3.1. PAM Adsorption on Kaolinite
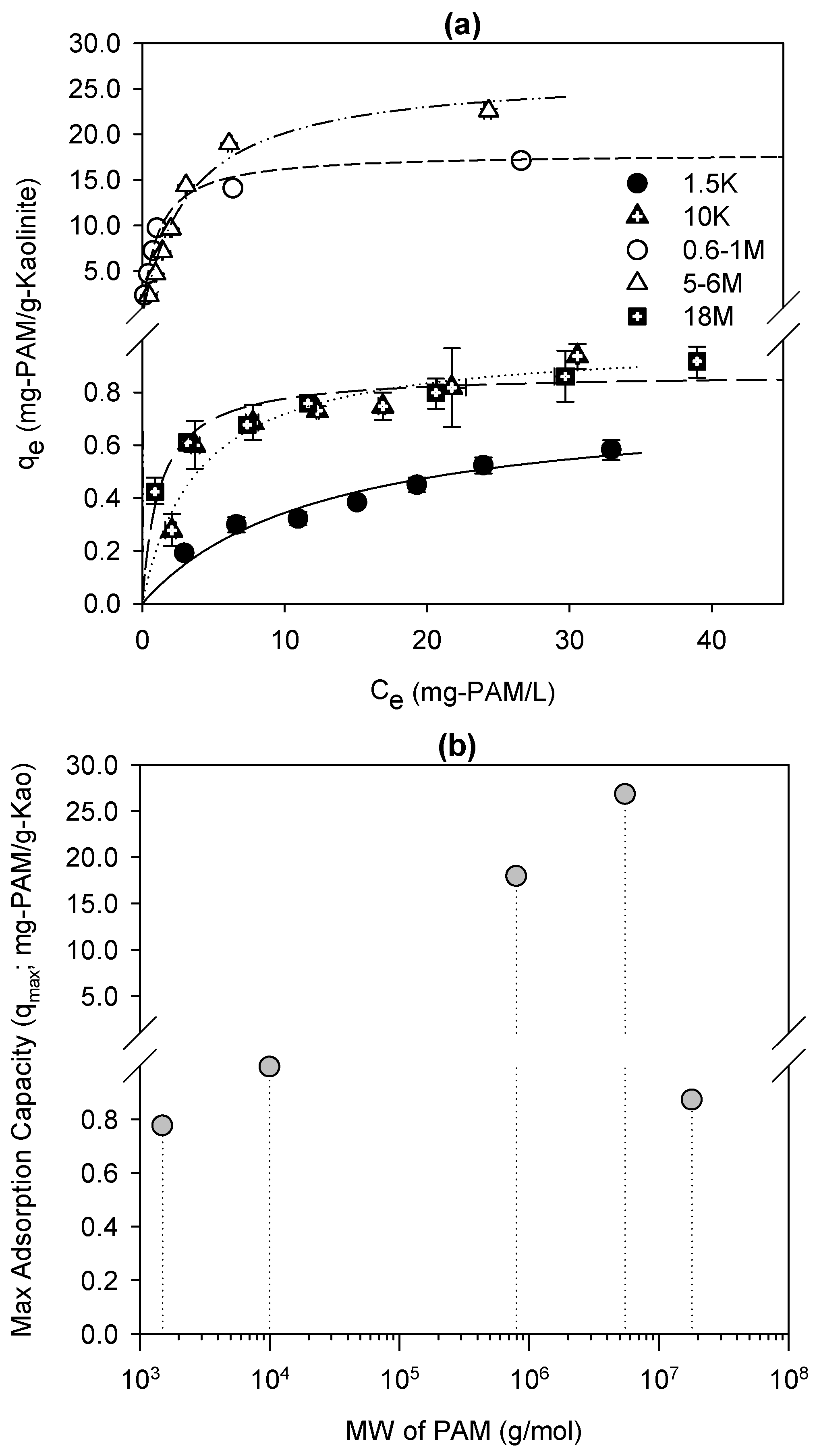
Figure 1a shows that the adsorption isotherms of the 10 K, 0.6–1 M, 5–6 M, and 18 M PAMs increased steeply and then reached a (pseudo) plateau, resembling the high-affinity adsorption isotherms of polymeric substances on solid surfaces [
20,
27,
28]. However, the rounded part of the adsorption isotherm between the initial steep rise and the plateau differs from high-affinity adsorption behavior. The polydispersity of a tested PAM,
i.e., the distribution of the PAM MW, has been reported to develop the rounded part of a high-affinity adsorption isotherm [
20]. Small polymeric molecules occupy more adsorption sites than larger polymeric molecules at a low polymer concentration, because of their higher mobility toward the solid surface. With increasing polymer concentration, large polymeric molecules start replacing small polymeric molecules, because of the thermodynamic advantages of their adsorption on the solid surface. This replacement process develops the rounded part of the high-affinity adsorption isotherm. All the high-affinity adsorption isotherms with rounded parts fitted the Langmuir isotherm well (
Table 1 and
Figure 1a). However, the adsorption isotherm of the smallest PAM,
i.e., 1.5 K PAM, was different from a high-affinity adsorption isotherm, increasing continuously without an apparent plateau. The adsorption isotherm of 1.5 K PAM might follow those of relatively small compounds rather than of large polymers.
