Catchment-Scale Modeling of Nitrogen Dynamics in a Temperate Forested Watershed, Oregon. An Interdisciplinary Communication Strategy
Abstract
:1. Introduction
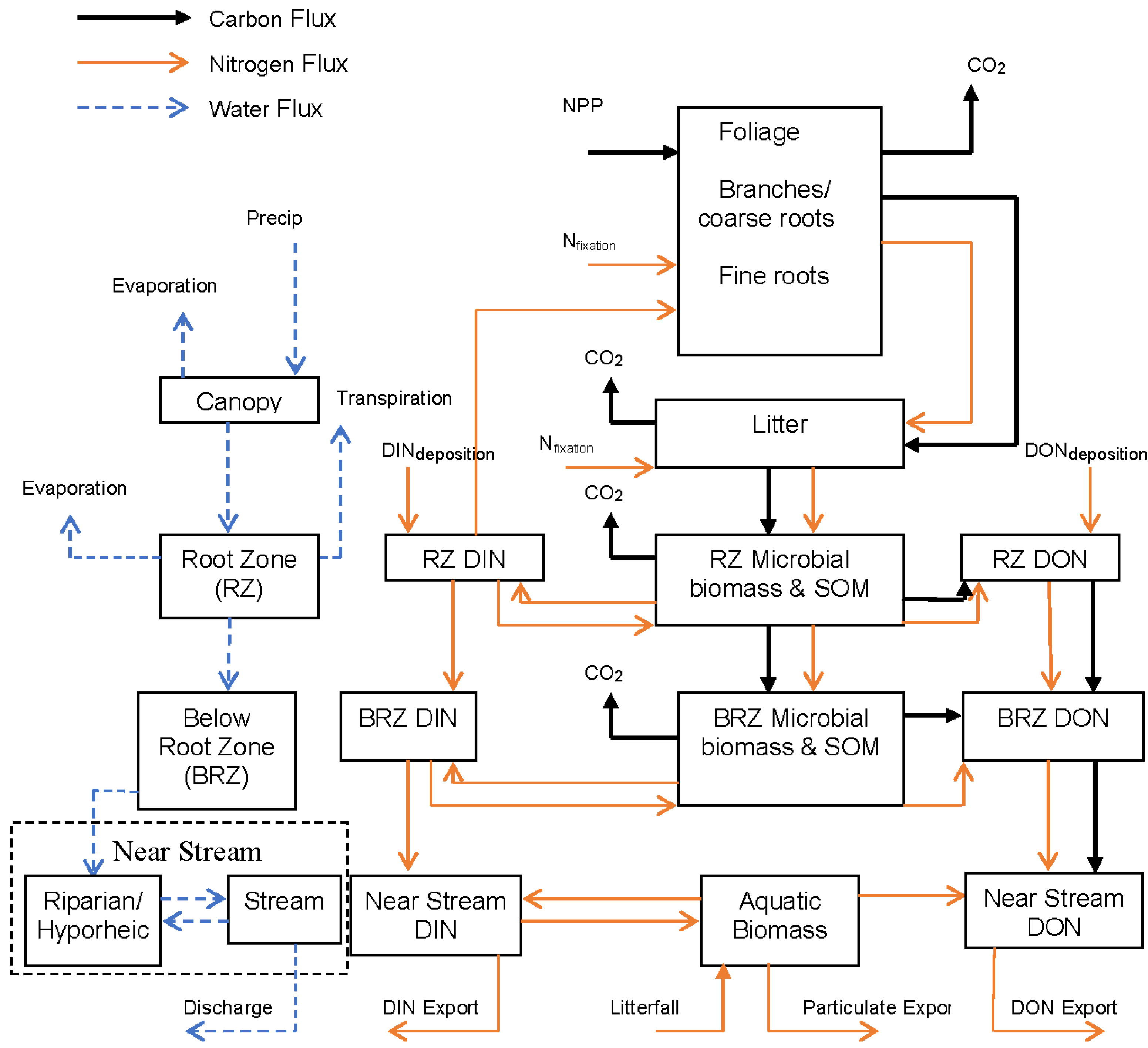
2. Materials and Methods
2.1. Ecosystem Modeling
2.2. Study Site
2.3. The HJA-N Model
| System Component | Mass | Additional Information | |||
|---|---|---|---|---|---|
| Name | Short Name | Name | Short Name | Short Name | Name |
| Biomass | B | Carbon | C | Root Zone | R |
| Detritus | D | Particulate Nitrogen | N | Below Root Zone | B |
| Soil Organic Matter (SOM) | S | Available Nitrogen (DIN) | Na | Hyporheic Zone | HY |
| Unavailable Nitrogen (DON) | Nu | In stream/aquatic | IS | ||
| Aquatic Biomass | A | Water | Wa | Canopy | C |
| Wood | w | ||||
| Fine root | r | ||||
| Foliage | f | ||||
| Equation | Defines |
|---|---|
| Carbon in biomass | |
| Nitrogen in biomass | |
| Carbon in detritus | |
| Nitrogen in detritus | |
| Carbon in the root zone SOM | |
| Nitrogen in the root zone SOM | |
| Carbon below the root zone SOM | |
| Nitrogen below the root zone SOM | |
| Dissolved available nitrogen in the root zone | |
| Dissolved unavailable nitrogen in the root zone | |
| Dissolved available nitrogen below the root zone | |
| Dissolved unavailable nitrogen below the root zone | |
| Dissolved available nitrogen in the aquatic environment | |
| Dissolved unavailable nitrogen in the aquatic environment | |
| Aquatic Biomass (particulate, algae,benthic) | |
| Water in the canopy | |
| Water in the root zone (we assume it moves vertically) | |
| Water below the root zone (we assume it moves horizontally) | |
| Water in the riparian/hyporheic zone | |
| Water in the channel |
| Rate | Definition | Units |
|---|---|---|
| DPW,C | Deposition of water (precipitation) | m/day |
| Throughfall from canopy | m/day | |
| Evaporation from canopy | m/day | |
| ETW,R | Evapotranspiration from root zone | m/day |
| Vertical movement of water within the root zone | m/day | |
| Long (time) flowpaths of water below the root zone | m/day | |
| Short (time) flowpaths of water below the root zone | m/day | |
| Hyporheic exchange of water from the stream | m/day | |
| Hyporheic exchange of water from the Hyporheic zone | m/day | |
| Discharge of water from the stream | m/day |
| Rate | Definition | Units |
|---|---|---|
