Effect of P-Reactive Drainage Aggregates on Green Roof Runoff Quality
Abstract
:1. Introduction
| State Assessment System of Water Quality | P concentration (mg PO4-P·L−1) | References |
|---|---|---|
| OECD | 0.035 | [13] |
| New Hapshire, USA | 0.020 | [14] |
| Canada | 0.035 | [15] |
| Waikato region, New Zealand | 0.020 | [16] |
| Type Of Green Roof | Location | Soil Substrate | Drainage Type | Roof Age [years] | Maitenance | P Concentration in Runoff | References |
|---|---|---|---|---|---|---|---|
| Extensive, Sedum plants | Malmö, Sweden | Crushed lava, natural calcareous soil, clay and shredded peat | Crushed brick | 1–2 | Fertilized | 0.25–0.28 mg PO4-P·L−1 0.2–0.3 mg P·L−1 | [24] |
| Extensive, Sedum plants | Malmö, Sweden | Crushed lava, natural calcareous soil, clay and shredded peat | Shingle (4–8 mm, gneiss-granite origin) | 1–2 | Fertilized | 0.25–0.35 mg PO4-P·L−1 0.3–0.7 mg P·L−1 | [24] |
| Extensive, Sedum plants | Malmö, Sweden | Crushed lava, natural calcareous soil, clay and shredded peat | Shingle (4–8 mm, gneiss-granite origin) | 9 | Non fertilized | No P release | [24] |
| Extensive, Sedum plants | Lund, Sweden | Crushed lava, natural calcareous soil, clay and shredded peat | Flor-Depot drainage (thickness of 3.5 mm) | 1–2 | Fertilized | 0.8–1.4 mg PO4-P·L−1 0.9–1.6 mg P·L−1 | [24] |
