Effects of Commercially Available Ultrasound on the Zooplankton Grazer Daphnia and Consequent Water Greening in Laboratory Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrasound
2.2. Effect on Daphnia Magna
2.2.1. Effect of Different Frequencies
2.2.2. Effect on Animals of Different Size
2.2.3. Effect of Size of Experimental Unit
2.3. Tank Experiment
3. Results and Discussion
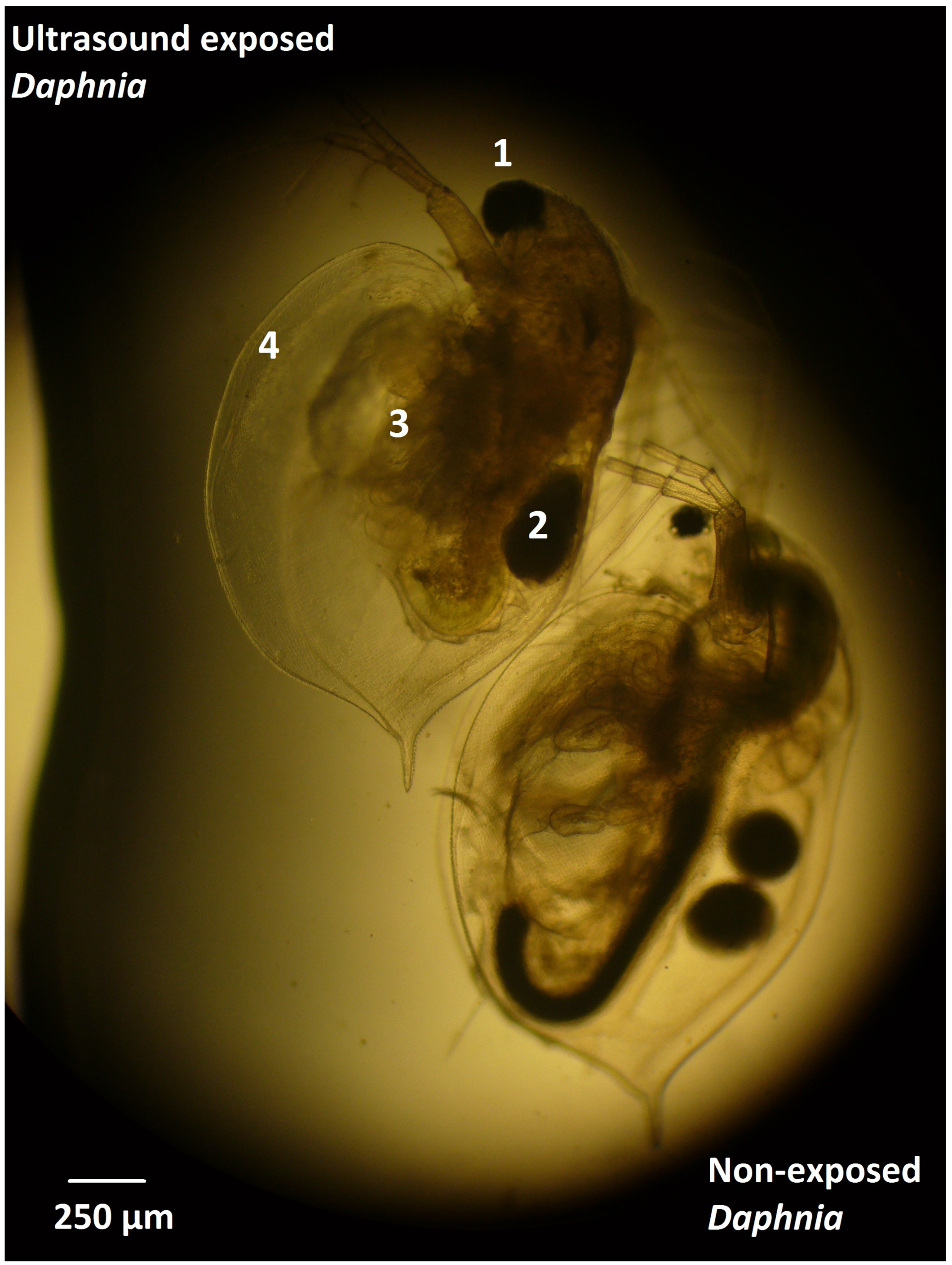
3.1. Effect of Ultrasound on Daphnia
3.1.1. Effect of Different Frequencies
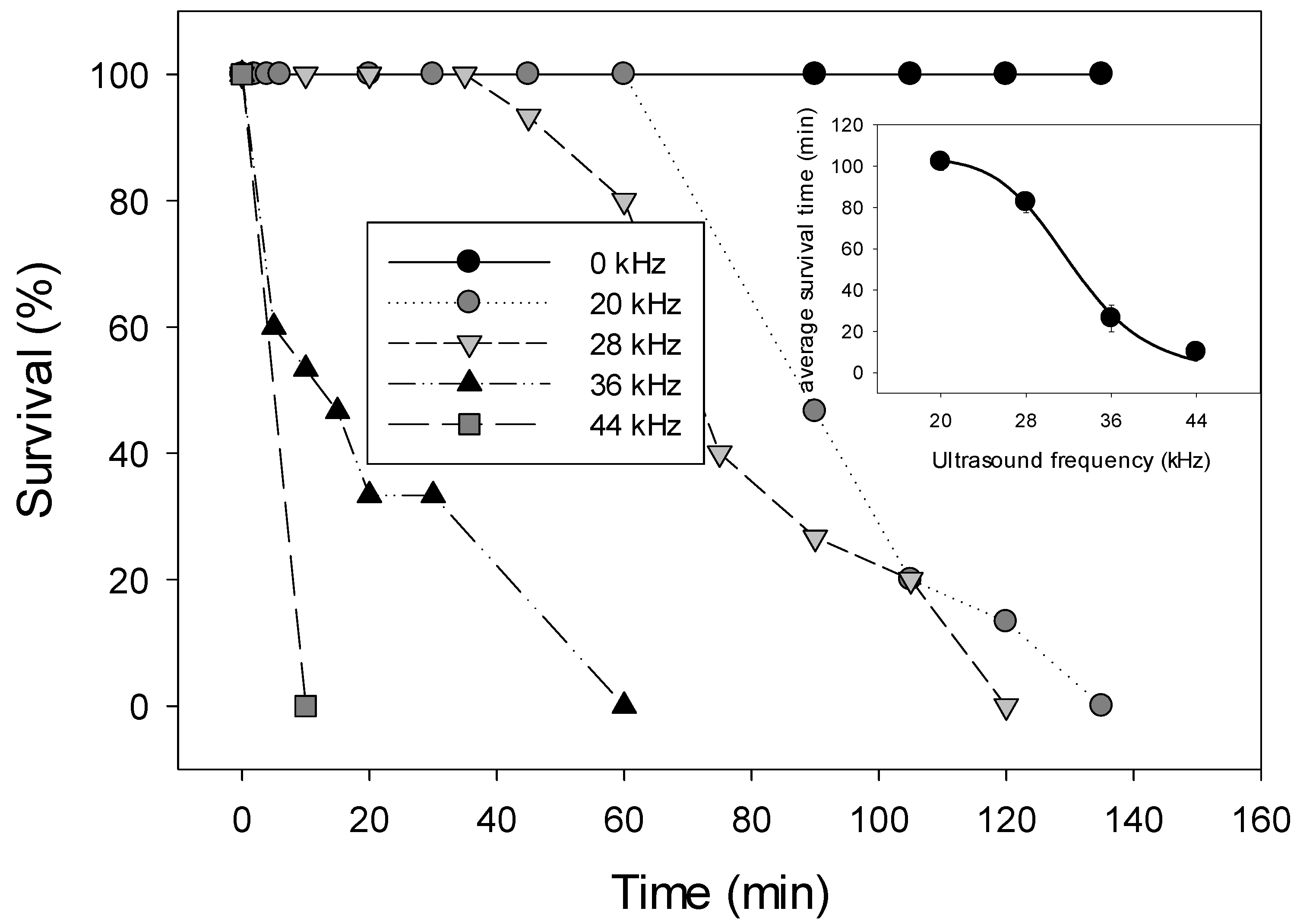
3.1.2. Effect on Animals of Different Size
| D. magna | Survival Time (min) | |
|---|---|---|
| Body-length (mm) | Control | 44 kHz |
| 0.69 (0.04) | >60 | 10.6 |
| 0.97 (0.07) | >60 | 4.9 |
| 1.11 (0.07) | >60 | 2.3 |
| 1.68 (0.05) | >60 | 2.4 |
| 2.05 (0.07) | >60 | 7.6 |
| 3.16 (0.11) | >60 | 17.1 |
3.1.3. Effect of Size of Experimental Unit
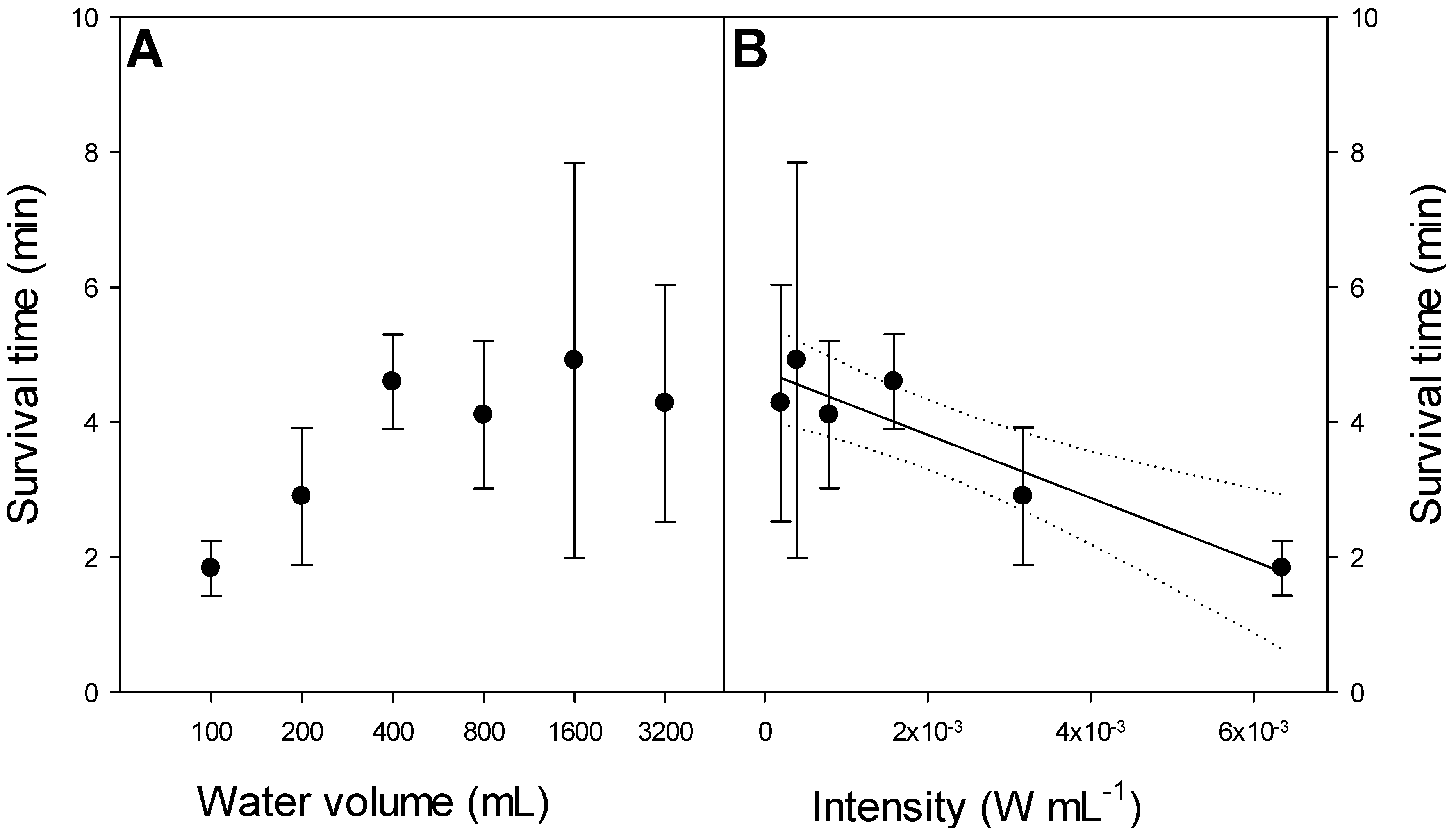
3.2. Effect of Ultrasound on a Plankton Community in 85 L Tanks
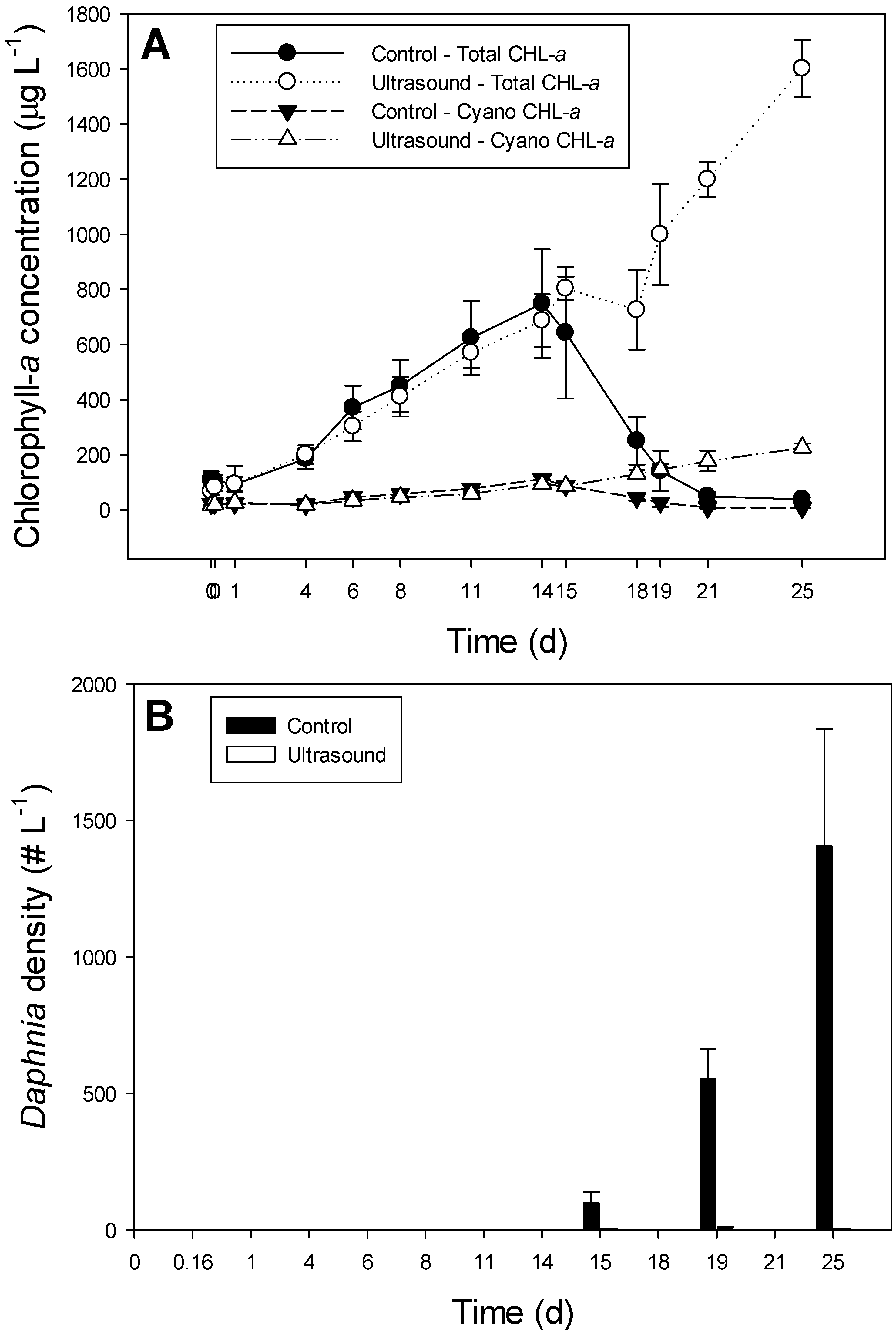
| Chlorophyll-a Concentration | Biovolume Concentration | |||||
|---|---|---|---|---|---|---|
| Tests of within-subjects Effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 12 | 63.5 | <0.001 | 11 | 24.8 | <0.001 |