| Pn allocated to foliage(i = f), wood (i = w) and fine roots (i = r) | kg C/m2 | |
| Turnover of C from foliage (i = f), wood (i = w) and fine roots (i = r) | kg C/m2 | |
| Uptake of N | kg N/m2 | |
| Uptake allocated to foliage (i = f), wood (i = w) and fine roots (i = r) | kg N/m2 | |
| Turnover of N from foliage (i = f), wood (i = w) and fine roots (i = r) | kg N/m2 | |
| Symbiotic fixation allocated to foliage (i = f), wood (i = w) and fine roots (i = r) | kg N/m2 | |
| Decomposition to SOM from foliage (i = f), wood (i = w) and fine roots (i = r) | kg C/m2 | |
| Decomposition of N from foliage (i = f), wood (i = w) and fine roots (i = r) | kg N/m2 | |
| Asymbiotic fixation allocated to foliage (i = f), wood (i = w) and fine roots (i = r) | kg N/m2 | |
| Decomposition as resp from foliage (i = f), wood (i = w) and fine roots (i = r) | kg C/m2 |
| Rate | Definition | Units |
|---|---|---|
| Respiration of C from root zone (i = R), below (i = B) | kg C/m2 | |
| Transfer of C from root zone to below | kg C/m2 | |
| Mobilization of unavailable N from RZ (i = R), below (i = B) | kg N/m2 | |
| Mobilization of available N from RZ (i = R), below (i = B) | kg N/m2 | |
| Transfer of N out of root zone | kg N/m2 | |
| Immobilization of available N (j = R), below (j = B) | kg N/m2 | |
| DPNa | Deposition of DIN | kg N/m2 |
| Breakdown of HMW DON from the root zone (i = R) or below (i = B) | kg N/m2 | |
| Denitrification from RZ (i = R), below (i = B), and instream (i = IS) | kg N/m2 | |
| Hydrologic transfer of N from RZ. (i = Na—unavailable. i = Nu—available) | kg N/m2 | |
| DPu | Deposition of DIN | kg N/m2 |
| Hydrologic transfer of N from brz. (i = Na represents unavailable, i = Nu represents available) | kg N/m2 |
| Rate | Definition | Units |
|---|---|---|
| Mobilization of available N from instream (i = Na represents unavailable, i = Nu represents available) | kg N/m2 | |
| Immobilization of available N from instream | kg N/m2 | |
| Discharge of available N from stream. (i = Na represents unavailable. i = Nu represents available) | kg N/m2 | |
| BIN | Biological input of N to the stream | kg N/m2 |
| Particulate export from the stream | kg N/m2 | |
| when , , Else | ||
| Term | Definition | Units |
|---|---|---|
| Incoming shortwave radiation | KJ/m2-day | |
| Photosynthetically active radiation (PAR) | KJ/m2-day | |