Table 1.
Results of nonlinear data fitting to Langmuir isotherms (qe = qmax × Ce/(Ks + Ce)) for 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M polyacrylamides (PAMs).
Table 1.
Results of nonlinear data fitting to Langmuir isotherms (qe = qmax × Ce/(Ks + Ce)) for 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M polyacrylamides (PAMs).
| MW (g/mol) | q,max (mgPAM/gKaolinite) | Ks (mgPAM/L) | R2 |
|---|
| 1.5 K † | 0.7753 | 12.542 | 0.9280 |
| 10 K | 0.9951 | 3.8749 | 0.9078 |
| 0.6~1 M ‡ | 17.941 | 1.0811 | 0.9851 |
| 5~6 M | 26.796 | 3.3032 | 0.9730 |
| 18 M | 0.8718 | 1.2365 | 0.9128 |
The maximum adsorption capacities of the tested PAMs on kaolinite increased with increasing PAM MW, except for the largest,
i.e., 18 M PAM (
Table 1 and
Figure 1b). The maximum adsorption capacities of the 0.6–1 M and 5–6 M PAMs were 20–30 times higher than those of the other tested PAMs. This supports the suggestion that a high-MW polymer has a higher adsorption capacity on the solid surface than a small-MW polymer does [
9,
19,
20,
29]. However, the maximum adsorption capacity of the highest-MW PAM,
i.e., 18 M PAM, decreased to those of the smallest-MW PAMs,
i.e., 1.5 K and 10 K PAMs. The 1 g/L stock solution of 18 M PAM was a gel with a very high viscosity, therefore it was hypothesized that the PAM concentration of the stock solution exceeded the critical entanglement concentration, above which the polymeric chains in the solution phase are entangled each other (semi-dilute) and not dissolved independently (dilute) [
26]. Entanglements among PAM chains might prevent PAM molecules from adsorbing on kaolinites.
Figure 1.
Experimental results of adsorption tests: (a) adsorption isotherm curves of 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M polyacrylamides (PAMs), representing the PAM adsorption capacity (i.e., the solid-phase equilibrium PAM concentration; qe) versus the solution-phase equilibrium PAM concentration (Ce) and (b) maximum adsorption capacity versus PAM molecular weight.
Figure 1.
Experimental results of adsorption tests: (a) adsorption isotherm curves of 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M polyacrylamides (PAMs), representing the PAM adsorption capacity (i.e., the solid-phase equilibrium PAM concentration; qe) versus the solution-phase equilibrium PAM concentration (Ce) and (b) maximum adsorption capacity versus PAM molecular weight.