| Time × treatment | 12 | 69.8 | <0.001 | 11 | 17.0 | <0.001 |
| Error | 48 | 44 | ||||
| Tests of between-subjects Effects | ||||||
| Source | df | F | p | df | F | p |
| Treatment | 1 | 40.2 | 0.003 | 1 | 17.1 | 0.014 |
| Error | 4 | 4 | ||||
| Particle concentration | Mean Particle Volume | |||||
| Tests of within-subjects Effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 11 | 29.4 | <0.001 | 11 | 7.06 | <0.001 |
| Time × treatments | 11 | 17.3 | <0.001 | 11 | 1.78 | 0.087 |
| Error | 44 | 44 | ||||
| Tests of between-subjects Effects | ||||||
| Source | df | F | p | df | F | p |
| Treatments | 1 | 10.1 | 0.034 | 1 | 0.09 | 0.784 |
| Error | 4 | 4 | ||||
| Cyanobacteria chlorophyll-a | Green algae chlorophyll-a | |||||
| Tests of within-subjects Effects | ||||||
| Source | df | F | p | df | F | p |
| Time | 12 | 62.4 | <0.001 | 12 | 56.1 | <0.001 |
| Time × treatment | 12 | 82.1 | <0.001 | 12 | 59.1 | <0.001 |
| Error | 48 | 48 | ||||
| Tests of between-subjects Effects | ||||||
| Source | df | F | p | df | F | p |
| Treatment | 1 | 53.9 | 0.002 | 1 | 31.9 | 0.005 |
| Error | 4 | 4 | ||||
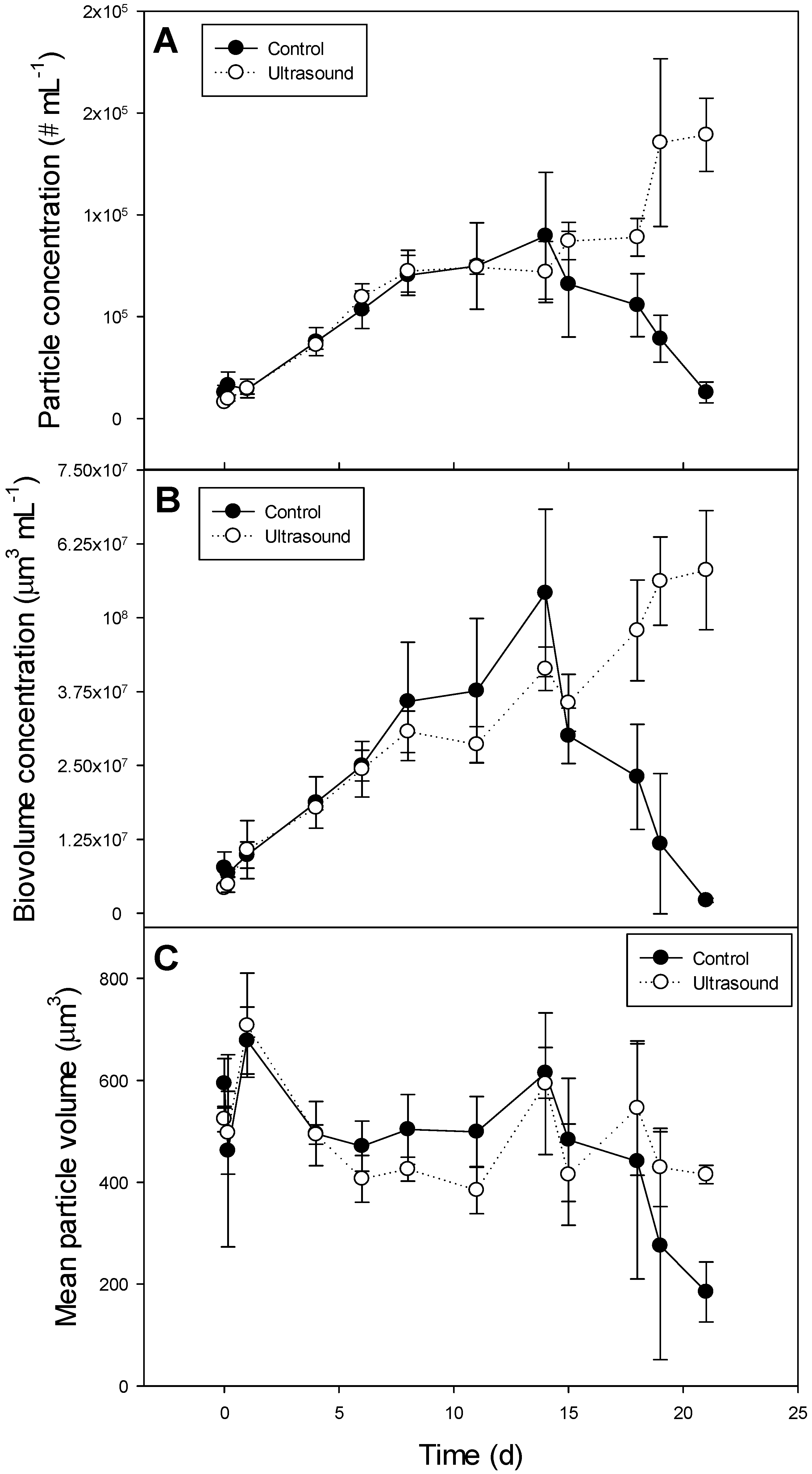
| Variable | Control | Ultrasound | F- and p values |
|---|---|---|---|
| pH | 8.2 (1.4) | 8.4 (1.4) | F1,4 = 1.97; p = 0.233 |