| Exstensive, herbaceous and Sedum species | Taipei, Taiwan | Sandy loam/expanded clay/vermiculite/waste cotton 2:3:3:1:1 | – | 3 | Irregular weeding and fertilization | 0.15 mgP·L−1 | [25] |
| Intensive, leave trees and bushes | Fukuoka, Japan | Aquasoil (inorganic lightweight soil made from perlite) | Plastic | 12 | – | 0 mg PO4-P·L−1 0.01 mg P·L−1 | [17] |
| Extensive, Sedum plants | Malmö, Sweden | Crushed lava, natural calcareous soil, clay and shredded peat | Shingle (coarse gravel) | 4 | Fertilized during first 2 years of operation | 0.27 mg PO4-P·L−1 0.31 mg P·L−1 | [17] |
| Extensive, Sedum plants | Storrs, United States | 75% lightweight expanded shale, 15% composted biosolids, 10% perlite | GreenGrid modules | 1–2 | Fertilized | 0.003–0.079 mg PO4-P·L−1 0.018–0.096 mg P·L−1 | [4] |
| Extensive, Sedum plants | Goldsboro, United States | 55% Perma Till, 30% Sand, 15% composted cow manure | Hydrodrain 300 | 1 | – | 0.6–1.4 mg P·L−1 | [26] |
| Extensive, Sedum plants | Tartu, Estonia | 66% LWA, 30% humus, 4% clay | Plastic wave and rock wool | 1–6 | – | 0.23 mg PO4-P·L−1 0.27 mg P·L−1 | [18,27] |
| Extensive, sod roof | Talinn, Estonia | Biolan black soil (horticultural peat, composted soil mix, sand, composted chicken dung, dolomite lime) | Plastic wave drainage | 2–5 | – | 0.18 mg PO4-P·L−1 0.24 mg P·L−1 | [27] |