3.2. Entanglements in High-MW Polymeric Solutions
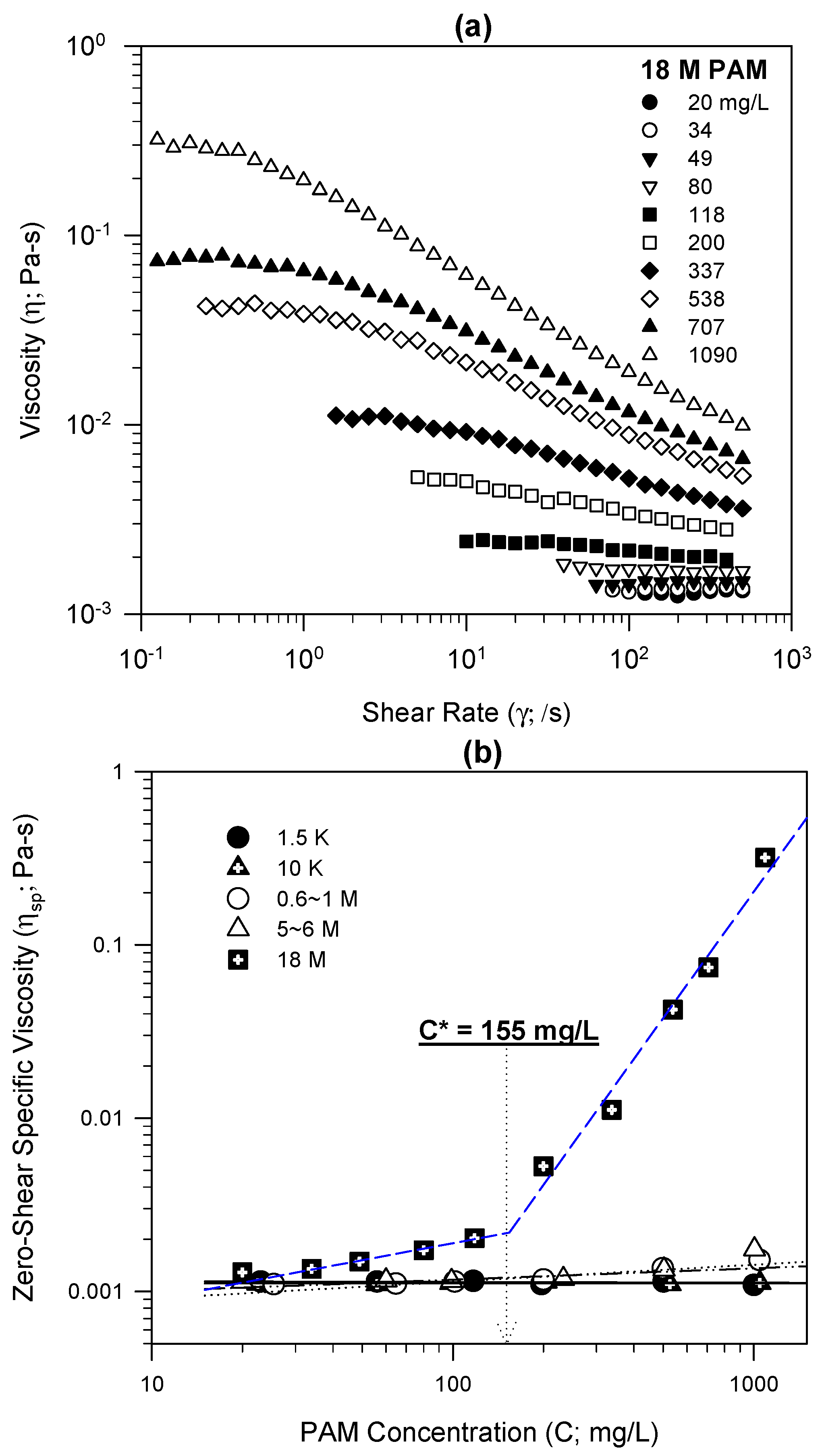
Steady-shear viscosity tests could provide scientific proof of polymeric chain entanglements in the 18 M PAM solution. Generally, the steady-shear viscosity curve of a high-MW polymeric solution increases, and then reaches a plateau as it approaches the zero shear rate (shear rate → 0).
Figure 2a shows typical steady-shear viscosity curves, which were measured using a series of 18 M PAM solutions prepared at different PAM concentrations. The zero-shear specific viscosities, obtained from the plateau (shear rate → 0) of the steady-shear viscosity curves, were then plotted against the solution-phase PAM concentration to identify the critical entanglement concentration separating dilute and semi-dilute regions (
Figure 2b).
The highest-MW PAM,
i.e., 18 M PAM, showed a typical zero-shear specific viscosity curve of a high-MW polymer,
i.e., the zero-shear specific viscosity curve increased with increasing PAM concentration and had an inflexion point at about 155 mg-PAM/L, separating two linear regions. This inflexion point is commonly called the critical entanglement concentration, and is the border between the two regimes of the polymeric solution,
i.e., the dilute and semi-dilute regimes [
24,
25,
26]. The 18 M PAM solution, with a concentration above 155 mg-PAM/L, was therefore in the semi-dilute region, and polymeric chain entanglements developed. However, the zero-shear specific viscosities of the 1.5 K, 10 K, 0.6–1 M, and 5–6 M PAMs were constant, near the level of pure water (≈0.001 Pa-s at 20 °C), irrespective of shear rates and PAM concentrations (see
Figure 2b). These PAM solutions remained in the dilute region, without polymeric chain entanglements.
