Determination of Perchlorate in Bottled Water from Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sample Preparation
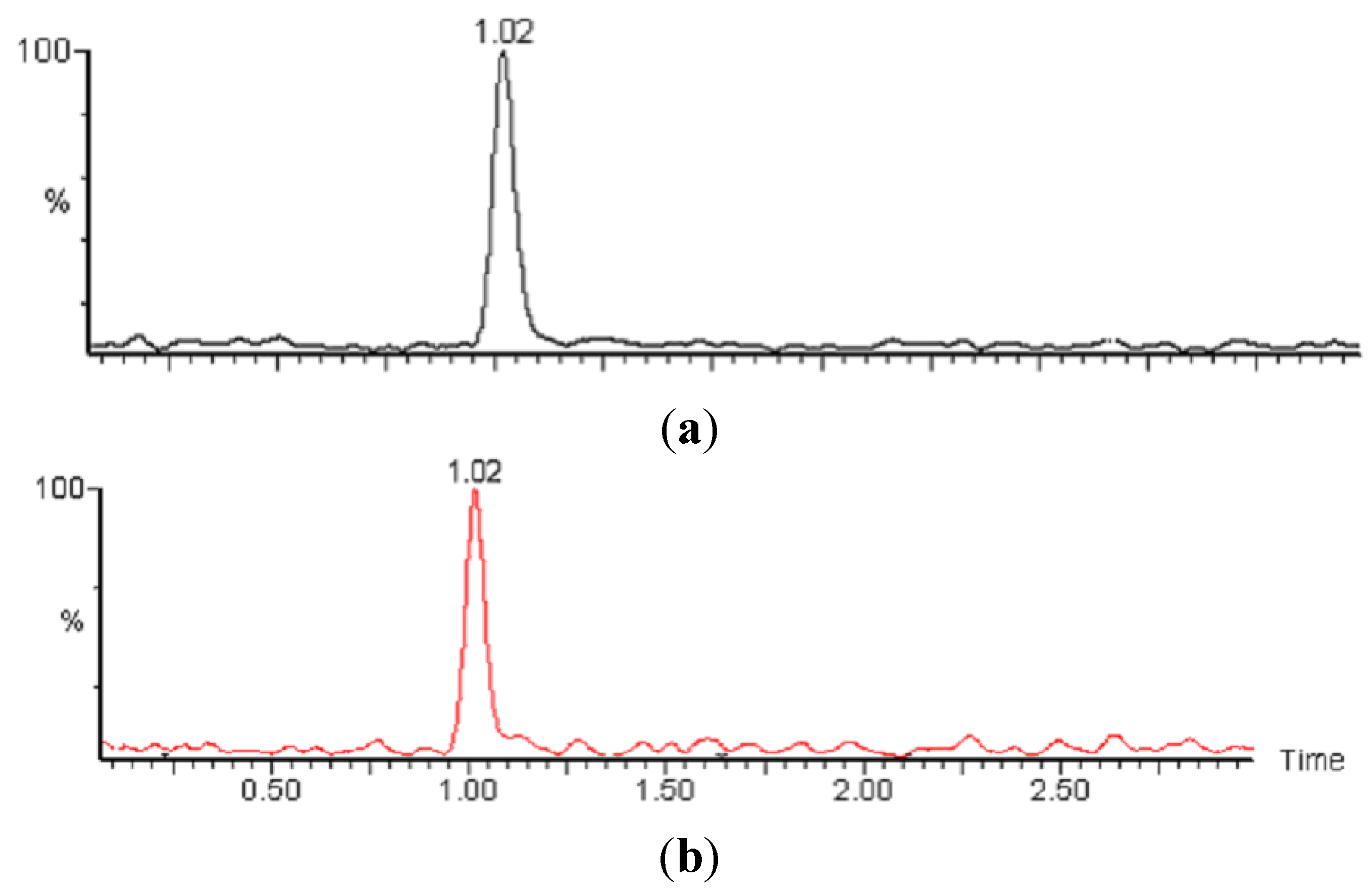