| Extensive, S. kamtschaticum, D. cooperi, T. calycinum | Texas, United States | Rooflite drain | TectaGreen modules | 1 | irrigated | 0.27–0.37 mg PO4-P·L−1 | [28] |
| Natural Materials | Recycled Materials | Manufactured Drainage Mats | Manufactured Aggregates |
|---|---|---|---|
| Gravel | Crushed brick | Plastic sheets with cups | LECA |
| Crushed rock | Shredded tires | Foam materials | Pollytag® |
| Crushed lava, etc. | Tumbled glass, etc. | Rockwool, etc. | Slag, etc. |
2. Materials and Methods

2.1. Tested Materials
2.2. Assessment of Physical Properties
| Material | Physical Parameters | Chemical Parameters | Column Experiment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grain Size | Porocity | Bulk Density | Moisture | Water Adsorption | Preliminary Sorption Test | Sorption Izoterm | Sorption Kinetic | ||
| Pollytag® | √ | √ | √ | √ | √ | √ | – | – | – |
| LECA | √ | √ | √ | √ | √ | √ | – | – | – |
| Chalcedony | √ | √ | √ | √ | √ | √ | – | – | – |
| Serpentinite | √ | √ | √ | √ | √ | √ | – | – | – |
| AAC | √ | √ | √ | √ | √ | √ | √ | √ | √ |
2.3. Assessment of P-Retention Capacity and Kinetics
2.4. Column Experiment

3. Results and Discussion
3.1. Physical Properties of Aggregates
| Material | Grain Size (mm) | Porosity (%) | Bulk Density (kg·m−3) | Moisture (%) | Water Adsorption (%) |
|---|---|---|---|---|---|
