An Estimate of Energy Available via Microbial Sulfate Reduction at a Quaternary Aquifer in Northern Japan considered for Low Temperature Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
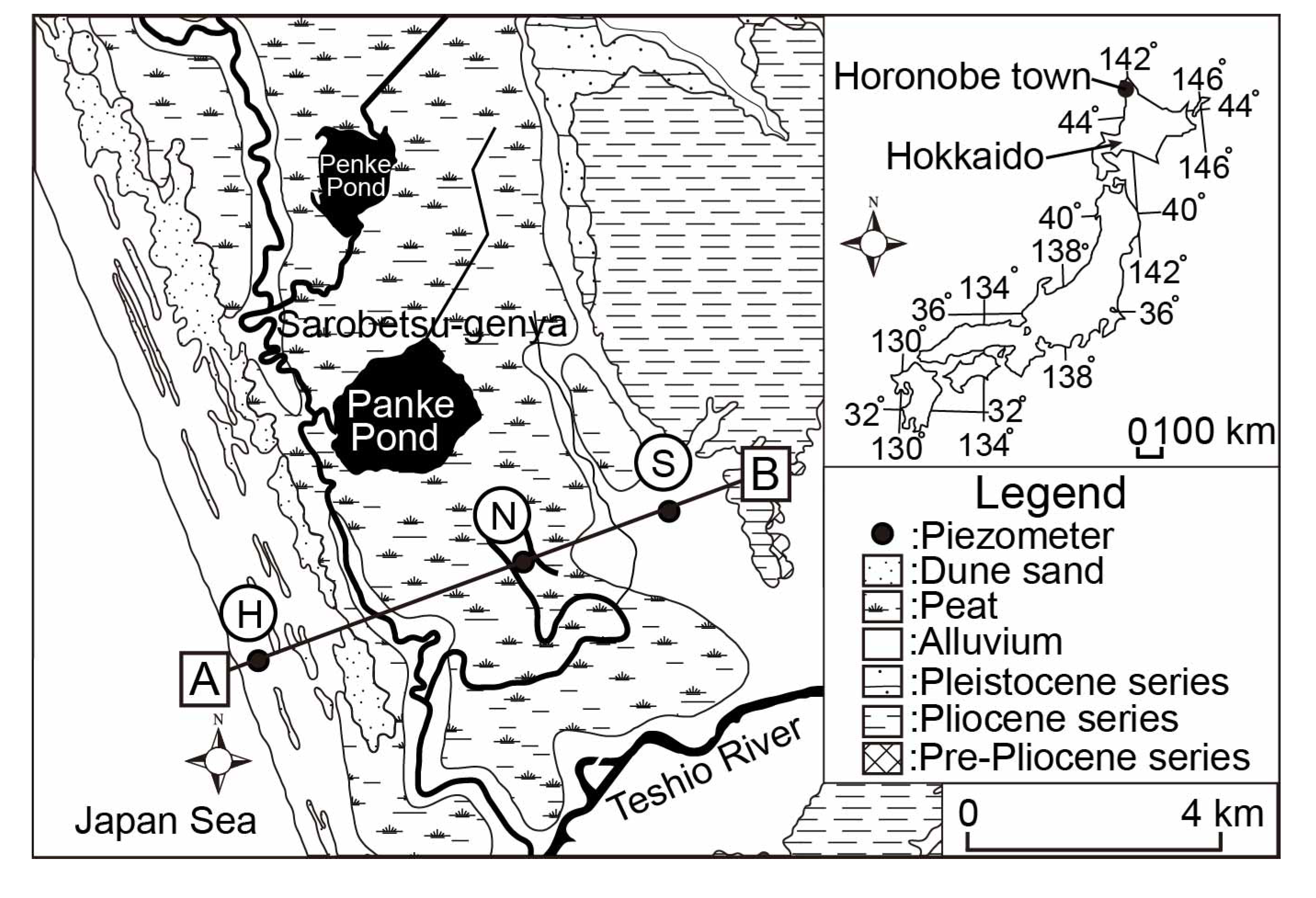
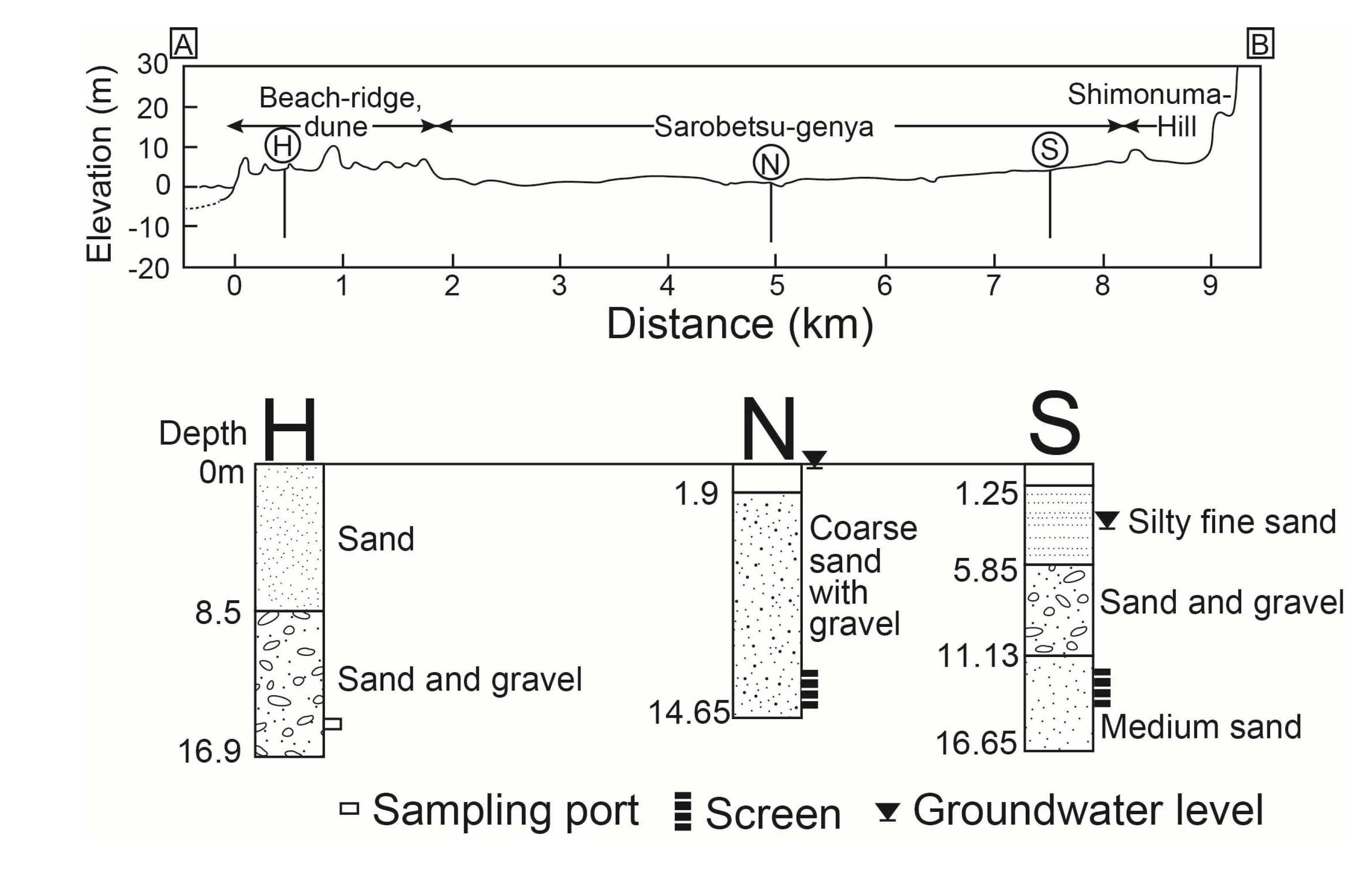
2.1. Study Site
| Site | Geological period | Depositional environment | Total organic carbon and total sulfur contents (wt%) near the screens |
|---|---|---|---|
| H | Holocene | Marine | 1.97 and 1.18 at depth of 15.15 m |
| N | Holocene | Freshwater | 1.24 and 0.14 at depth of 10.50 m |
| S | Holocene | Marine | 0.48 and 0.21 at depth of 13.25 m |
2.2. Sampling Method
2.3. Analyses
2.4. Geochemical Modeling of Energy Availability
| Sulfate reduction | ΔG0 (kJmol−1) | Temperature (°C) |
|---|---|---|
| CH3COO− + SO42− + H+ → 2HCO3− + H2S(aq) | −84.77 | 9 |
| −87.03 | 20 | |
| −91.45 | 40 | |
| −96.27 | 60 |
3. Results and Discussion
| Sampling date | 26 August 2009 | 10 August 2009 | 24 July 2009 |
|---|---|---|---|
| Location | H | N b | S |
| pH a | 8.04 | 6.70 | 6.62 |
| DOC | 2.05 | 2.39 | 0.72 |
| CH3COO− | 0.026 | 0.039 | 0.027 |
| F− | 0.123 | 0.046 | 0.063 |
| Cl− | 39.2 | 54.1 | 20.0 |
| NO3− | n/d | n/d | n/d |
| SO42− | 0.024 | 6.50 | 5.45 |
| S2− | 0.047 | 0.095 | 0.088 |
| HCO3− | 127 | 152 | 81.8 |
| Na+ | 22.1 | 29.2 | 12.7 |
| NH4+ | 0.691 | 0.928 | 0.304 |
| K+ | 10 | 6.2 | 1.6 |
| Mg2+ | 13.8 | 17.6 | 10.9 |
| Ca2+ | 16.6 | 9.84 | 3.85 |
| P | 0.803 | 0.644 | 0.636 |
| Fe | 0.129 | 28.4 | 11.3 |
| Mn | 0.101 | 0.595 | 0.242 |