| Conductivity (µS·cm−1) | 236 (8) | 230 (4) | F1,4 = 0.05; p = 0.839 |
| Oxygen (mg·L−1) | 13.9 (5.1) | 14.8 (4.4) | F1,4 = 0.78; p = 0.426 |
| Temperature (°C) | 21.5 (2.1) | 21.3 (2.1) | F1,4 = 0.87; p = 0.404 |
4. Discussion
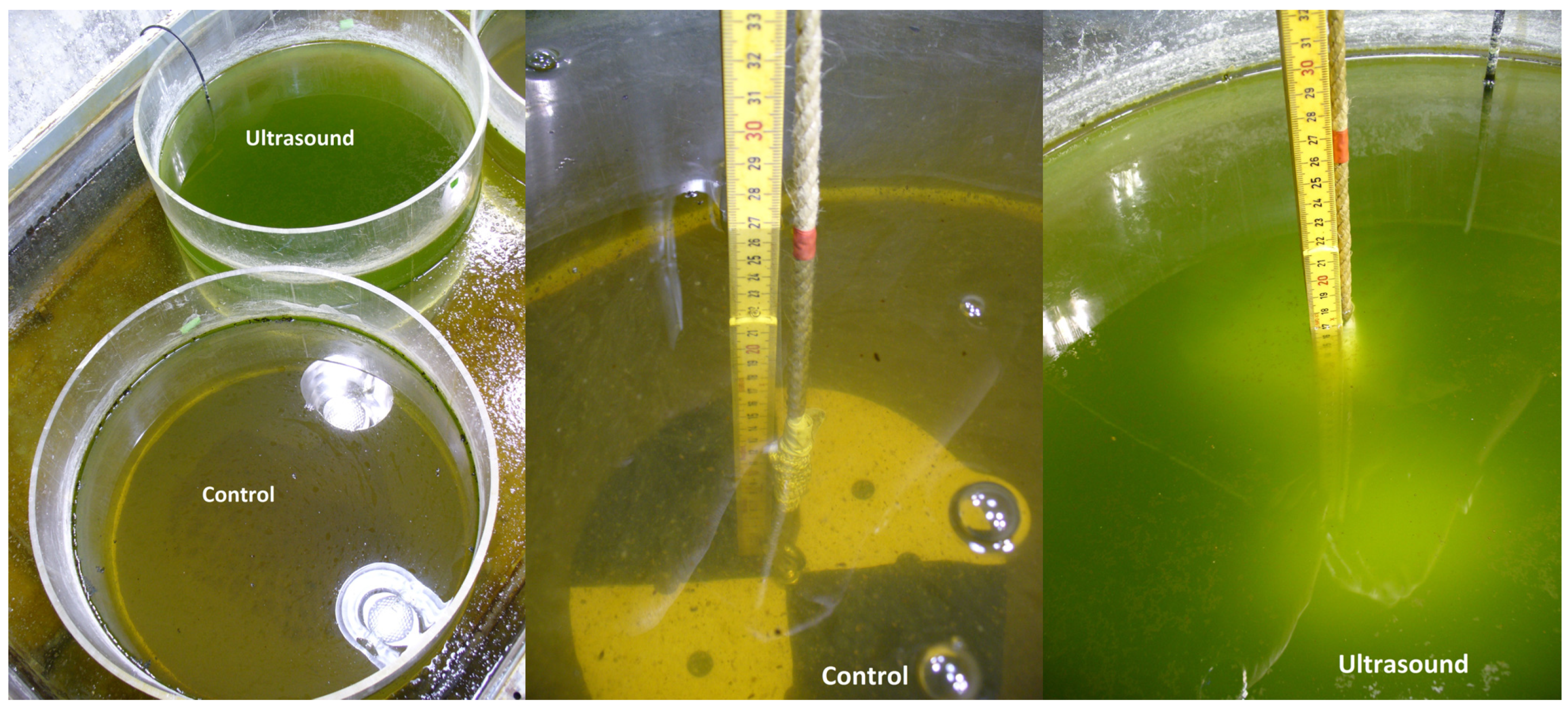
5. Conclusions
- Ultrasound had an acute lethal effect on D. magna;
- Higher ultrasound frequencies caused faster death than lower frequencies;
- Survival time of differently sized Daphnia exposed to ultrasound in different volumes varied only slightly;
- Ultrasound strongly suppressed Daphnia in 85 L tanks and freed phytoplankton from grazing control causing a turbid phytoplankton dominated water, whereas phytoplankton in the controls was under strong Daphnia control;
- The commercial available ultrasound transducers for clearing lakes and ponds are not environmentally friendly and are ineffective in controlling phytoplankton.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [PubMed]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Codd, G.A. Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol. Eng. 2000, 16, 51–60. [Google Scholar] [CrossRef]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Directive 2000/60/EG of the European Parliament and of the Council Establishing a Framework for the Community Action in the Field of Water Policy of. 23 October; European Union: Brussels, Belgium, 2000; pp. 1–72.
- Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC; European Union: Brussels, Belgium, 2006; pp. 37–51.
- Ibelings, B.W.; Stroom, J.M.; Lürling, M.F.L.L.W.; Kardinaal, W.E.A. Netherlands: Risks of toxic cyanobacterial blooms in recreational waters and guidelines. In Current Approaches to Cyanotoxin Risk Management, Risk Management and Regulations in Different Countries; Chorus, I., Ed.; Federal Environment Agency: Dessau Roβlau, Germany, 2012; pp. 82–96. [Google Scholar]
- Wu, X.; Joyce, E.M.; Mason, T.J. The effects of ultrasound on cyanobacteria. Harmful Algae 2011, 10, 738–743. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. A review of the use of sonication to control cyanobacterial blooms. Water Res. 2013, 46, 4319–4329. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Beating the blues: Is there any music in fighting cyanobacteria with ultrasound? Water Res. 2014, 66, 361–373. [Google Scholar] [CrossRef]
- Flexidal Technics. Available online: http://flexidal.be/nl/produktenvanflexidal_algen.asp?rubriek=algen&fotoid=8 (accessed on 12 July 2014).
- Kikuchi, T.; Uchida, T. Calorimetric method for measuring high ultrasonic power using water as a heating material. J. Phys. Conf. Ser. 2011, 279. [Google Scholar] [CrossRef]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Tollrian, R. Neckteeth formation in Daphnia pulex as example of continuous phenotypic plasticity: Morphological effects of Chaoborus kairomone concentration and their quantification. J. Plankton Res. 1993, 15, 1309–1318. [Google Scholar] [CrossRef]
- Lürling, M.; Beekman, W. Palmelloids formation in Chlamydomonas reinhardtii: Defence against rotifer predators? Ann. Limnol. 2006, 42, 65–72. [Google Scholar] [CrossRef]
- Govaert, E.; Vanderstukken, M.; Muylaert, K. Evaluatie van Effecten van Ultrasone Straling op Het Ecosysteem; KU Leuven Kortrijk: Kortrijk, Belgium, 2007; p. 20. (In Dutch) [Google Scholar]
- Hoyer, O.; Clasen, J. The application of new technologies in the water treatment process of a modern waterworks. Water Sci. Technol. 2002, 2, 63–69. [Google Scholar]
- Holm, E.R.; Stamper, D.M.; Brizzolara, R.A.; Barnes, L.; Deamer, N.; Burkholder, J.M. Sonication of bacteria, phytoplankton and zooplankton: Application to treatment of ballast water. Mar. Pollut. Bull. 2008, 56, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.N.T. The effect of ultrasonic irradiation on the survival of Daphnia magna. Exp. Biol. 1968, 49, 61–70. [Google Scholar]