| Pollytag® | 8–11 | 62.32 | 660 | 0.51 | 29.60 |
| LECA | 8–16 | 52.20 | 950 | 2.64 | 14.60 |
| Chalcedony | 1–9 | 54.55 | 1110 | 0.20 | 20.11 |
| Serpentinite | 6–17 | 52.67 | 1240 | 0.33 | 7.60 |
| AAC | 1–6 | 83.75 | 300 | 6.05 | 83.74 |
3.2. P-Retention Capacity of Aggregates
| Initial P Concentration (mg·L−1) | Sorption of P (mg P-PO4 Per 1 g of Material) | ||||
|---|---|---|---|---|---|
| Pollytag® | LECA | Chalcedony | Serpentinite | AAC | |
| 1 | 0 | 0 | 0 | 0 | 0.03 |
| 2 | 0 | 0 | 0 | 0 | 0.05 |
| 5 | – | – | – | – | 0.18 |
| 10 | 0.34 | 0 | 0 | 0 | 0.53 |
| 20 | 0.89 | 0 | 0 | 0 | 0.26 |
| 50 | 2.67 | 0 | 0 | 0 | 0.35 |
3.3. Phosphorus Sorption by AAC
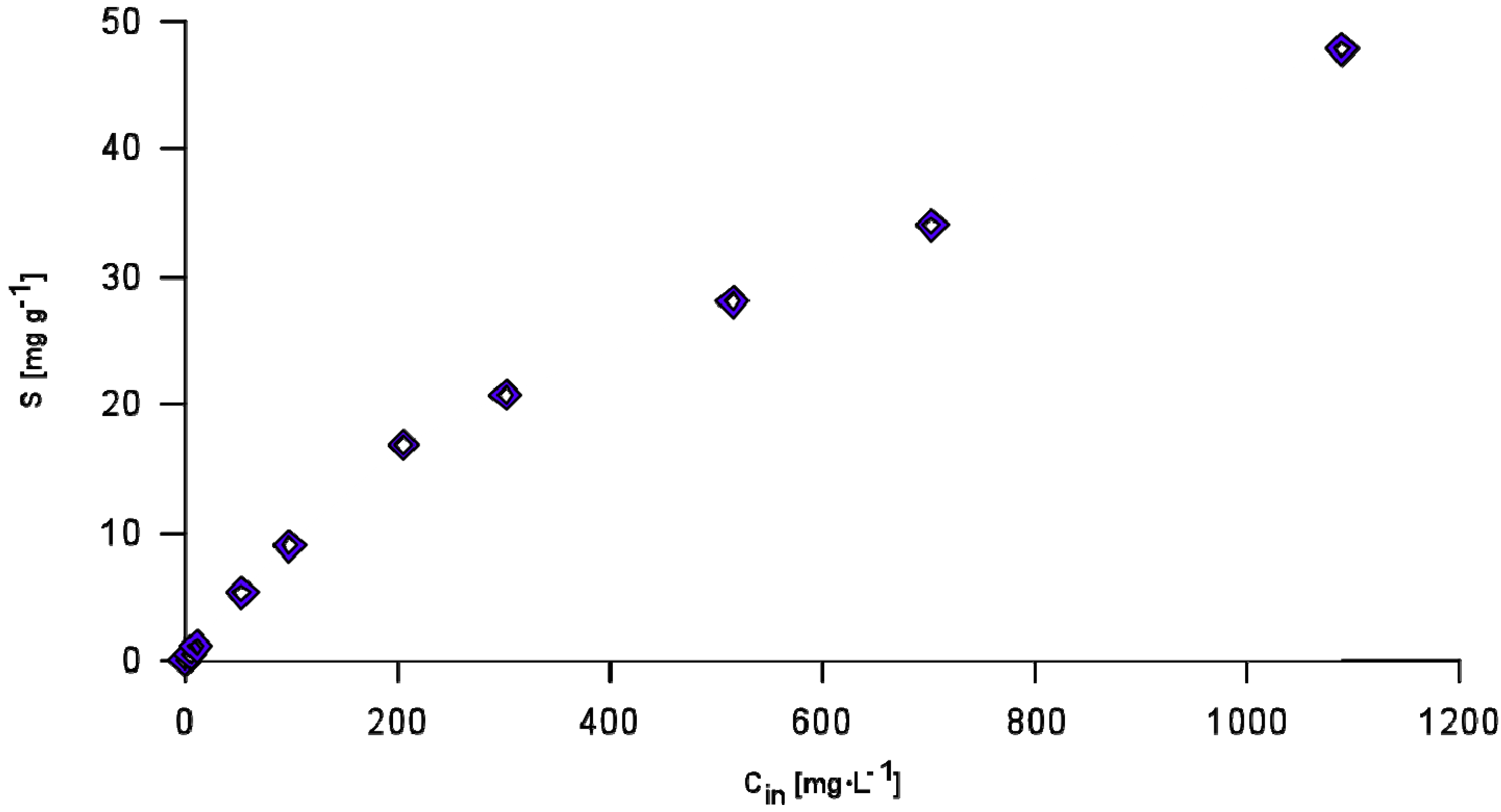
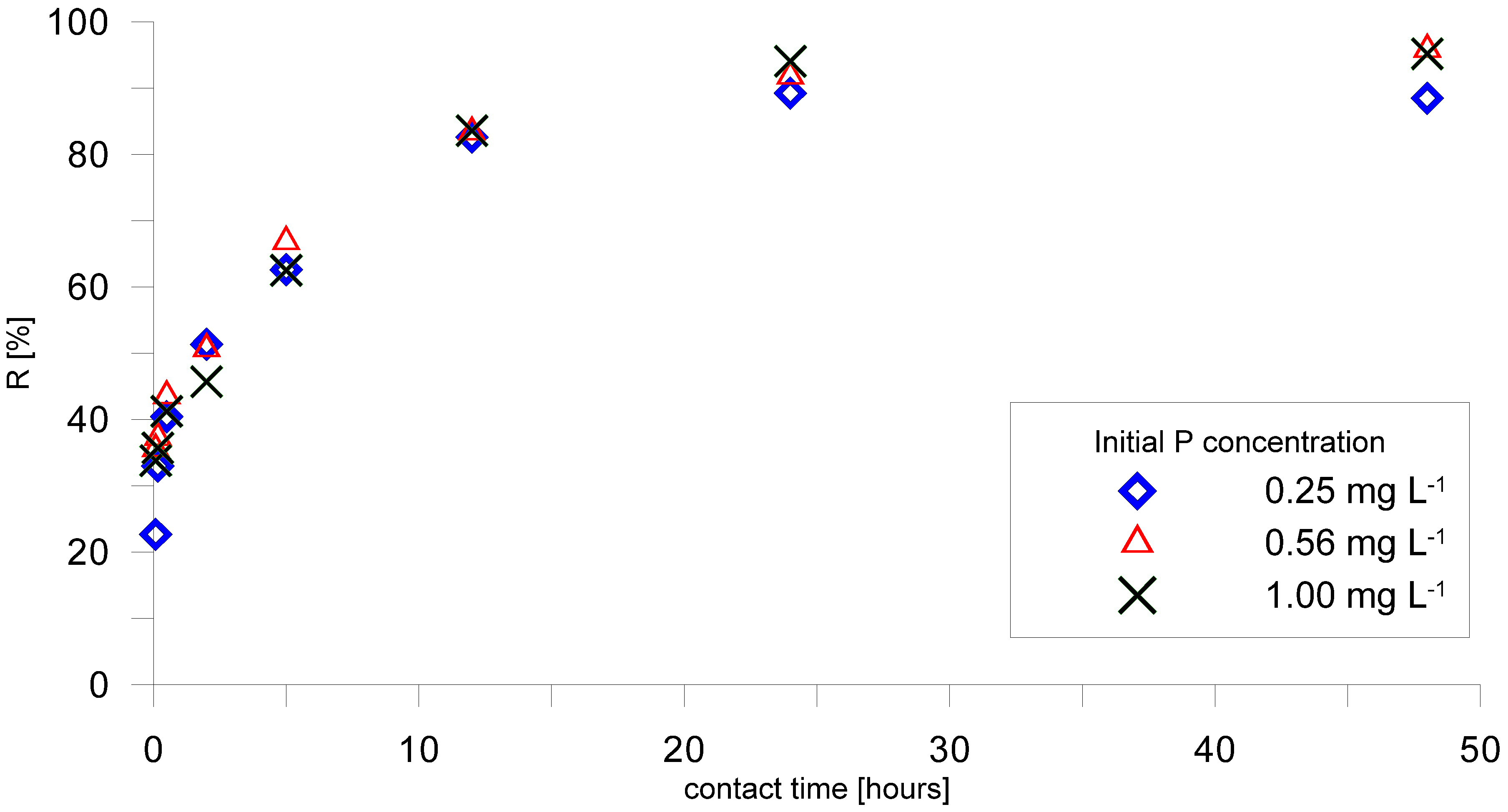
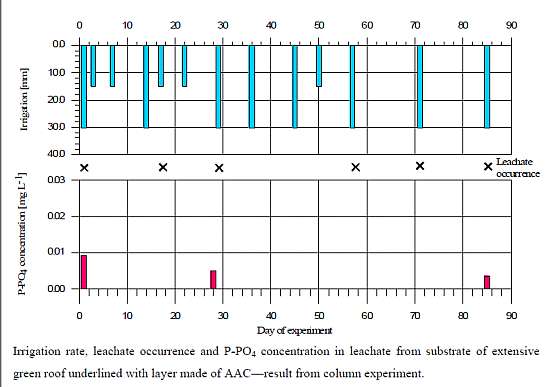
3.4. Column Study
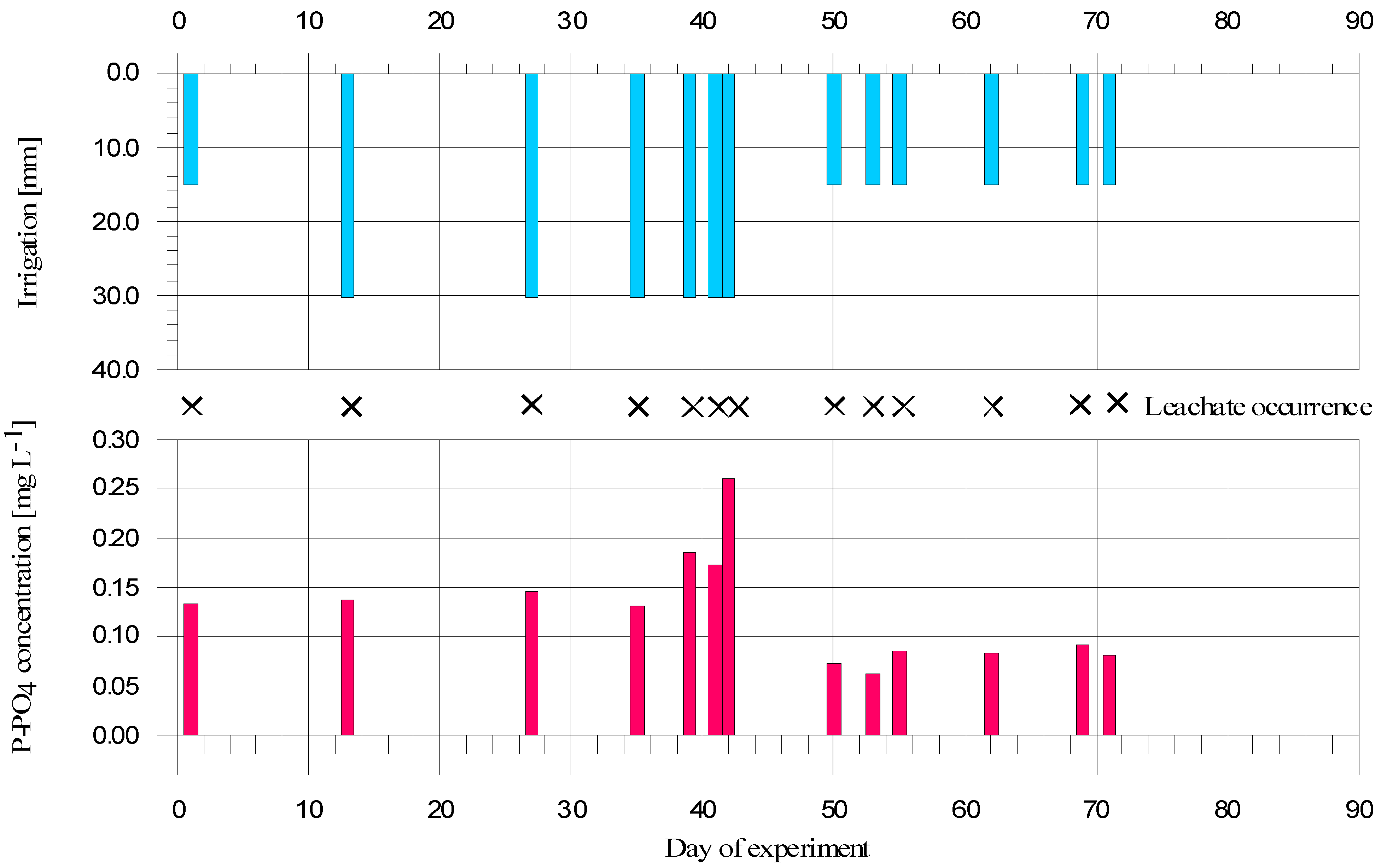
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Czemiel Berndtsson, J. Green roof performance towards management of runoff water quantity and quality: A review. Ecol. Eng. 2010, 36, 351–360. [Google Scholar] [CrossRef]
- Molineux, C.J.; Fentiman, C.H.; Gange, A.C. Characterizing alternative recycled waste materials for use as green roof growing media in the U.K. Ecol. Eng. 2009, 35, 1507–1513. [Google Scholar]