Figure 2.
Experimental results of steady-shear viscosity measurements: (a) steady-shear viscosity versus shear rate at different polyacrylamide (PAM) concentrations for 18 M PAM and (b) zero-shear specific viscosity versus PAM concentration for 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M PAMs.
Figure 2.
Experimental results of steady-shear viscosity measurements: (a) steady-shear viscosity versus shear rate at different polyacrylamide (PAM) concentrations for 18 M PAM and (b) zero-shear specific viscosity versus PAM concentration for 1.5 K, 10 K, 0.6–1 M, 5–6 M, and 18 M PAMs.
The concentration of the stock solution of 18 M PAM was 1 g/L, above the critical entanglement concentration (155 mg/L) in the adsorption tests, so the small adsorption capacity of 18 M PAM might be caused by polymeric chain entanglements. Even after mixing the PAM stock solution and kaolinite suspensions in the adsorption tests, PAM molecules might remain entangled as a gel during these tests, without enough energy or time for disentangling the polymeric chains. This further indicates that the preparation and handling of excessively high-MW PAMs need to be performed with caution, so they may not be suitable for
in situ applications [
18].
3.3. PAM-Mediated Particle Flocculation
The flocs in kaolinite suspensions with 0.6–1 M or 5–6 M PAMs were large enough to be visible in the bottle-point adsorption tests, but flocs with 1.5 K, 10 K, or 18 M PAMs were not. The 1.5 K and 10 K PAMs might be confined within the electrostatic repulsion layer, because of their short polymeric chain structures, and therefore unable to develop polymeric bridges between particles and decrease the flocculation capability. However, 18 M PAM might reduce the flocculation capability, probably because of polymeric chain entanglements and the low adsorption capacity (see also
Section 3.1 and
Section 3.2). Only the 0.6–1 M and 5–6 M PAMs were therefore used in the flocculation tests.
The PAM MWs and flow turbulent shear rates were selected as the experimental parameters in the flocculation tests, and their effects on the adsorption capacities and flocculation capabilities, particularly nonequilibrium flocculation [
14,
16,
21], were investigated. Equilibrium flocculation occurs after stepwise completion of adsorption and reconfiguration of the adsorbed polymeric chains, but nonequilibrium flocculation occurs before completion of adsorption and reconfiguration (
Figure 3) [
8,
15]. Nonequilibrium flocculation therefore enhances the flocculation capability, because of stretching and waving of the polymeric chains before they settle on the solid surfaces. A high-MW PAM increases the time for polymer reconfiguration, but a higher fluid shear rate decreases the time for particle collision and attachment, because of the thermodynamics of the polymeric chain structure. A high-MW PAM is therefore more prone to nonequilibrium flocculation, and this increases the flocculation capability compared with that of a low-MW PAM. The PAM MWs and fluid shear rates were therefore investigated as the experimental parameters in the flocculation tests.
Figure 3.
Schematic diagram of equilibrium and nonequilibrium flocculation mechanisms in kaolinite suspensions dosed with polyacrylamides.
Figure 3.
Schematic diagram of equilibrium and nonequilibrium flocculation mechanisms in kaolinite suspensions dosed with polyacrylamides.