- Kamenskii, I.V. Influence of ultrasound on eggs and larvae of some fish trematodes. Byulleten’ Vsesoyuznogo Instituta Gel’mintologii im 1970, 4, 47–50. (In Russian) [Google Scholar]
- Miller, D.L. A review of the ultrasonic bioeffects of microsonation, gas-body activation, and related cavitation-like phenomena. Ultrasound Med. Biol. 1987, 13, 443–470. [Google Scholar] [CrossRef]
- Wells, R.M.G.; Hudson, M.J.; Brittain, T. Function of the hemoglobin and the gas bubble in the backswimmer Anisops assimilis (Hemiptera: Notonectidae). J. Comp. Physiol. B 1981, 142, 515–522. [Google Scholar] [CrossRef]
- Knudsen, F.R.; Larsson, P.; Jakobson, P.J. Acoustic scattering from a larval insect (Chaoborus flavicans) at six echosounder frequencies: Implication for acoustic estimates of fish abundance. Fish. Res. 2006, 79, 84–89. [Google Scholar] [CrossRef]
- Chislock, M.F.; Sarnelle, O.; Jernigan, L.M.; Wilson, A.E. Do high concentrations of microcystin prevent Daphnia control of phytoplankton? Water Res. 2013, 47, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Lürling, M. Effects of microcystin-free and microcystin-containing strains of the cyanobacterium Microcystis aeruginosa on growth of the grazer Daphnia magna. Environ. Toxicol. 