- Aslup, S.E.; Ebbs, S.D.; Battaglia, L.L.; Retzlaff, W.A. Heavy metals in leachate from simulated green roof systems. Ecol. Eng. 2011, 37, 1709–1717. [Google Scholar]
- Gregoire, B.G.; Clausen, J.C. Effect of modular extensive green roof on stormwater runoff and water quality. Ecol. Eng. 2011, 37, 963–969. [Google Scholar]
- Solano, L.; Ristvey, A.G.; Lea-Cox, J.D.; Cohan, S.M. Sequestering zinc from recycled crumb rubber in extensive green roof media. Ecol. Eng. 2012, 47, 284–290. [Google Scholar]
- Gnecco, I.; Palla, A.; Lanza, L.G.; la Barbera, P. The Role of Green Roofs as a Source/Sink of Pollutants in Storm Water Outflows. Water Resour. Manag. 2013, 27, 4715–4730. [Google Scholar]
- Beck, D.A.; Johnson, G.R.; Spolek, G.A. Amending greenroof soil with biochar to affect runoff water quantity and quality. Environ. Pollut. 2011, 159, 2111–2118. [Google Scholar]
- Karczmarczyk, A.; Baryła, A.; Charazińska, P.; Bus, A.; Frak, M. Influence of the green roof substrate on runoff quality. Infrastruct. Ecol. Rural Areas 2012, 3, 7–15. [Google Scholar]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy, Official Journal L 327; European Union: Brussels, Belgium, 2000; pp. 1–73. [Google Scholar]
- Drizo, A. Innovative phosphorous removal technologies. Aust. J. Clean Technol. 2012, 3. Available online: http://www.azocleantech.com/article.aspx?ArticleId=226 (accessed on 18 August 2014).