| Sr | 0.125 | 0.147 | 0.040 |
| Ba | 0.009 | 0.218 | 0.052 |
| Si | 13.2 | 18.8 | 20 |
| CH4 | 1.11 | 9.8 | 0.225 |
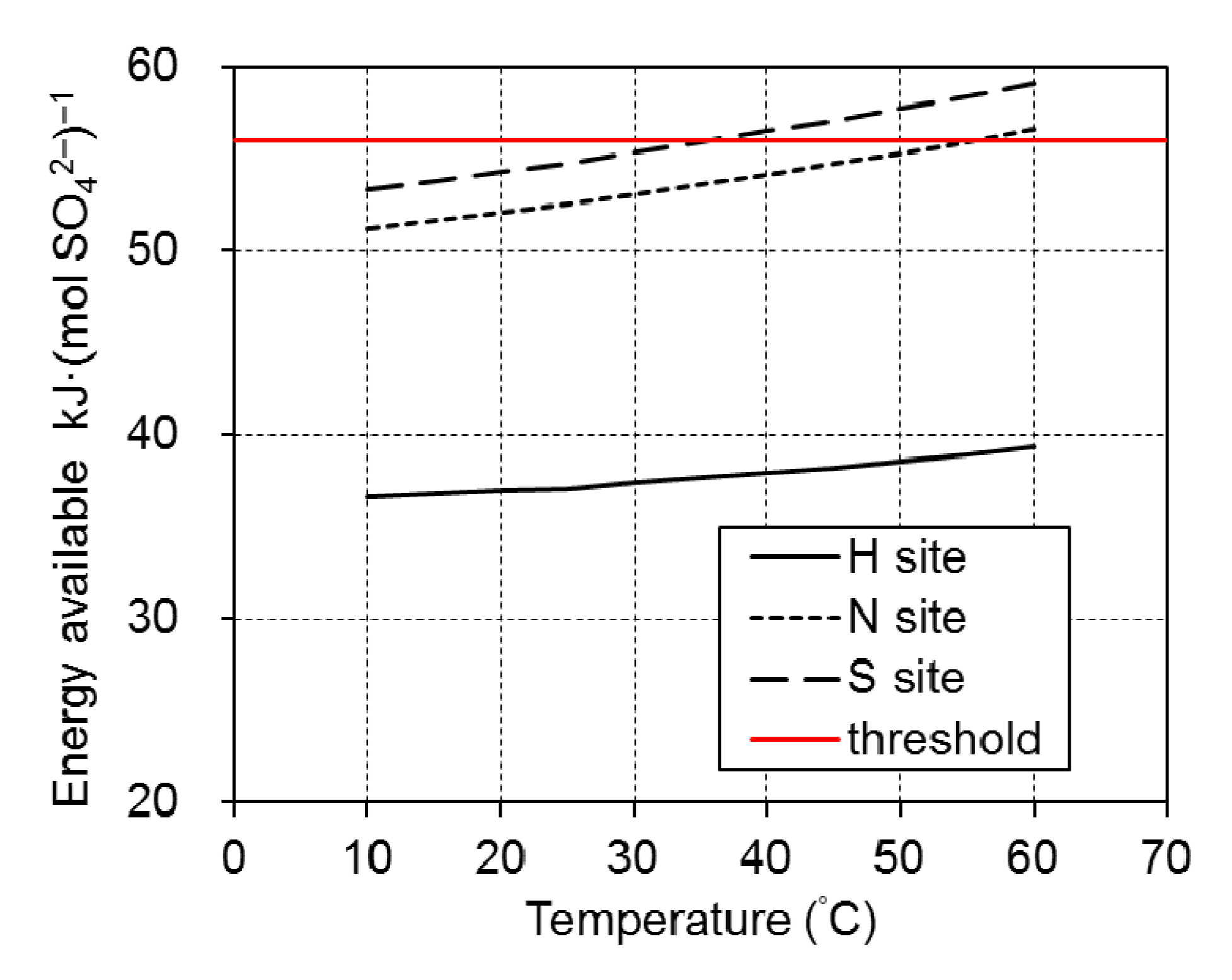
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jesußek, A.; Grandel, S.; Dahmke, A. Impact of subsurface heat storage on aquifer hydrogeochemistry. Environ. Earth Sci. 2013, 69, 1999–2012. [Google Scholar] [CrossRef]
- Gleeson, T.; VanderSteen, J.; Sophocleous, M.A.; Taniguchi, M.; Alley, W.M.; Allen, D.M.; Zhou, Y. Groundwater sustainability strategies. Nature Geosci. 2010, 3, 378–379. [Google Scholar] [CrossRef]
- Edmunds, W.M. Geochemistry’s vital contribution to solving water resource problem. Appl. Geochem. 2009, 24, 1058–1073. [Google Scholar] [CrossRef]
- Bonte, M.; Stuyfzand, P.J.; Hulsmann, A.; Van Beelen, P. Underground thermal energy storage: Environmental risks and policy developments for drinking in the Netherlands and the EU. Ecol. Soc. 2011, 16, Article 22. [Google Scholar]
- Haehnlein, S.; Bayer, P.; Blum, P. International legal status of the use of shallow geothermal energy. Renew. Sust. Energ. Rev. 2010, 14, 2611–2625. [Google Scholar] [CrossRef]
- Park, J.; Sanford, R.A.; Bethke, C.M. Geochemical and microbial zonation of the Middendorf aquifer, South Carolina. Chem. Geol. 2006, 230, 88–104. [Google Scholar] [CrossRef]
- Jakobsen, R.; Cold, L. Geochemistry at the sulfate reduction–methanogenesis transition zone in an anoxic aquifer—A partial equilibrium interpretation using 2D reactive transport modeling. Geochim. Cosmochim. Acta 2007, 71, 1949–1966. [Google Scholar] [CrossRef]