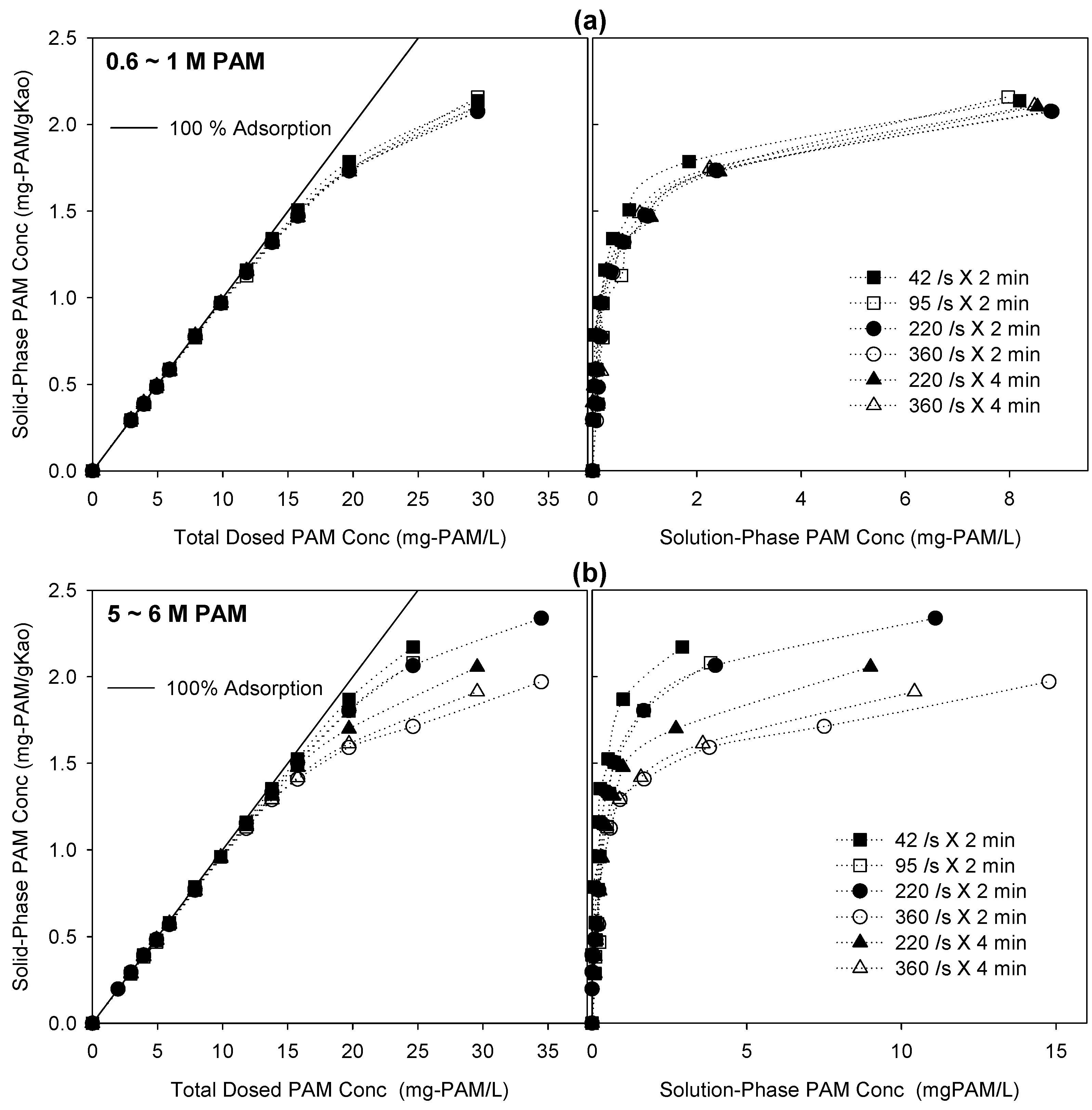
The adsorption capacities obtained from the flocculation tests are plotted in
Figure 4 for different PAM MWs (0.6–1 M and 5–6 M PAMs), shear rates (42–360 s
−1), and contact times (2–4 min). The relationship between adsorption capacity (
i.e., the solid-phase PAM concentration) and the solution-phase PAM concentration (the right-hand panels of
Figure 4) was typical high-affinity adsorption behavior, similar to the adsorption isotherms obtained in the bottle-point adsorption tests (see also
Section 3.2). However, because of the short reaction time (2 or 4 min), the adsorption capacities were about an order of magnitude less in the flocculation tests than in the bottle-point adsorption tests. The major finding is that the adsorption capacities of the higher-MW 5–6 M PAM were scattered for different shear rates and contact times, in particular, deviating from the 100% adsorption line and the y-axis, but those of the lower-MW 0.6–1 M PAM remained constant (
Figure 4). The adsorption capacity of the higher-MW 5–6 M PAM decreased with increasing shear rate and contact time.
Figure 4.
Experimental results of flocculation tests performed using 0.6–1 M and 5–6 M polyacrylamides (PAMs) at different shear rates and contact times. (a,b) represent PAM adsorption capacity versus PAM concentration for 0.6–1 M and 5–6 M, respectively. The x-axis represents the total dosed PAM concentrations in the left-hand panels, and solution-phase PAM concentrations in the right-hand panels.