- Quinn, J.M. Guidelines for the control of undesired biological growths in water. In New Zealand National Institute of Water and Atmospheric Research; Consultancy Report No. 6213/2; Ministry for the Environment: Wellington, New Zealand, 1991. [Google Scholar]
- Sosiak, A.J. Long-term response of periphyton and macrophytes to reduced municipal nutrient loading to the Bow River (Alberta, Canada). Can. J. Fish. Aquat. Sci. 2002, 59, 987–1001. [Google Scholar]
- Vollenweider, R.; Kereks, J. Eutrophication of Waters, Monitoring, Assessment and Control; OECD: Paris, France, 1982. [Google Scholar]
- New Hampshire Department of Environmental Services. Layman’s Guide for Measuring Lake’s Trophic State; BB-27; New Hampshire Department of Environmental Services: Concord, NH, USA, 1997. [Google Scholar]
- Galvez-Cloutier, R.; Sanchez, M. Trophic Status Evaluation for 154 Lakes in Quebec, Canada: Monitoring and Recommendations. Water Qual. Res. J. Can. 2007, 42, 252–268. [Google Scholar]
- Waikato Regional Council: Water Quality Glossary. Available online: www.waikatoregion.govt.nz/Environment/Natural-resources/Water/Lakes/Water-quality-glossary (accessed on 3 July 2014).
- Czemiel Berndtsson, J.; Bengtsson, L.; Jinno, K. Runoff water quality from intensive and extensive vegetated roofs. Ecol. Eng. 2009, 35, 369–380. [Google Scholar]
- Teemusk, A.; Mander, Ü. Rainwater runoff quantity and quality performance from a greenroof: The effects of short-terms events. Ecol. Eng. 2007, 30, 271–277. [Google Scholar] [CrossRef]
- Moran, A.; Hunt, B.; Smith, J.T. Hydrological and water quality performance from green roofs in Goldsboro and Raleigh, North Carolina. Washington, DC, USA, 19–22 July 2005; Proceedings of the Green Roofs for Healthy Cities Conference.
- Getter, K.L.; Rowe, D.B. The role of extensive green roofs in sustainable development. HortScience 2006, 41, 1276–1285. [Google Scholar]
- Farrell, C.; Mitchell, R.E.; Szota, C.; Rayner, J.P.; Williams, N.S.G. Green roofs for hot and dry climates: Interacting effects of plant water use, succulence and substrate. Ecol. Eng. 2012, 49, 270–276. [Google Scholar]
- Köhler, M.; Poll, P.H. Long-term performance of selected old Berlin greenroofs in comparison to younger extensive greenroofs in Berlin. Ecol. Eng. 2010, 36, 722–729. [Google Scholar]
- Bianchini, F.; Hewage, K. How “green” are green roofs? Lifecycle analysis of green roof materials. Build. Environ. 2012, 48, 57–65. [Google Scholar]
- Czemiel Berndtsson, J.; Emilsson, T.; Bengtsson, L. The influence of extensive vegetated roofs on runoff water quality. Sci. Total Environ. 2006, 355, 48–63. [Google Scholar]
- Chen, C.-F. Performance evaluation and development strategies for green roofs in Taiwan: A review. Ecol. Eng. 2013, 52, 51–58. [Google Scholar]
- Hathaway, A.M.; Hunt, W.F.; Jennings, G.D. A field study of green roof hydrologic and water quality performance. Trans. Am. Soc. Agric. Biol. Eng. 2008, 51, 37–44. [Google Scholar]
- Teemusk, A.; Mander, Ü. The Influence of Green Roofs on Runoff Water Quality: A Case Study from Estonia. Water Resour. Manag. 2011, 25, 3699–3713. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.; Dvorak, B.D.; Volder, A.; Stanley, N.C. Chemistry of growth medium and leachate from green roof systems in south-central Texas. Urban Ecosyst. 2011, 14, 17–33. [Google Scholar]
- Bus, A. Removal of Phosphates from Surface Water by Using Selected Reactive Materials. Ph.D. Thesis, Warsaw University of Life Sciences-SGGW, Warsaw, Poland, May 2013. [Google Scholar]
- Karczmarczyk, A.; Bus, A. Filter for Removal of Pollutants, especially from Small Water Bodies. Patent Application P.403571, 17 April 2013. [Google Scholar]
- Karczmarczyk, A.; Bus, A. Testing of reactive materials for phosphorus removal from water and wastewater—Comparative study. Ann. Warsaw Univ. Life Sci. SGGW Land Reclam. 2014, 46, 57–67. [Google Scholar]
- Pollytag S.A. Homepage. Available online: www.pollytag.com.pl (accessed on 17 May 2014).