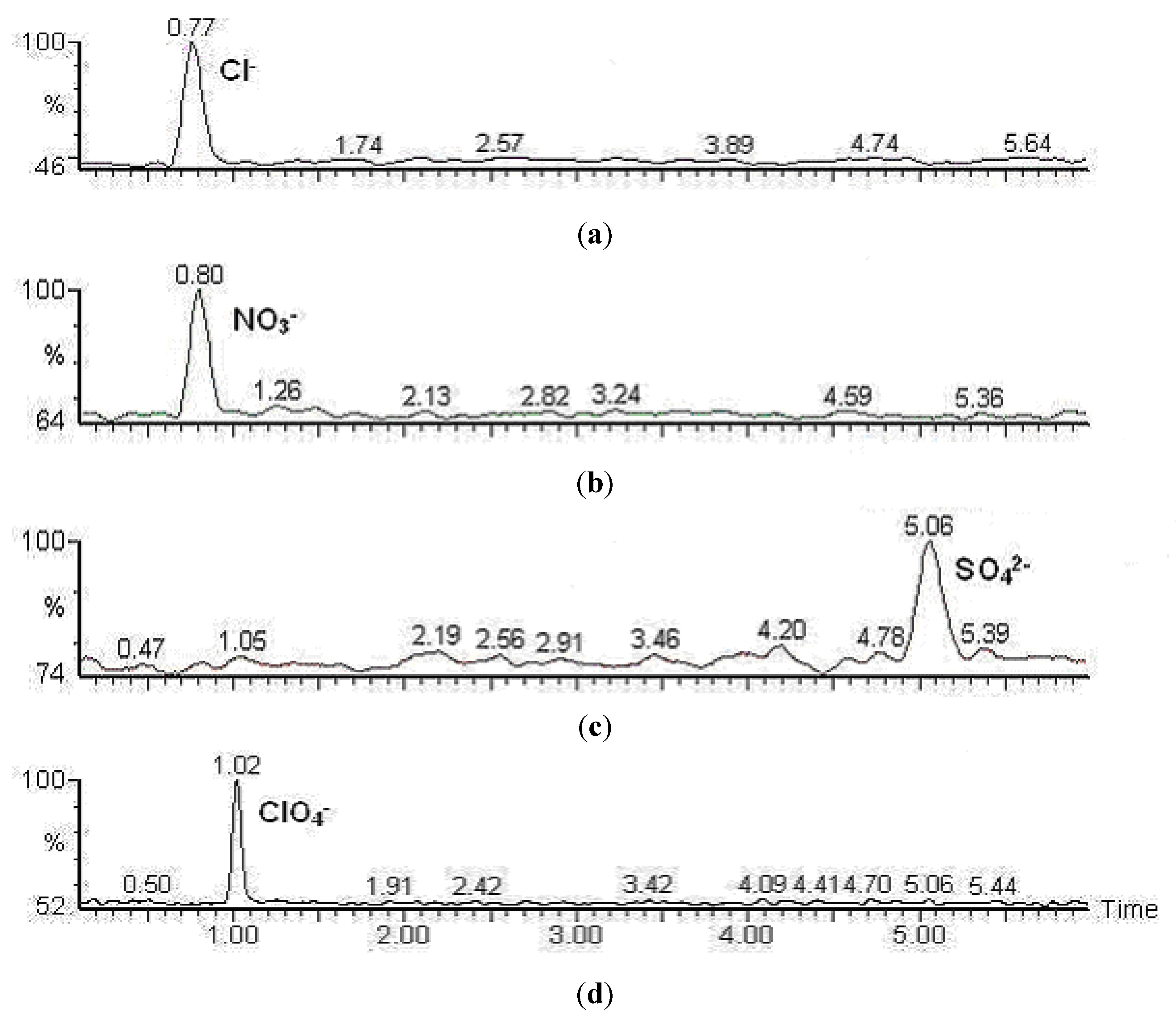
2.3. Liquid Chromatography
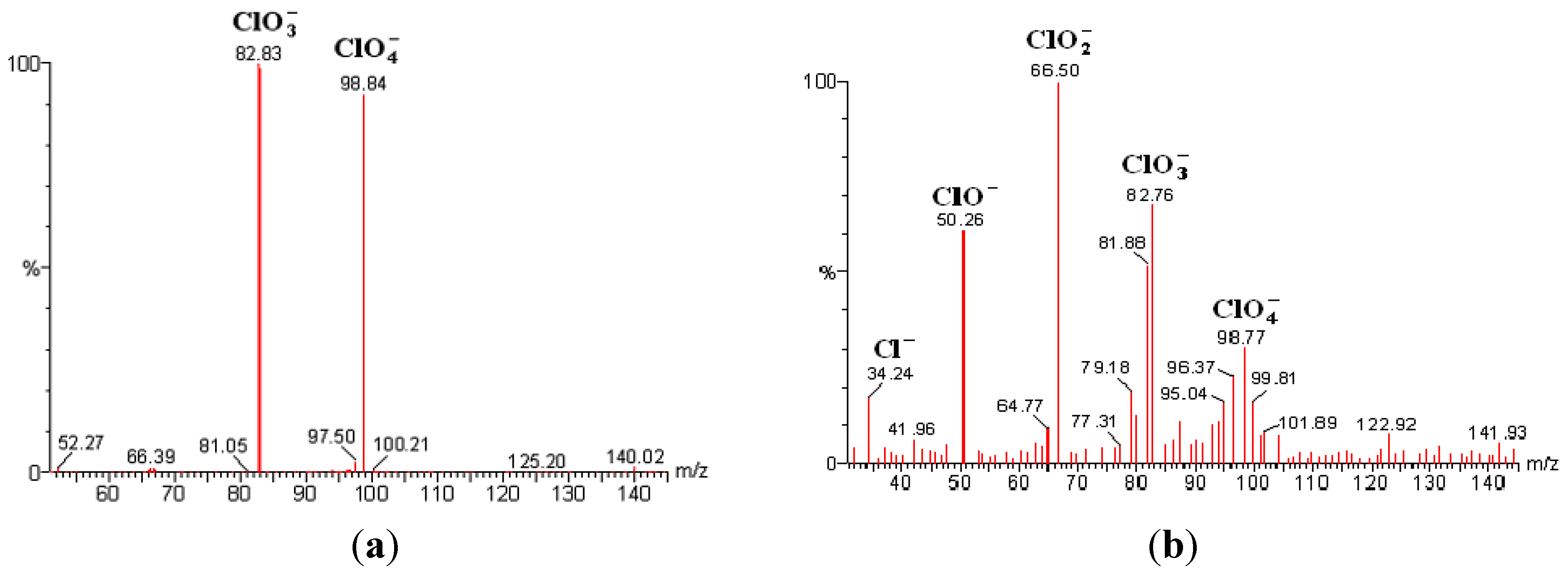
2.4. Mass Spectrometry
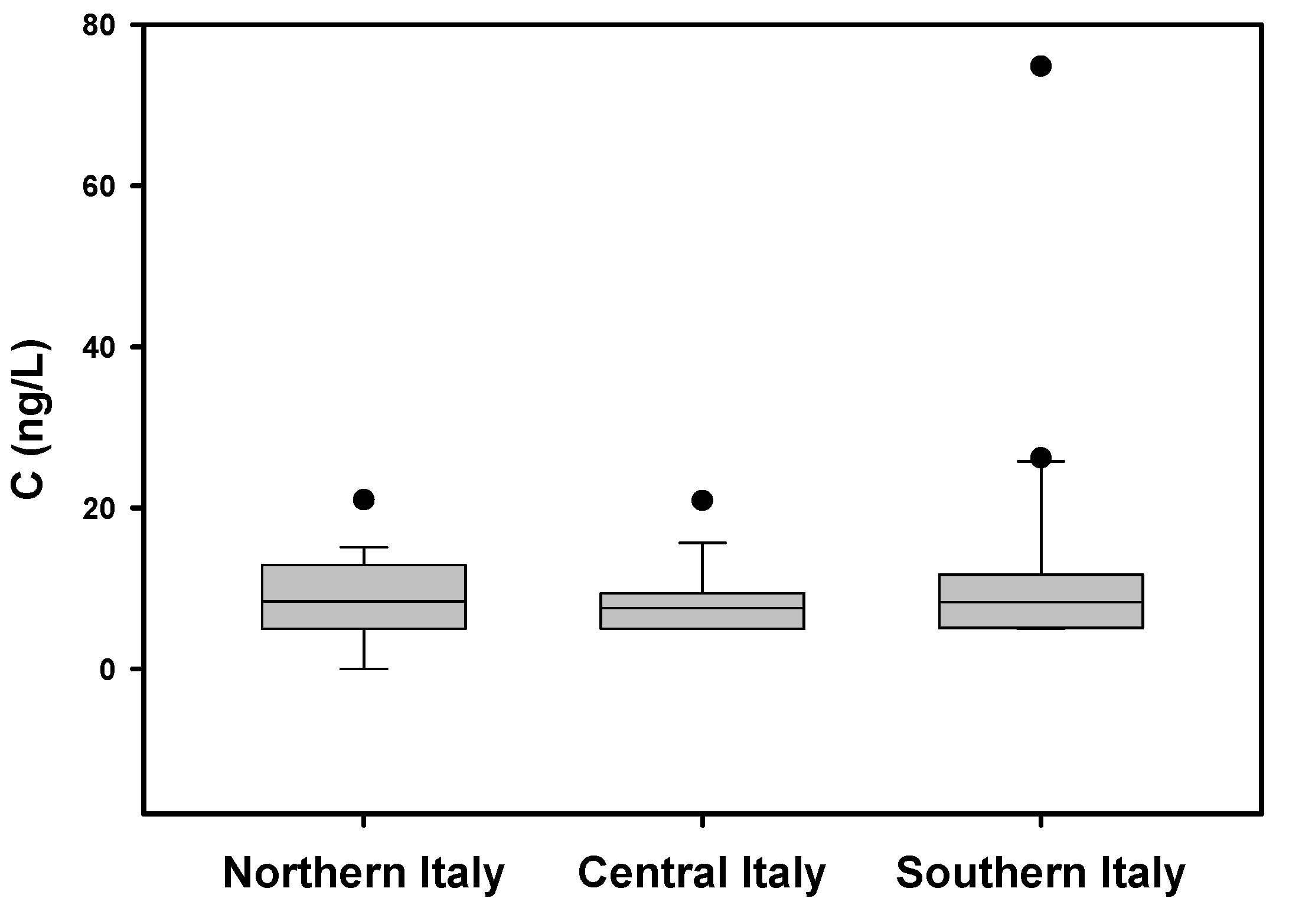