| Absorbed PAR (Beer’s Law) | KJ/m2-day | |
| k | Light extinction coefficient | – |
| Leaf area | m2/m2 | |
| SLA | Specific leaf area | kg/m2 |
| Utilized absorbed PAR | KJ/m2-day | |
| Growth modifier | – | |
| Gross Primary Productivity (GPP) | kg/ha | |
| Canopy quantum efficiency | Mol C/mol photon | |
| Net canopy production (from 3PG) | kg C/m2 | |
| Y | Respiration fraction of GPP | – |
| Temperature modifier | – | |
| Soil water modifier | – | |
| Vapor pressure deficit modifier | – | |
| Age modifier | – | |
| If | ||
| Nutrient modifier | - | |
| otherwise | ||
| Allocation fraction of foliage where i = C for Carbon and i = N for Nitrogen | – | |
| Allocation fraction of wood where i = C for Carbon and i = N for Nitrogen | – | |
| Allocation fraction root C | – | |
| Allocation fraction root N | – | |
| C (i = C) or N (i = N) remaining allocated to wood | – | |
| Temperature modifier | – | |
| Root allocation parameters (hyperbola) | – |
| Term | Definition | Units |
|---|---|---|
| Max uptake rate | kg N/m2 | |
| Uptake ½ saturation | kg N/m2 | |
| Turnover rate constants | year−1 | |
| Decomposition rate constants | day−1 | |
| Coef by which D increases each 10 C | kg/m2 | |
| T | temperature | C |
| Fraction of D as CO2 | – | |
| CN ratios of detrital pools | – | |
| Critical CN, above which N is retained | – | |
| Soil respiration coefficient | year−1 | |
| Transfer rate of particulates | year−1 | |
| CN ratios of SOM pools | – | |
| Max immobilization rate | year−1 | |
| Immobilization ½ saturation | kg N/ha | |
| Denitrification rate constant | year−1 | |
| Sorption rate constant | year−1 | |
| Decomposition rate of dissolved unavailailable N | day−1 | |
| Instream mobilization rate constant | day−1 | |
| Instream immobilization rate constant | day−1 | |
| Hydro factor for particulate export | – | |
| Max particulate export rate constant | day−1 | |
| Hydro threshold | m/m | |
| Hydro factor | – | |
| Hydro factor | – | |
| Canopy interception and evaporation rate terms | day−1 | |
| Vertical, long, and short flowpath | day−1 | |
| Hyporheic and instream exchange | day−1 | |
| Instream water retention rate constant | day−1 |
2.3.1. Measured Data
2.3.2. Hydrologic Model
2.3.3. Vegetation Model
2.3.4. Soils Model
2.3.5. Aquatic Environment Model
