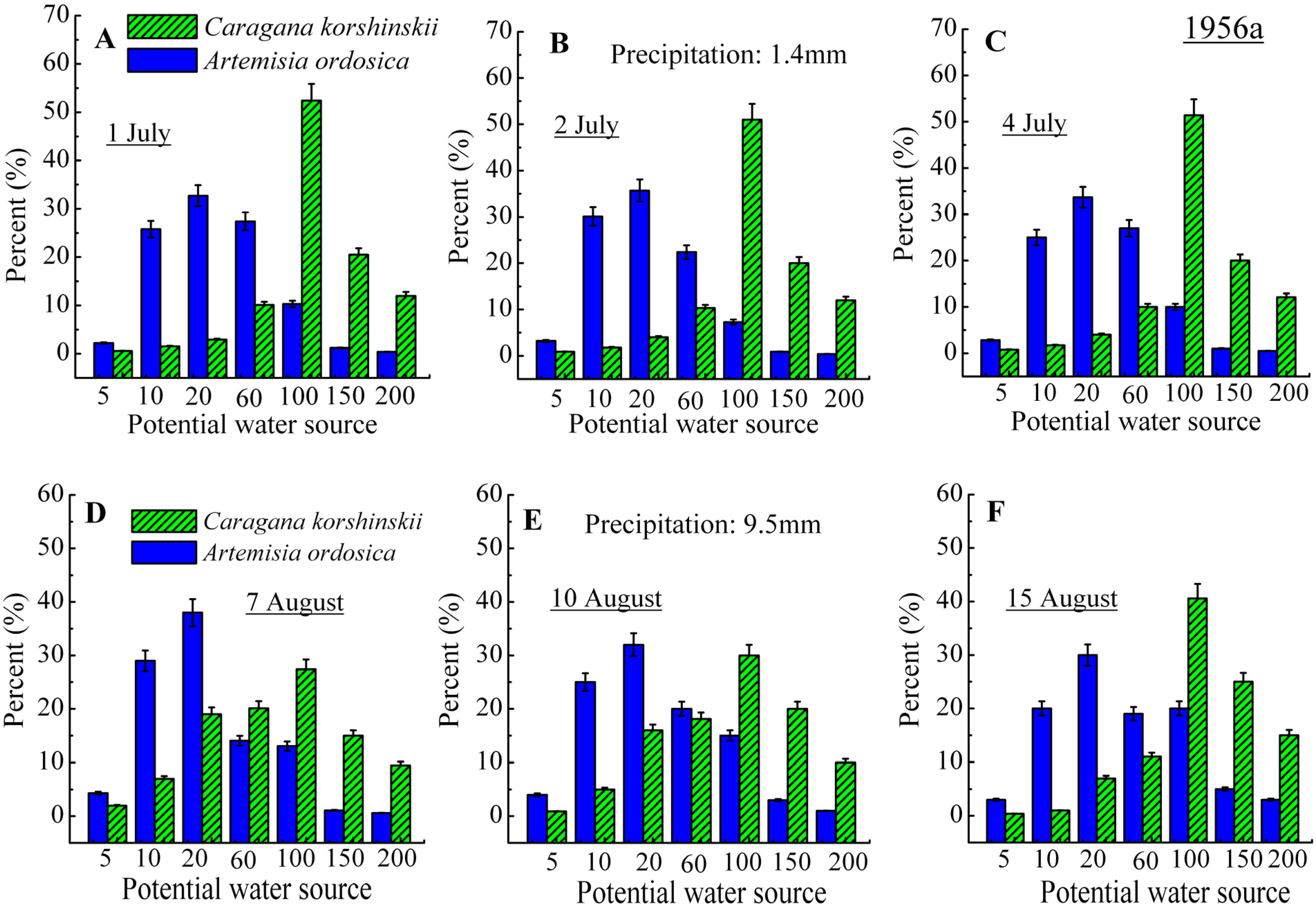
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Appendix
Conflicts of Interest
References
- Le Houerou, H.N.L. Man-made deserts: Desertification processes and threats. Arid Land Res. Manag. 2002, 16, 1–36. [Google Scholar]
- Li, X.R.; Xiao, H.L.; Zhang, J.G.; Wang, X.P. Long-term ecosystem effects of sand-binding vegetation in the Tengger Desert, northern China. Restor. Ecol. 2004, 12, 376–390. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, Z.S.; Huang, L.; Liu, L.C.; Wang, X.P. The ecohydrology of the soil–vegetation system restoration in arid zones: A review. Sci. Cold Arid Reg. 2009, 1, 199–206. [Google Scholar]
- Li, X.R.; Zhang, Z.S.; Huang, L.; Wang, X.P. Review of the ecohydrological processes and feedback mechanisms controlling sand-binding vegetation systems in sandy desert regions of China. Chin. Sci. Bull. 2013, 58, 1483–1496. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.S.; Li, X.R. Sap flow of Artemisia ordosica and the influence of environmental factors in a revegetated desert area: Tengger Desert, China. Hydrol. Process. 2010, 24, 1248–1253. [Google Scholar]
- Schwinning, S.; Ehleringer, J.R. Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J. Ecol. 2001, 89, 464–480. [Google Scholar] [CrossRef]
- Loik, M.E.; Breshears, D.D.; Lauenroth, W.K.; Belnap, J. A multiscale perspective of water pulses in dryland ecosystems: Climatology and ecohydrology of the western USA. Oecologia 2004, 141, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Siegwolf, R.T.W. Stable Isotopes as Indicators of Ecological Change; Academic Press, Elsevier: San Diego, CA, USA, 2007. [Google Scholar]
- Resco, V.; Querejeta, J.I.; Ogle, K.; Voltas, J.; Sebastià, M.; Serrano-Ortiz, P.; Linares, J.C.; Moreno-Gutiérrez, C.; Herrero, A.; Carreira, J.A.; et al. Stable isotope views on ecosystem function: Challenging or challenged? Biol. Lett. 2010, 6, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Schnyder, H.; Cuntz, M.; Keitel, C.; Zeeman, M.J.; Dawson, T.E.; Badeck, F.W.; Brugnoli, E.; Ghashghaie, J.; Grams, T.E.E.; et al. Progress and challenges in using stable isotopes to trace plant carbon and water relations across scales. Biogeosciences 2012, 9, 3083–3111. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Herczeg, A.L.; Leaney, F.W. Review: Environmental tracers in arid-zone hydrology. Hydrogeol. J. 2011, 19, 17–29. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plant Cell Environ. 1992, 15, 1073–1082. [Google Scholar] [CrossRef]
- Chimner, R.A.; Cooper, D.J. Using stable oxygen isotopes to quantify the water source used for transpiration by native shrubs in the San Luis Valley, Colorado USA. Plant Soil 2004, 260, 225–236. [Google Scholar] [CrossRef]
- Schwinning, S. The water relations of two evergreen tree species in a karst savanna. Oecologia 2008, 158, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Fang, J.; Liu, S.; Zhao, X.Y.; Li, S.G. Stable isotopic observation of water use sources of Pinus sylvestris var. mongolica in Horqin Sandy Land, China. Trees Struct. Funct. 2013, 27, 1249–1260. [Google Scholar] [CrossRef]