- Brons, H.J.; Griffioen, J.; Appelo, C.A.J.; Zehnder, A.J.B. (Bio)geochemical reactions in aquifer material from a thermal energy storage site. Wat. Res. 1991, 25, 729–736. [Google Scholar] [CrossRef]
- Jesußek, A.; Köber, R.; Grandel, S.; Dahmke, A. Aquifer heat storage: Sulphate reduction with acetate at increased temperatures. Environ. Earth Sci. 2013, 69, 1763–1771. [Google Scholar] [CrossRef]
- Bonte, M.; Röling, W.F.M.; Zaura, E.; Van der Wielen, P.W.J.J.; Stuyfzand, P.J.; Van Breukelen, B.M. Impacts of shallow geothermal energy production on redox processes and microbial communities. Environ. Sci. Technol. 2013, 47, 14476–14484. [Google Scholar] [CrossRef]
- Poulton, S.W.; Krom, M.D.; Rijn, J.V.; Raiswell, R. The use of hydrous iron (III) oxides for the removal of hydrogen sulphide in aqueous systems. Wat. Res. 2002, 36, 825–834. [Google Scholar] [CrossRef]
- Jin, Q.; Bethke, C.M. Cellular energy conservation and the rate of microbial sulfate reduction. Geology 2010, 37, 1027–1030. [Google Scholar]
- Ochifuji, K. Present status and problems of underground thermal energy use and storage. J. Geotherm. Res. Soc. Japan. 2002, 24, 315–327. (in Japanese). [Google Scholar]
- Oguchi, T.; Saito, K.; Kadomura, H.; Grossman, M. Fluvial geomorphology and paleohydrology in Japan. Geomorphology 2001, 39, 3–19. [Google Scholar] [CrossRef]