2003, 18, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.S.; Lürling, M.; Panosso, R.; Huszar, V. Effects of the cyanobacterium Cylindrospermopsis raciborskii on feeding and life-history characteristics of the grazer Daphnia magna. Ecotoxicol. Environ. Saf. 2009, 72, 1183–1189. [Google Scholar]
- Purcell, D.; Parsons, S.A.; Jefferson, B.; Holden, S.; Campbell, A.; Wallen, A.; Chipps, M.; Holden, B.; Ellingham, A. Experiences of algal bloom control using green solutions barley straw and ultrasound, an industry perspective. Water Environ. J. 2013, 27, 148–156. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Joung, S.-H.; Choi, A.; Kim, H.-S.; Jang, K.-Y.; Oh, H.-M. Selective control of cyanobacteria in eutrophic pond by a combined device of ultrasonication and water pumps. Environ. Technol. 2007, 28, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Lee, T.J.; Matsumara, M. In situ algal bloom control by the integration of ultrasonic radiation and jet circulation to flushing. Environ. Sci. Technol. 2001, 35, 4941–4946. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Nakano, K.; Matsumara, M. A novel strategy for cyanobacterial bloom control by ultrasonic irradiation. Water Sci. Technol. 2002, 46, 207–215. [Google Scholar] [PubMed]