- Leca Homepage. Available online: www.leca.ae (accessed on 17 May 2014).
- Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau (FLL). Guidelines for the Planning, Construction and Maintenance of Green Roofing—Green Roofing Guideline; FLL: Bonn, Germany, 2008. [Google Scholar]
- McKay, G. Use of Adsorbents for Removal Pollutants from Wastewater; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Franus, M. Physical and mechanical properties LECA obtained with added glauconite. Civ. Eng. Archit. Mag. 2012, 10, 5–14. [Google Scholar]
- Kus, H.; Nygren, K.; Norberg, P. In-use performance assessment of rendered autoclaved aerated concrere walls by long-term moisture monitoring. Build. Environ. 2004, 39, 677–687. [Google Scholar]
- Ioannu, I.; Hamilton, A.; Hall, C. Capillary absorption of water and n-decane by autoclaved aerated concrete. Cem. Concr. Res. 2008, 38, 766–771. [Google Scholar]
- Bezpalko, N. Application of TDR to Study the Processes of Mass and Energy Transfer through the Selected Barrier Construction. Ph.D. Thesis, Lublin University of Technology, Lublin, Poland, April 2009. [Google Scholar]
- Huisman, J.; Sperl, C.; Bouten, W.; Verstraten, J. Soil water content measurements at different scales: Accuracy of time domain reflectometry and ground-penetrating radar. J. Hydrol. 2001, 245, 48–58. [Google Scholar]
- Drizo, A.; Frost, C.A.; Grace, J.; Smith, K.A. Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems. Water Res. 1999, 33, 3595–3602. [Google Scholar]
- Johansson, L. The use of LECA (Light Expanded Clay Aggregates) for the removal of phosphorus from wastewater. Water Sci. Technol. 1997, 35, 87–93. [Google Scholar]
- Zhu, T.; Jenssen, P.D.; Mæhlum, T.; Krogstad, T. Phosphorus sorption and chemical characteristic of light-weight aggregates (LWA)—potential filter media in treatment wetlands. Water Sci. Technol. 1997, 35, 103–108. [Google Scholar]
- Renman, G.; Renman, A. Sustainable use of crushed autoclaved aerated concrete (CAAC) as a filter medium in wastewater purification. In Proceedings of the WASCON 2012 Conference, Gothenburg, Sweden, 30 May–1 June 2012.
- Johansson Westholm, L. Substrate for phosphorus removal—Potential benefits for on-site wastewater treatment? Water Res. 2006, 40, 23–36. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karczmarczyk, A.; Baryła, A.; Bus, A. Effect of P-Reactive Drainage Aggregates on Green Roof Runoff Quality. Water 2014, 6, 2575-2589. https://doi.org/10.3390/w6092575
Karczmarczyk A, Baryła A, Bus A. Effect of P-Reactive Drainage Aggregates on Green Roof Runoff Quality. Water. 2014; 6(9):2575-2589. https://doi.org/10.3390/w6092575
Chicago/Turabian StyleKarczmarczyk, Agnieszka, Anna Baryła, and Agnieszka Bus. 2014. "Effect of P-Reactive Drainage Aggregates on Green Roof Runoff Quality" Water 6, no. 9: 2575-2589. https://doi.org/10.3390/w6092575