- Machida, I.; Suzuki, Y.; Takeuchi, M. The 14C age of confined groundwater in a sandy-muddy Pleistocene aquifer. In Groundwater Response to Changing Climate; Taniguchi, M., Holman, I.P., Eds.; CRC Press: Balkema, the Netherlands, 2008. [Google Scholar]
- Hama, K.; Kunimaru, T.; Metcalfe, R.; Martin, A.J. The hydrogeochemistry of argillaceous rock formations at the Horonobe URL site, Japan. Phys. Chem. Earth 2007, 32, 170–180. [Google Scholar] [CrossRef]
- New Energy Vision in Horonobe Area; Horonobe Town: Horonobe Town, Japan, 2002. (in Japanese)
- Sakoh, S.; Hirota, T.; Sagayama, T.; Yokoyama, E.; Suga, K.; Matsunami, H.; Terashima, K. Hydrogeological Maps of Hokkaido, No. 1, Wakkanai; Geological Survey of Hokkaido: Sapporo, Japan, 1983. (in Japanese) [Google Scholar]
- Sakai, T.; Ioka, S.; Igarashi, T. Hydrogeological structure and groundwater flow of alluvium in the south Sarobetsu Moor. J. Jpn. Soc. Eng. Geol. 2012, 53, 172–182. (in Japanese). [Google Scholar] [CrossRef]
- Sakai, T.; Ioka, S.; Ishijima, Y.; Igarashi, T. Geological analysis of alluvium in the Sarobetsu Moor. J. Jpn. Soc. Eng. Geol. 2011, 52, 2–15. (in Japanese). [Google Scholar] [CrossRef]
- Parkhurst, J.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3―A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods, Book 6; U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Denver, CO, USA, 2013. [Google Scholar]
- Chen, W.-F.; Liu, T.-K. Ion activity products of iron sulfides in groundwaters: Implication from the Choshui fan-delta, Western Taiwan. Geochim. Cosmochim. Acta 2005, 69, 3535–3544. [Google Scholar] [CrossRef]