2.3.6. Control Capacity
3. Results and Discussion
3.1. Results
3.1.1. Evaluation of Budget Estimates of N and C Stocks
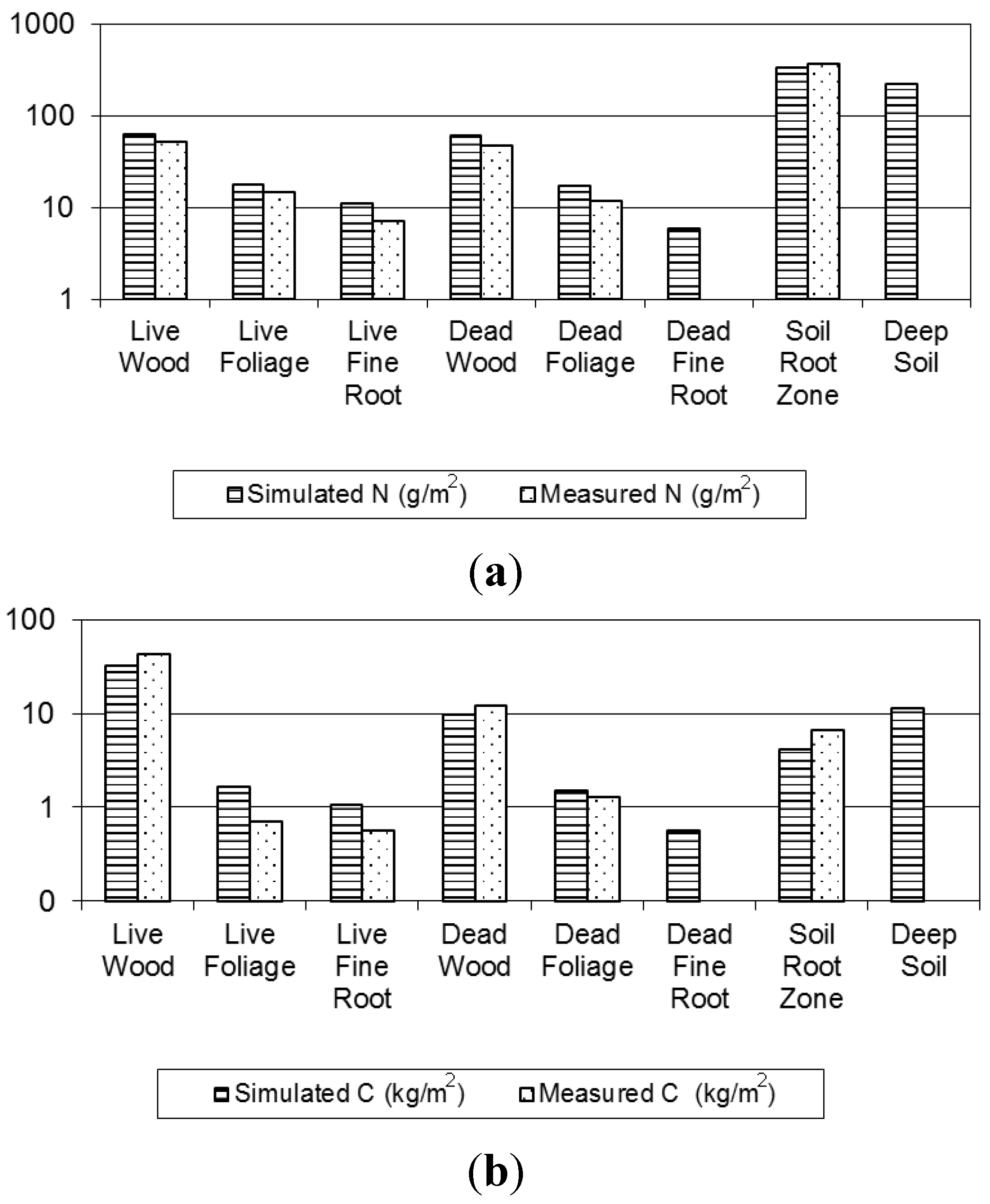

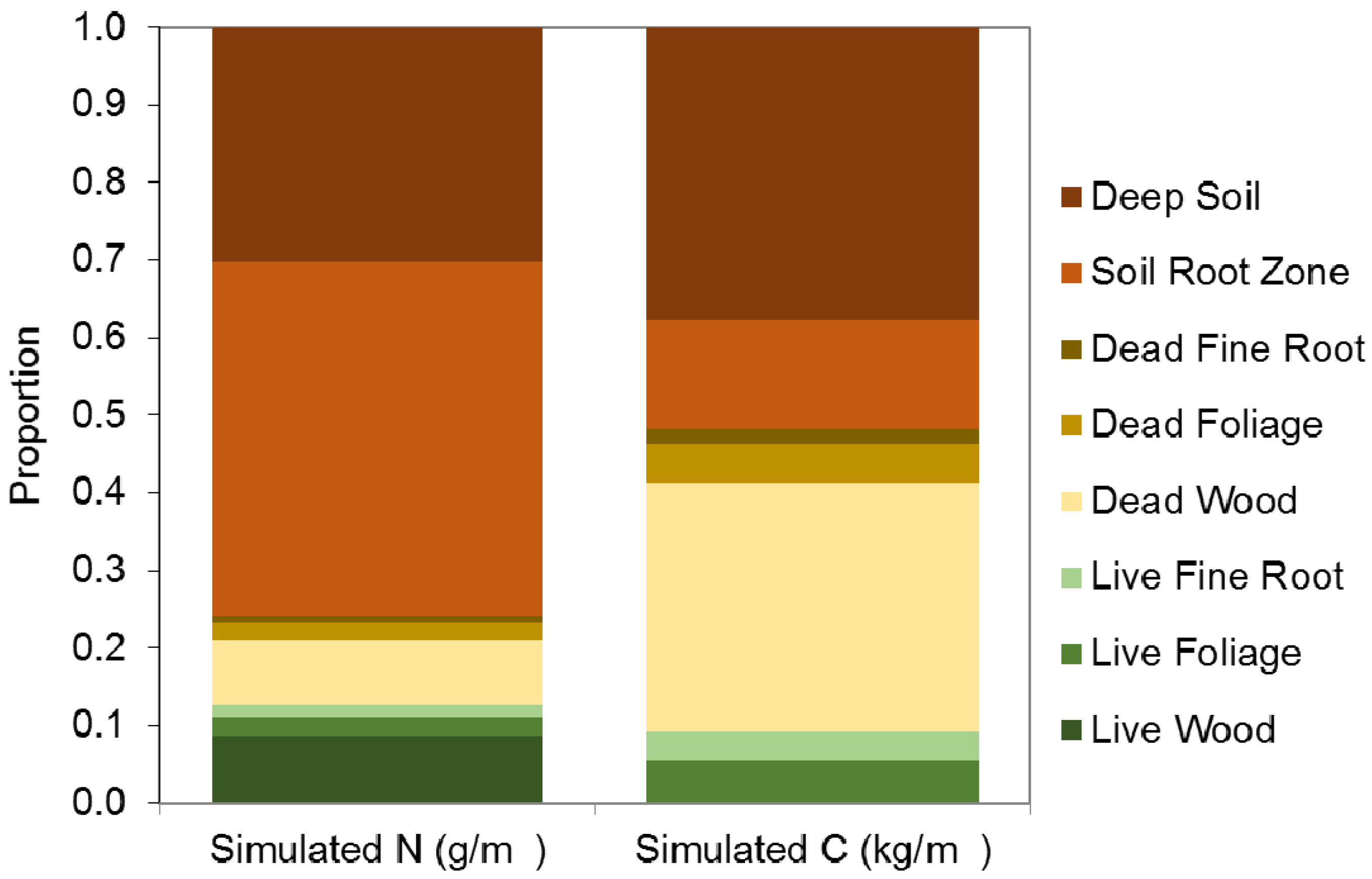
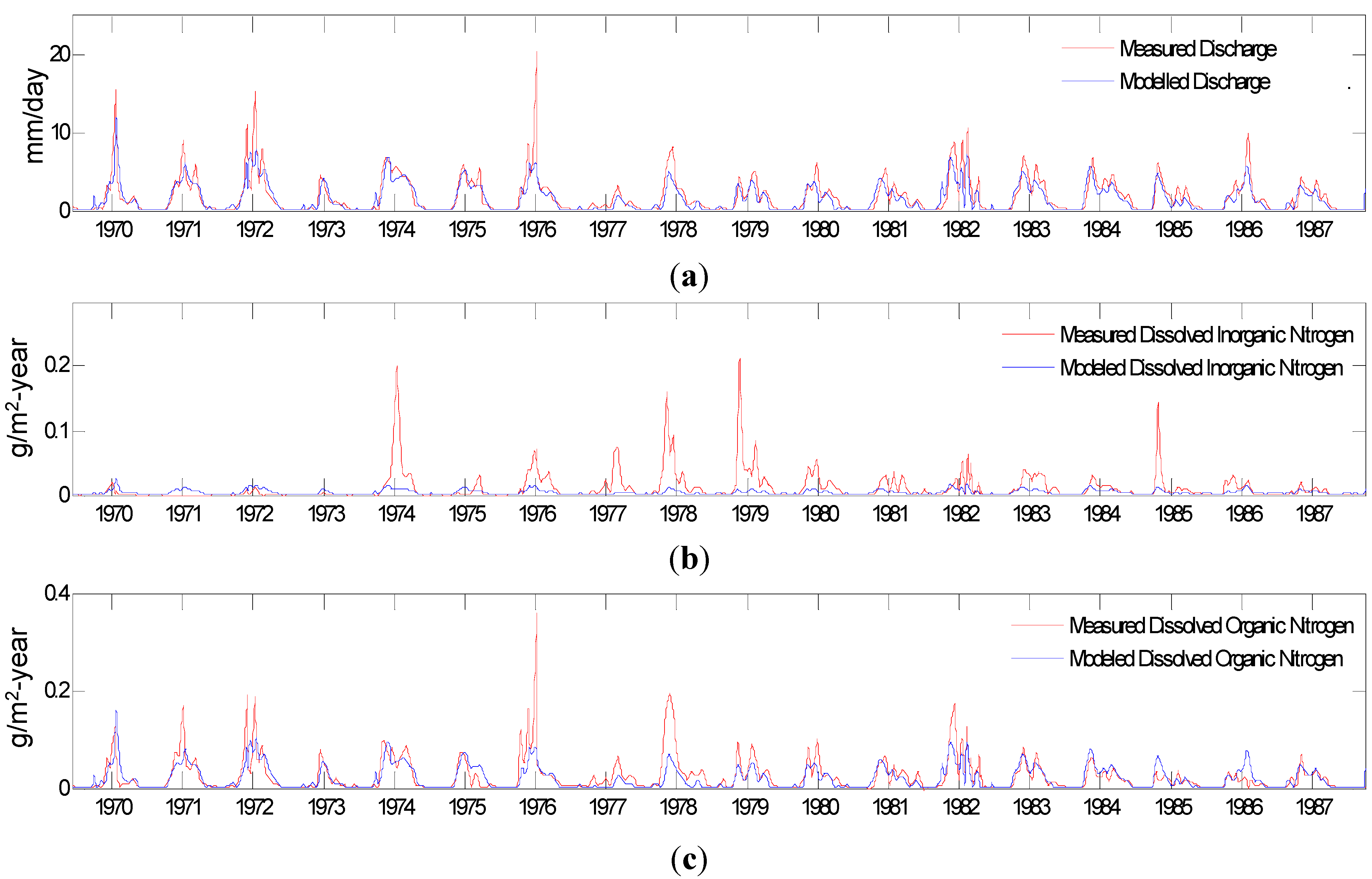
3.1.2. Model Evaluation against Observations
Evaluation of against Budget Estimates of N and C Fluxes
| Ecosystem Component | C (kg/m2) | N (g/m2) | CN | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | Model | a | Model | a | Model | ||
| Vegetation | Wood | 42.29 | 37.87 | 31.77 (0.04) | 52.17 | 63.3 (0.11) | 810.63 | 501.57 |
| Foliage | 0.69 | 0.94 | 2.17 (0.04) | 14.44 | 18.2 (0.38) | 48.13 | 119.20 | |
| Fine Roots | 0.06 | 0.36 | 1.12 (0.04) | 0.69 | 11.7 (0.16) | 80.65 | 96.34 | |
| Detritus | Wood | 13.31 | 9.87 | 9.54 (0.05) | 47.1 | 61.1 (0.58) | 282.59 | 156.05 |
| Foliage | 1.28 | 1.78 | 2.01 (0.02) | 11.76 | 18.1 (0.15) | 108.84 | 110.73 | |
| Fine Roots | 0.48 | 0.593 (0.01) | 6.4 (0.11) | 93.06 | ||||
| Soil | Root Zone | 6.65 | 9.3 | 4.28 (0.06) | 372.4 | 338.5 (4.23) | 17.88 | 12.65 |
| Deep Soil | 12.80 (0.33) | 220.5 (5.39) | 58.07 | |||||

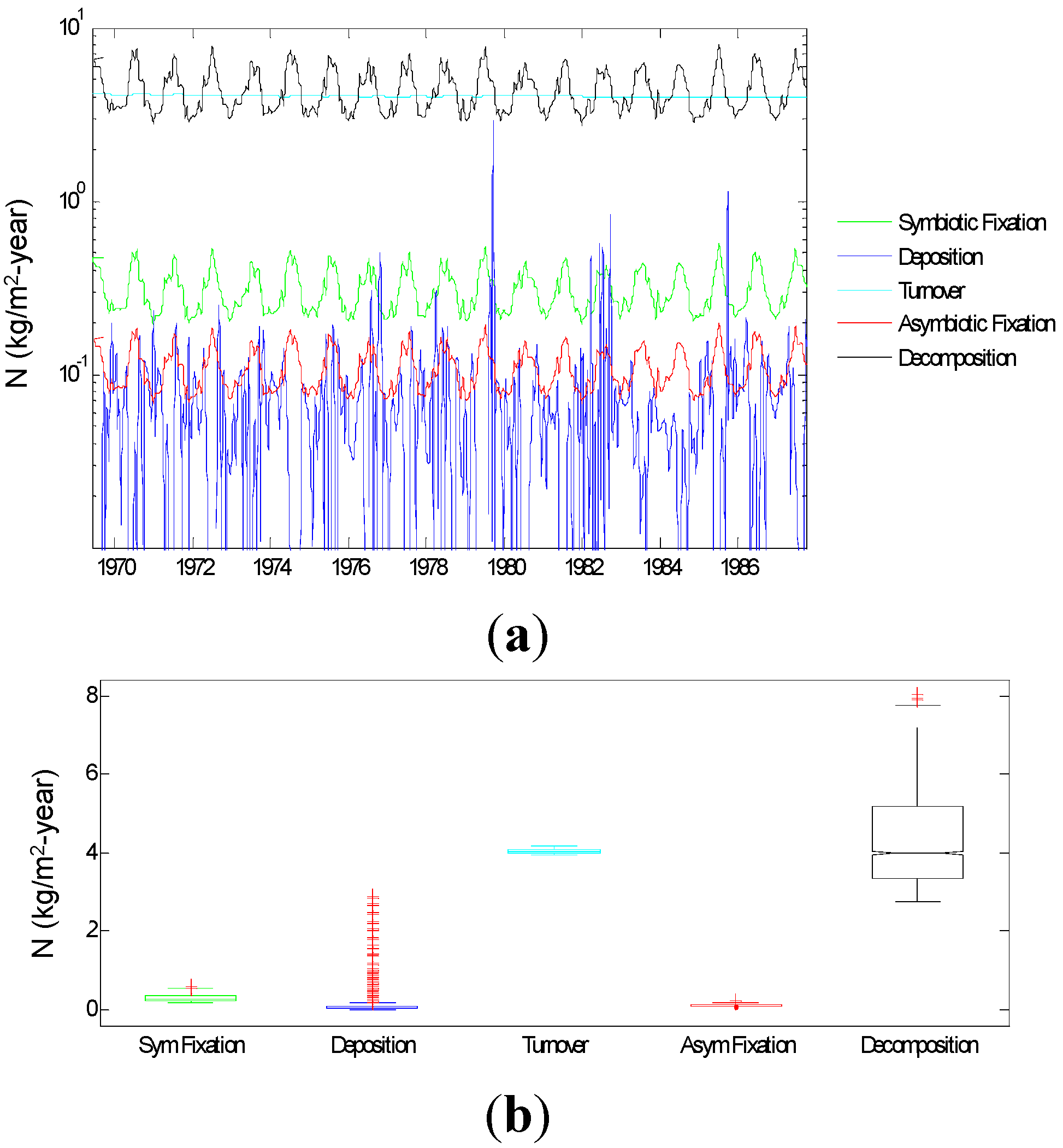


| Rate | g/m2-year |
|---|---|
| Gross primary production | 935.28 |
| Growth wood | 44.34 |
| Growth foliage | 177.34 |
| Growth fine roots | 217.96 |
| Total growth (NPP) | 439.63 |
| Mortality wood | 32.56 |
| Mortality foliage | 176.62 |
| Mortality fine roots | 217.35 |
| Total mortality | 426.53 |
| Decomposition from wood | 4.37 |
| Decomposition from foliage | 127.54 |
| Decomposition from fine roots | 185.66 |
| Total decomposition | 672.16 |
| Respiration from wood | 39.35 |
| Respiration from foliage | 0.01 |
| Respiration from fine roots | 32.76 |
| Respiration from SOM RZ | 173.98 |
| Respiration from SOM BRZ | 44.16 |
| Respiration heterotrophic | 332.78 |
| Respiration Autotrophic | 495.70 |
| Total respiration | 828.48 |
| DOC Production RZ | 17.4 |
| DOC Production BRZ | 4.42 |
| Total DOC production | 21.82 |
| Rate | g/m2-year |
|---|---|
| DON_Deposition | 0.04 |
| DIN_Deposition | 0.03 |
| Total deposition | 0.08 |
| Symbiotic fixation allocated to wood | 0 |
| Symbiotic fixation allocated to foliage | 0 |
| Symbiotic fixation allocated to roots | 0.43 |
| Total symbiotic fixation | 0.43 |
| Uptake allocated to wood | 0.04 |