- Kardinaal, E.; de Haan, M.; Ruiter, H. Maatregelen ter voorkoming blauwalgen werken onvoldoende. H2O 2008, 7, 4–7. (In Dutch) [Google Scholar]
- Mackay, E.B.; Maberly, S.C.; Pan, G.; Reitzel, K.; Bruere, A.; Corker, N.; Douglas, G.; Egemose, S.; Hamilton, D.; Hatton-Ellis, T.; et al. Geoengineering in lakes: Welcome attraction or fatal distraction? Inland Waters 2014, 4, 349–356. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lürling, M.; Tolman, Y. Effects of Commercially Available Ultrasound on the Zooplankton Grazer Daphnia and Consequent Water Greening in Laboratory Experiments. Water 2014, 6, 3247-3263. https://doi.org/10.3390/w6113247
Lürling M, Tolman Y. Effects of Commercially Available Ultrasound on the Zooplankton Grazer Daphnia and Consequent Water Greening in Laboratory Experiments. Water. 2014; 6(11):3247-3263. https://doi.org/10.3390/w6113247
Chicago/Turabian StyleLürling, Miquel, and Yora Tolman. 2014. "Effects of Commercially Available Ultrasound on the Zooplankton Grazer Daphnia and Consequent Water Greening in Laboratory Experiments" Water 6, no. 11: 3247-3263. https://doi.org/10.3390/w6113247