- Bethke, C.M. Geochemical and Biogeochemical Reaction Modeling, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- McMahon, P.B.; Chapelle, F.H. Redox processes and water quality of selected principal aquifer systems. Ground Water 2008, 46, 259–271. [Google Scholar] [CrossRef]
- Ioka, S.; Sakai, T.; Igarashi, T.; Ishijima, Y. Long-term continuous in-situ potentiometrically measured redox potential in anoxic groundwater with high methane and iron contents. Environ. Earth Sci. 2011, 64, 143–149. [Google Scholar] [CrossRef]
- Jakobsen, R.; Postma, D. In situ rate of sulfate reduction in an aquifer (Rømø, Denmark) and implications for the reactivity of organic matter. Geology 1994, 22, 1103–1106. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ioka, S.; Muraoka, H. An Estimate of Energy Available via Microbial Sulfate Reduction at a Quaternary Aquifer in Northern Japan considered for Low Temperature Thermal Energy Storage. Water 2014, 6, 858-867. https://doi.org/10.3390/w6040858
Ioka S, Muraoka H. An Estimate of Energy Available via Microbial Sulfate Reduction at a Quaternary Aquifer in Northern Japan considered for Low Temperature Thermal Energy Storage. Water. 2014; 6(4):858-867. https://doi.org/10.3390/w6040858
Chicago/Turabian StyleIoka, Seiichiro, and Hirofumi Muraoka. 2014. "An Estimate of Energy Available via Microbial Sulfate Reduction at a Quaternary Aquifer in Northern Japan considered for Low Temperature Thermal Energy Storage" Water 6, no. 4: 858-867. https://doi.org/10.3390/w6040858