| Uptake allocated to foliage | 1.79 |
| Uptake allocated to roots | 1.87 |
| Total uptake | 3.70 |
| Mortality wood | 0.06 |
| Mortality foliage (litterfall) | 1.81 |
| Mortality roots | 2.33 |
| Total turnover | 4.21 |
| Asymbiotic fixation allocated to wood | 0.08 |
| Asymbiotic fixation allocated to foliage | 0.08 |
| Asymbiotic fixation allocated to roots | 0.076 |
| Total asymbiotic fixation | 0.22 |
| Decomposition of wood | 0.27 |
| Decomposition of foliage | 1.93 |
| Decomposition of roots | 2.42 |
| Total decomposition | 4.64 |
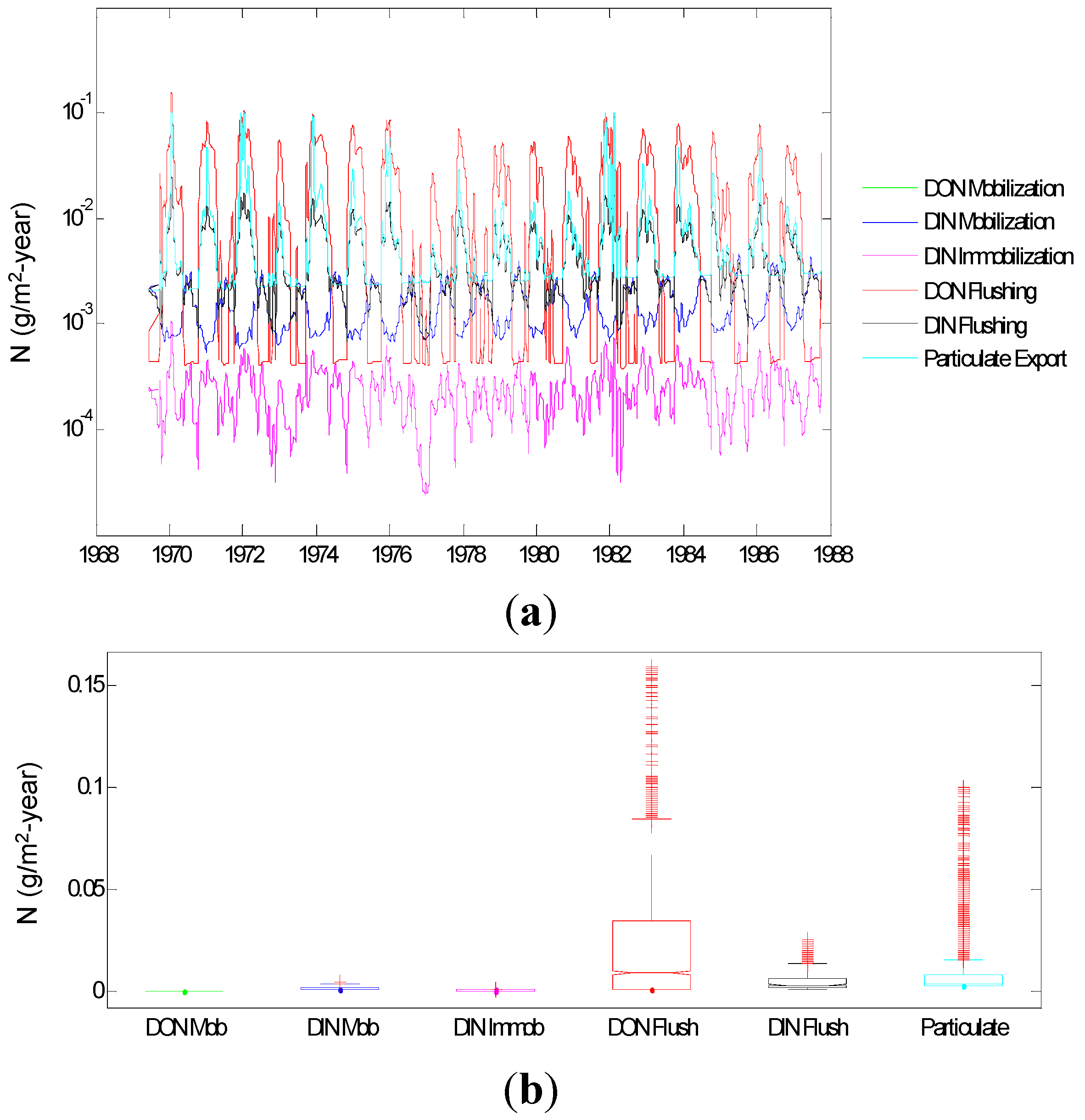
| DON mobilization (RZ) | 2.58 |
| DIN mobilization (RZ) | 2.58 |
| DIN Immobilization (RZ) | 1.19 |
| DON breakdown to DIN (RZ) | 2.28 |
| DON transport in RZ | 0.34 |
| DIN transport in RZ | 0.00 |
| Denitrification RZ | 0.00 |
| DON mobilization (BRZ) | 1.92 |
| DIN mobilization (BRZ) | 1.17 |
| DIN Immobilization (BRZ) | 2.67 |
| DON breakdown to DIN (BRZ) | 2.23 |
| DON transport in BRZ | 0.62 |
| DIN transport in BRZ | 0.020 |
| Denitrification BRZ | 0.01 |
| Particulate input to aquatic | 1.88 |
| DON mobilization | 0.00 |
| DIN immobilization | 0.00 |
| DIN mobilization | 0.00 |
| Denitrification from aquatic | 0.00 |
| DIN export | 0.00 |
| DON export | 0.02 |
| Particulate export | 0.02 |
3.1.3. Carbon Fluxes
3.1.4. Input of N to SOM
3.1.5. Root Zone N Dynamics
3.1.6. N Dynamics below the Root Zone
3.1.7. Aquatic Environment
3.2. Discussion
3.2.1. Relative Importance of Various System Components
Control Capacity

3.2.2. Limitations of the Modeling Framework
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar]
- Matson, P.; Lohse, K.A.; Hall, S.J. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. AMBIO J. Hum. Environ. 2002, 31, 113–119. [Google Scholar] [CrossRef]
- Van Breemen, N.; Boyer, E.W.; Goodale, C.L.; Jaworski, N.A.; Paustian, K.; Seitzinger, S.P.; Lajtha, K.; Mayer, B.; van Dam, D.; Howarth, R.W.; et al. Where did all the nitrogen go? Fate of nitrogen inputs to large watersheds in the northeastern USA. Biogeochemistry 2002, 57, 267–293. [Google Scholar] [CrossRef]
- Perakis, S.S.; Sinkhorn, E.R. Biogeochemistry of a temperate forest nitrogen gradient. Ecology 2011, 92, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. BioScience 1989, 39, 378–286. [Google Scholar] [CrossRef]