Figure 4.
Experimental results of flocculation tests performed using 0.6–1 M and 5–6 M polyacrylamides (PAMs) at different shear rates and contact times. (a,b) represent PAM adsorption capacity versus PAM concentration for 0.6–1 M and 5–6 M, respectively. The x-axis represents the total dosed PAM concentrations in the left-hand panels, and solution-phase PAM concentrations in the right-hand panels.
The higher-MW 5–6 M PAM might need higher mechanical energy and a longer time for chain reconfiguration and settlement on kaolinite than the lower-MW 0.6–1 M PAM, because of the thermodynamic disadvantages of the polymeric chain structure for reconfiguration and settlement. At a higher shear rate, resulting in a shorter particle collision time (e.g., 360 s
−1 in
Figure 4b), the higher-MW 5–6 M PAM might quickly start flocculation, before completing polymeric chain reconfiguration and settlement. The aggregated and closed structure of the floc might then inhibit PAM molecules from approaching and adsorbing on kaolinite particles, thereby reducing the adsorption capacity (
Figure 4b). The lower-MW 0.6–1 M PAM, however, might start flocculation after rapid completion of polymeric chain reconfiguration, producing similar adsorption isotherms irrespective of the shear rate and contact time. In fact, the higher-MW 5–6 M PAM was more susceptible to fluid shear rates and likely to develop different adsorption isotherms than the low-MW 0.6–1 M PAM was, because of the unstable polymeric structures on the solid surfaces.
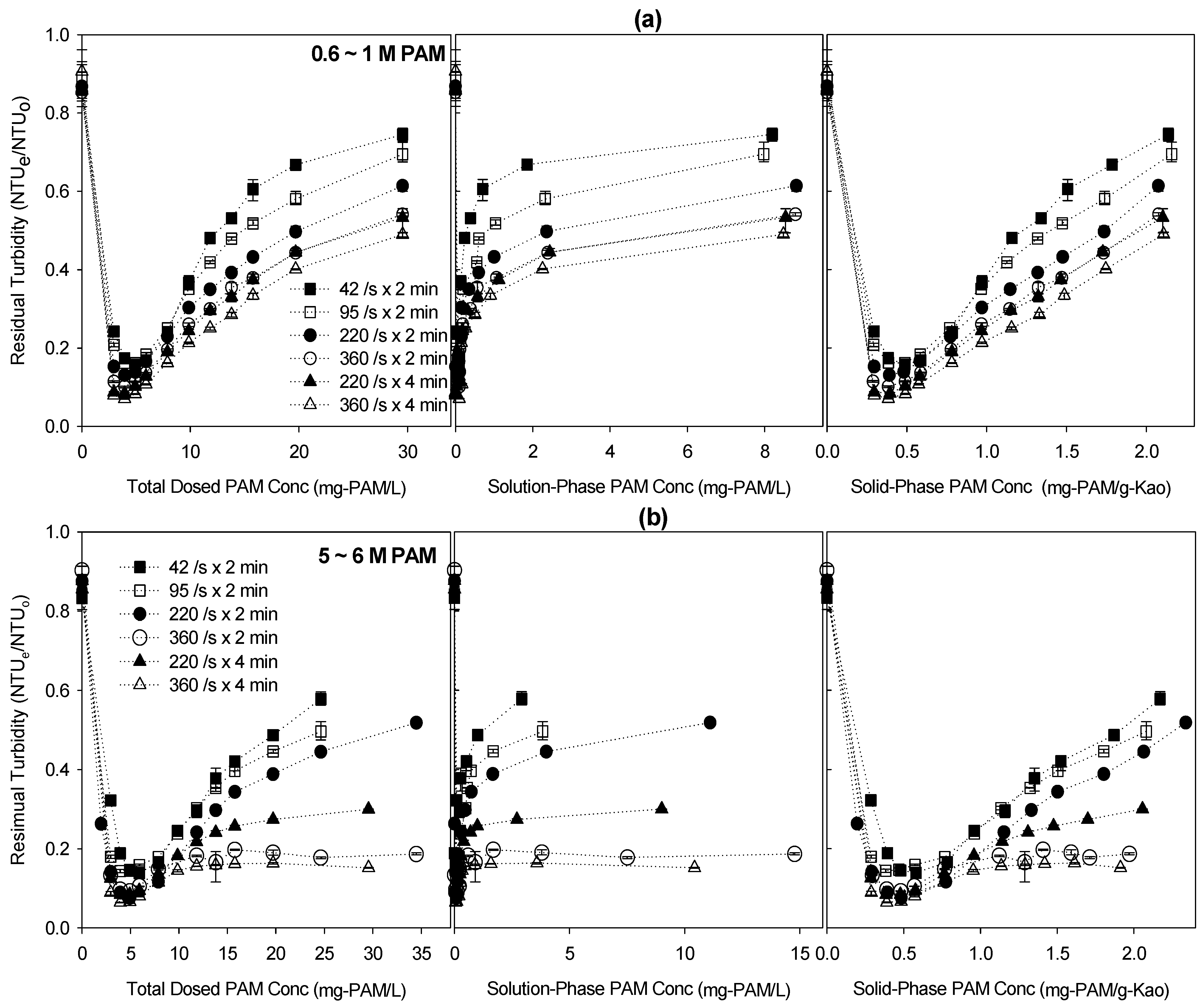
The residual turbidity (final turbidity/initial turbidity) was used as an experimental index of the flocculation capability of PAM-dosed kaolinite suspensions and plotted against different x-axes for different shear rates and contact times (
Figure 5). The residual turbidity generally decreased to a minimum at a total dosed PAM concentration of about 5 mg-PAM/L and continuously increased (the left-hand panels in
Figure 5). When plotted against the solution-phase PAM concentration, the residual turbidity curves sharply decreased to a minimum near the zero solution-phase PAM concentration, but increased and approached the plateau with increasing solution-phase PAM concentration (the middle panels). The plot of the residual turbidity against the solid-phase PAM concentration (the right-hand panels) became more linear in the restabilization phase, after passing the minimum, than in the plot against the total dosed PAM concentration (the left panels). This might indicate that particle restabilization is linearly correlated with the amount of adsorbed PAM molecules.