- Williams, D.G.; Ehleringer, J.R. Intra- and interspecific variation for summer precipitation use in pinyon–juniper woodlands. Ecol. Monogr. 2000, 70, 517–537. [Google Scholar]
- Mensforth, L.J.; Thorbum, P.J.; Tyerman, S.D.; Walker, G.R. Sources of water used by riparian Eucalyptus camaldulensis overlying highly saline groundwater. Oecologia 1994, 100, 21–28. [Google Scholar] [CrossRef]
- Thorbum, P.J.; Walker, G.R. Variations in stream water uptake by Eucalyptus camaldulensis with differing access to stream water. Oecologia 1994, 100, 293–301. [Google Scholar] [CrossRef]
- Smith, S.D.; Wellington, A.B.; Nachlinger, J.A.; Fox, C.A. Functional responses of riparian vegetation to streamflow diversions in the eastern Sierra Nevada. Ecol. Appl. 1991, 1, 89–97. [Google Scholar] [CrossRef]
- Busch, D.E.; Ingraham, N.L.; Smith, S.D. Water uptake in woody riparian phreatophytes of the southwestem United States: A stable isotope study. Ecol. Appl. 1992, 2, 450–459. [Google Scholar] [CrossRef]
- Ohte, N.; Koba, K.; Yoshikawa, K.; Sugimoto, A.; Matsuo, N.; Kabeya, N.; Wang, L.H. Water utilization of natural and planted trees in the semiarid desert of Inner Mongolia, China. Ecol. Appl. 2003, 13, 337–351. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.S.; Li, X.R. The extrapolation of the leaf area-based transpiration of two xerophytic shrubs in a revegetated desert area in the Tengger Desert, China. Hydrol. Res. 2014. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Osmond, C.B. Stable isotopes. In Plant Physiological Ecology: Field Methods and Instrumentation; Pearcy, R.W., Ehleringer, J., Mooney, H.A., Rundel, P.W., Eds.; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Socki, R.A.; Romanek, C.S.; Gibson, E.K., Jr. On-line technique for measuring stable oxygen and hydrogen isotopes from microliter quantities of water. Anal. Chem. 1999, 71, 2250–2253. [Google Scholar] [CrossRef] [PubMed]
- Coplen, T.B.; Wildman, J.D.; Chen, J. Improvements in the gaseous hydrogen–water equilibration technique for hydrogen isotope ratio analysis. Anal. Chem. 1991, 63, 910–912. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef]
- Phillips, D.L.; Newsome, S.D.; Gregg, J.W. Combining sources in stable isotope mixing models: Alternative methods. Oecologia 2005, 144, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Craig, H. Isotope variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Allison, G.; Barnes, C.; Hughes, M. The distribution of deuterium and 18O in dry soil 2. Experimental. J. Hydrol. 1983, 64, 377–397. [Google Scholar] [CrossRef]
- Dawson, T.E.; Ehleringer, J.R. Streamside trees that do not use stream water. Nature 1991, 350, 335–337. [Google Scholar] [CrossRef]
- Jackson, P.C.; Meinzer, F.C.; Bustamante, M.; Goldstein, G.; Franco, A.; Rundel, P.W.; Caldas, L.; Igler, E.; Causin, F. Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiol. 1999, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E. Determining water use by trees and forests from isotopic, energy balance and transpiration analyses: The role of tree size and hydraulic lift. Tree Physiol. 1996, 16, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Li, X.R.; Liu, L.C.; Jia, R.L.; Zhang, J.G.; Wang, T. Distribution, biomass, and dynamics of roots in a revegetated stand of Caragana korshinskii in the Tengger Desert, northwestern China. J. Plant Res. 2009, 122, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Lugo, A.E. Ecology of tropical dry forests. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Castellanos, J.; Maass, M.; Kummerow, J. Root biomass of a dry deciduous tropical forest in Mexico. Plant Soil 1991, 131, 225–235. [Google Scholar] [CrossRef]