- Stoddard, J.L. Long-term changes in watershed retention of nitrogen. Its causes and aquatic consequences. In Environmental Chemistry of Lakes and Reservoirs, Edition: Advances in Chemistry Series, Edition Advances in Chemistry Series; American Chemical Society: Washington, DC, USA, 1994; Volume 237, pp. 223–284. [Google Scholar]
- Meixner, T.; Fenn, M. Biogeochemical budgets in a Mediterranean catchment with high rates of atmospheric N deposition—Importance of scale and temporal asynchrony. Biogeochemistry 2004, 70, 331–356. [Google Scholar] [CrossRef]
- Curtis, C.J.; Evans, C.D.; Goodale, C.L.; Heaton, T.H. What have stable isotope studies revealed about the nature and mechanisms of N saturation and nitrate leaching from semi-natural catchments? Ecosystems 2011, 14, 1021–1037. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Lapworth, D.J.; Skeffington, R.A.; Wade, A. Excess nitrogen leaching and C/N decline in the Tillingbourne catchment, southern England: INCA process modelling for current and historic time series. Hydrol. Earth Syst. Sci. 2002, 6, 455–466. [Google Scholar] [CrossRef]
- Cosby, B.J.; Ferrier, R.C.; Jenkins, A.; Emmett, B.A.; Wright, R.F.; Tietema, A. Modelling the ecosystem effects of nitrogen deposition: Model of Ecosystem Retention and Loss of Inorganic Nitrogen (MERLIN). Hydrol. Earth Syst. Sci. Discuss. 1997, 1, 137–158. [Google Scholar] [CrossRef]
- Fenn, M.E.; Haeuber, R.; Tonnesen, G.S.; Baron, J.S.; Grossman-Clarke, S.; Hope, D.; Jaffe, D.A.; Copeland, S.; Geiser, L.; Rueth, H.M.; et al. Nitrogen emissions, deposition, and monitoring in the Western United States. BioScience 2003, 53, 391–403. [Google Scholar] [CrossRef]
- Fenn, M.E.; Baron, J.S.; Allen, E.B.; Rueth, H.M.; Nydick, K.R.; Geiser, L.; Bowman, W.D.; Sickman, J.O.; Meixner, T.; Johnson, D.W.; et al. Ecological effects of nitrogen deposition in the Western United States. BioScience 2003, 53, 404–420. [Google Scholar] [CrossRef]
- Heemskerk, M.; Wilson, K.; Pavao-Zuckerman, M. Conceptual models as tools for communication across disciplines. Conserv. Ecol. 2003, 7, 8. [Google Scholar]
- Grant, R.F.; Roulet, N.T. Methane efflux from boreal wetlands: Theory and testing of the ecosystem model Ecosys with chamber and tower flux measurements. Glob. Biogeochem. Cycles 2002, 16. [Google Scholar] [CrossRef]