The residual turbidity of the higher-MW 5–6 M PAM decreased more,
i.e., the flocculation capability increased more, with increasing shear rate and contact time, than did those of the lower-MW 0.6–1 M PAM. The relative turbidity curves of the higher-MW 5–6 M PAM changed from a U-shape to an L-shape at a higher shear rate and contact time (
Figure 5b), whereas the curves of the lower-MW 0.6–1 M PAM remained U-shaped (
Figure 5a). Particle restabilization resulting in U-shaped residual turbidity curves is common in colloidal suspensions with polymeric substances, especially under polymer-over-dosed conditions, because the saturated polymeric layers on the particle surfaces inhibit formation of polymeric bridges and increase the repulsive forces among particles [
14,
21]. In contrast, the higher-MW 5–6 M PAM developed L-shaped residual turbidity curves, especially at high shear rates (e.g., 220 or 360 s
−1), indicating a higher flocculation capability. As shown in
Figure 3, the higher-MW 5–6 M PAM might be more susceptible to nonequilibrium flocculation, and thus enhance particle flocculation but reduce restabilization, especially under polymer-over-dosed conditions at high shear rates, because of stretching and waving of the polymeric chain structures on the solid surfaces. In contrast, the lower-MW 0.6–1 M PAM might be governed by equilibrium flocculation, and thus reduce particle flocculation but enhance restabilization at higher shear rates, quickly reconfiguring and settling on the solid surfaces.
Figure 5.
Experimental results of flocculation tests performed using 0.6–1 M and 5–6 M polyacrylamides (PAMs) at different shear rates and contact times. (a,b) represent the residual turbidity (final turbidity/initial turbidity) versus PAM concentrations for 0.6–1 M and 5–6 M, respectively. The x-axis represents the total dosed PAM concentrations in the left-hand panels, the solution-phase PAM concentrations in the middle panels, and the solution-phase PAM concentrations in the right-hand panels.
Figure 5.
Experimental results of flocculation tests performed using 0.6–1 M and 5–6 M polyacrylamides (PAMs) at different shear rates and contact times. (a,b) represent the residual turbidity (final turbidity/initial turbidity) versus PAM concentrations for 0.6–1 M and 5–6 M, respectively. The x-axis represents the total dosed PAM concentrations in the left-hand panels, the solution-phase PAM concentrations in the middle panels, and the solution-phase PAM concentrations in the right-hand panels.