- Stratton, L.C.; Goldstein, G.; Meinzer, F.C. Temporal and spatial partitioning of water resources among eight woody species in a Hawaiian dry forest. Oecologia 2000, 124, 309–317. [Google Scholar] [CrossRef]
- Ruan, Y.F.; Zhao, L.J.; Xiao, H.L.; Cheng, G.D.; Zhou, M.X.; Wang, F. Water sources of plants and groundwater in typical ecosystems in the lower reaches of the Heihe River Basin. Sci. Cold Arid Reg. 2014, 6, 0226–0235. [Google Scholar]
- Walker, B.H.; Ludwig, D.; Holling, C.S.; Peterman, R.M. Stability of semi-arid savanna grazing systems. J. Ecol. 1981, 69, 473–498. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Kemp, P.R.; Tenhunen, J.D. Effects of long-term rainfall variability on evapotranspiration and soil water distribution in the Chihuahuan Desert: A modeling analysis. Plant Ecol. 2000, 150, 145–159. [Google Scholar] [CrossRef]
- Ogle, K.; Reynolds, J.F. Plant responses to precipitation in desert ecosystems: Integrating functional types, pulses, thresholds, and delays. Oecologia 2004, 141, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Huang, L.; Zhang, Z.S.; Li, X.R. Soil CO2 concentration in biological soil crusts and its driving factors in a revegetated area of the Tengger Desert, Northern China. Environ. Earth Sci. 2014, 72, 767–777. [Google Scholar] [CrossRef]
- Horton, J.L.; Hart, S.C. Hydraulic lift: A potentially important ecosystem process. Trends Ecol. Evol. 1998, 13, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Armas, C.; Padilla, F.M.; Pugnaire, F.I.; Jackson, R.B. Hydraulic lift and tolerance to salinity of semiarid species: Consequences for species interactions. Oecologia 2010, 162, 11–21. [Google Scholar] [CrossRef]
- Dawson, T.E. Hydraulic lift and the water use by plants: Implications for water balance, performance and plant–plant interactions. Oecologia 1993, 95, 565–574. [Google Scholar] [CrossRef]
- Li, X.R.; He, M.Z.; Duan, Z.H.; Xiao, H.L.; Jia, X.H. Recovery of topsoil physiochemical properties in revegetated sites in the sand-burial ecosystems of the Tengger Desert, northern China. Geomorphology 2007, 88, 254–265. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zhang, Z. Stable Isotopic Analysis on Water Utilization of Two Xerophytic Shrubs in a Revegetated Desert Area: Tengger Desert, China. Water 2015, 7, 1030-1045. https://doi.org/10.3390/w7031030
Huang L, Zhang Z. Stable Isotopic Analysis on Water Utilization of Two Xerophytic Shrubs in a Revegetated Desert Area: Tengger Desert, China. Water. 2015; 7(3):1030-1045. https://doi.org/10.3390/w7031030
Chicago/Turabian StyleHuang, Lei, and Zhishan Zhang. 2015. "Stable Isotopic Analysis on Water Utilization of Two Xerophytic Shrubs in a Revegetated Desert Area: Tengger Desert, China" Water 7, no. 3: 1030-1045. https://doi.org/10.3390/w7031030