- Eshleman, K.N. A linear model of the effects of disturbance on dissolved nitrogen leakage from forested watersheds. Water Resour. Res. 2000, 36, 3325–3335. [Google Scholar] [CrossRef]
- Landsberg, J.J.; Waring, R.H. A generalised model of forest productivity using simplified concepts of radiation-use efficiency, carbon balance and partitioning. For. Ecol. Manag. 1997, 95, 209–228. [Google Scholar] [CrossRef]
- Aber, J.D.; Ollinger, S.V.; Driscoll, C.T. Modeling nitrogen saturation in forest ecosystems in response to land use and atmospheric deposition. Ecol. Model. 1997, 101, 61–78. [Google Scholar] [CrossRef]
- Parton, W.J. The CENTURY model. In Evaluation of Soil Organic Matter Models; Springer: Berlin, Germany; Heidelberg, Germany, 1996; pp. 283–291. [Google Scholar]
- Rastetter, E.B.; Ryan, M.G.; Shaver, G.R.; Melillo, J.M.; Nadelhoffer, K.J.; Hobbie, J.E.; Aber, J.D. A general biogeochemical model describing the responses of the C and N cycles in terrestrial ecosystems to changes in CO2, climate, and N deposition. Tree Physiol. 1991, 9, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Running, S.W.; Coughlan, J.C. A general model of forest ecosystem processes for regional applications I. Hydrologic balance, canopy gas exchange and primary production processes. Ecol. Model. 1988, 42, 125–154. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Wilson, E.J.; Butterfield, D. A semi-distributed Integrated Nitrogen model for multiple source assessment in Catchments (INCA): Part I—Model structure and process equations. Sci. Total Environ. 1998, 210, 547–558. [Google Scholar] [CrossRef]
- Band, L.E.; Tague, C.L.; Groffman, P.; Belt, K. Forest ecosystem processes at the watershed scale: Hydrological and ecological controls of nitrogen export. Hydrol. Process. 2001, 15, 2013–2028. [Google Scholar] [CrossRef]
- Rastetter, E.B.; Shaver, G.R. A model of multiple-element limitation for acclimating vegetation. Ecology 1992, 73, 1157–1174. [Google Scholar] [CrossRef]
- Ranken, D.W. Hydrologic Properties of Soil and Subsoil on a Steep, Forested Slope. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1974. [Google Scholar]
- Swanson, F.J.; James, M.E. Geology and Geomorphology of the HJ Andrews Experimental Forest, Western Cascades, Oregon. 1975. Available online: http://catalog.hathitrust.org/Record/007405299 (accessed on 20 March 2015).
- McGuire, K.J.; McDonnell, J.J.; Weiler, M.; Kendall, C.; McGlynn, B.L.; Welker, J.M.; Seibert, J. The role of topography on catchment-scale water residence time. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef]
- Vanderbilt, K.L.; Lajtha, K.; Swanson, F.J. Biogeochemistry of unpolluted forested watersheds in the Oregon Cascades: Temporal patterns of precipitation and stream nitrogen fluxes. Biogeochemistry 2003, 62, 87–117. [Google Scholar] [CrossRef]
- Grier, C.C.; Logan, R.S. Old-growth Pseudotsuga menziesii communities of a Western Oregon watershed: Biomass distribution and production budgets. Ecol. Monogr. 1977, 47, 373–400. [Google Scholar] [CrossRef]
- Sollins, P.; Grier, C.C.; McCorison, F.M.; Cromack, K., Jr.; Fogel, R.; Fredriksen, R.L. The internal element cycles of an old-growth Douglas-fir ecosystem in Western Oregon. Ecol. Monogr. 1980, 50, 261–285. [Google Scholar] [CrossRef]
- Triska, F.J.; Sedell, J.R.; Cromack, K., Jr.; Gregory, S.V.; McCorison, F.M. Nitrogen budget for a small coniferous forest stream. Ecol. Monogr. 1984, 54, 119–140. [Google Scholar] [CrossRef]
- Richmond, B.; Peterson, S. An Introduction to Systems Thinking; High Performance Systems, Incorporated: Honolulu, HI, USA, 2001. [Google Scholar]
- Bergström, S.; Singh, V.P. The HBV model. In Computer Models of Watershed Hydrology; Singh, V.P., Ed.; Water Resources Publications: Highlands Ranch, CO, USA, 1995; pp. 443–476. [Google Scholar]
- Shuttleworth, W.J. Evaporation. Handbook of Hydrology; Maidment, D.R., Ed.; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Harr, R.D. Water flux in soil and subsoil on a steep forested slope. J. Hydrol. 1977, 33, 37–58. [Google Scholar] [CrossRef]
- Chen, H.; Harmon, M.E.; Griffiths, R.P. Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: A chronosequence approach. Can. J. For. Res. 2001, 31, 246–260. [Google Scholar] [CrossRef]
- Hedin, L.O.; Armesto, J.J.; Johnson, A.H. Patterns of nutrient loss from unpolluted, old-growth temperate forests: Evaluation of biogeochemical theory. Ecology 1995, 76, 493–509. [Google Scholar] [CrossRef]
- Perakis, S.S. Nutrient limitation, hydrology and watershed nitrogen loss. Hydrol. Process. 2002, 16, 3507–3511. [Google Scholar]
- Neff, J.C.; Chapin, F.S., III; Vitousek, P.M. Breaks in the cycle: Dissolved organic nitrogen in terrestrial ecosystems. Front. Ecol. Environ. 2003, 1, 205–211. [Google Scholar] [CrossRef]
- Bond, B.J.; Jones, J.A.; Moore, G.; Phillips, N.; Post, D.; McDonnell, J.J. The zone of vegetation influence on baseflow revealed by diel patterns of streamflow and vegetation water use in a headwater basin. Hydrol. Process. 2002, 16, 1671–1677. [Google Scholar] [CrossRef]
- Haggerty, R.; Wondzell, S.M.; Johnson, M.A. Power-law residence time distribution in the hyporheic zone of a 2nd-order mountain stream. Geophys. Res. Lett. 2002, 29, 18. [Google Scholar] [CrossRef]
- Harmon, M.E.; Bible, K.; Ryan, M.G.; Shaw, D.C.; Chen, H.; Klopatek, J.; Li, X. Production, respiration, and overall carbon balance in an old-growth Pseudotsuga-Tsuga forest ecosystem. Ecosystems 2004, 7, 498–512. [Google Scholar] [CrossRef]
- McCuen, R.H.; Knight, Z.; Cutter, A.G. Evaluation of the Nash–Sutcliffe efficiency index. J. Hydrol. Eng. 2006, 11, 597–602. [Google Scholar] [CrossRef]
- Sollins, P.; McCorison, F.M. Nitrogen and carbon solution chemistry of an old growth coniferous forest watershed before and after cutting. Water Resour. Res. 1981, 17, 1409–1418. [Google Scholar] [CrossRef]
- Coops, N.C.; Black, T.A.; Jassal, R.P.S.; Trofymow, J.T.; Morgenstern, K. Comparison of MODIS, eddy covariance determined and physiologically modelled gross primary production (GPP) in a Douglas-fir forest stand. Remote Sens. Environ. 2007, 107, 385–401. [Google Scholar] [CrossRef]
- Abdelnour, A.; McKane, R.B.; Stieglitz, M.; Pan, F.; Cheng, Y. Effects of harvest on carbon and nitrogen dynamics in a Pacific Northwest forest catchment. Water Resour. Res. 2013, 49, 1292–1313. [Google Scholar] [CrossRef]
- Hicks, W.T.; Harmon, M.E.; Griffiths, R.P. Abiotic controls on nitrogen fixation and respiration in selected woody debris from the Pacific Northwest, USA. Ecoscience 2003, 10, 66–73. [Google Scholar]
- Ashkenas, L.R.; Johnson, S.L.; Gregory, S.V.; Tank, J.L.; Wollheim, W.M. A stable isotope tracer study of nitrogen uptake and transformation in an old-growth forest stream. Ecology 2004, 85, 1725–1739. [Google Scholar] [CrossRef]
- Hornberger, G.M.; Bencala, K.E.; McKnight, D.M. Hydrological controls on dissolved organic carbon during snowmelt in the Snake River near Montezuma, Colorado. Biogeochemistry 1994, 25, 147–165. [Google Scholar] [CrossRef]
- Lajtha, K.; Crow, S.E.; Yano, Y.; Kaushal, S.S.; Sulzman, E.; Sollins, P.; Spears, J.D.H. Detrital controls on soil solution N and dissolved organic matter in soils: A field experiment. Biogeochemistry 2005, 76, 261–281. [Google Scholar] [CrossRef]
- Bosatta, E.; Ågren, G.I. Theoretical analyses of interactions between inorganic nitrogen and soil organic matter. Eur. J. Soil Sci. 1995, 46, 109–114. [Google Scholar] [CrossRef]
- Kaye, J.P.; Hart, S.C. Competition for nitrogen between plants and soil microorganisms. Trends Ecol. Evol. 1997, 12, 139–143. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaché, K.; Breuer, L.; Jones, J.; Sollins, P. Catchment-Scale Modeling of Nitrogen Dynamics in a Temperate Forested Watershed, Oregon. An Interdisciplinary Communication Strategy. Water 2015, 7, 5345-5377. https://doi.org/10.3390/w7105345
Vaché K, Breuer L, Jones J, Sollins P. Catchment-Scale Modeling of Nitrogen Dynamics in a Temperate Forested Watershed, Oregon. An Interdisciplinary Communication Strategy. Water. 2015; 7(10):5345-5377. https://doi.org/10.3390/w7105345
Chicago/Turabian StyleVaché, Kellie, Lutz Breuer, Julia Jones, and Phil Sollins. 2015. "Catchment-Scale Modeling of Nitrogen Dynamics in a Temperate Forested Watershed, Oregon. An Interdisciplinary Communication Strategy" Water 7, no. 10: 5345-5377. https://doi.org/10.3390/w7105345