3. Results and Discussion
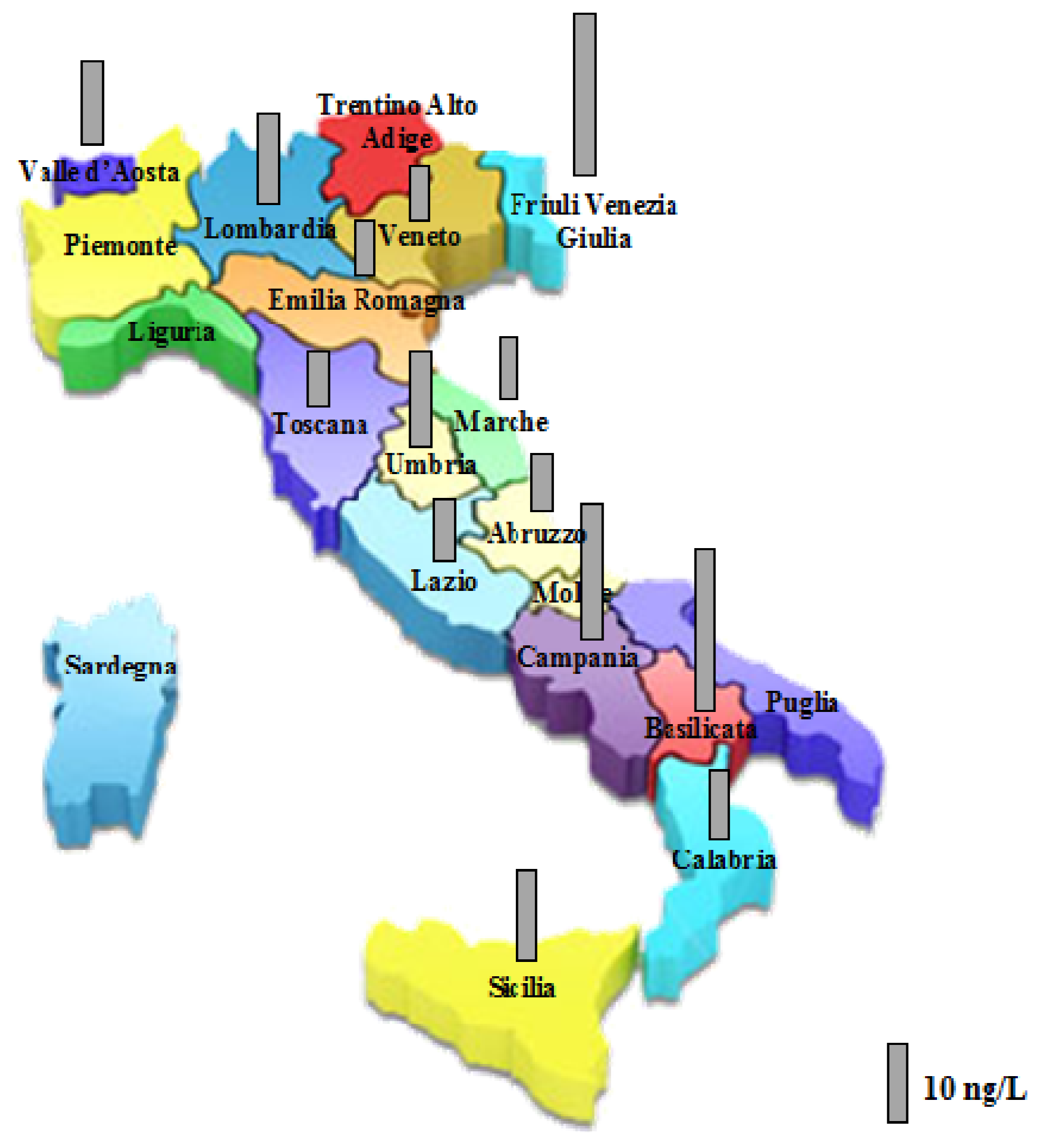
| Region | Perchlorate a (ng/L) | Region | Perchlorate a (ng/L) |
|---|---|---|---|
| Valle D’Aosta | <LOQ | Umbria | <LOQ |
| 7 ± 0.3 | <LOQ | ||
| 13 ± 0.4 | 9 ± 0.2 | ||
| 9 ± 0.4 | 9 ± 0.2 | ||
| 13 ± 0.3 | 15 ± 0.3 | ||
| Piemonte | <LOQ | 6 ± 0.5 | |
| Lombardia | 15 ± 0.6 | 21 ± 0.2 | |
| 7 ± 0.1 | <LOQ | ||
| <LOQ | Marche | 8 ± 0.3 | |
| <LOQ | 8 ± 0.3 | ||
| 11 ± 0.3 | Lazio | <LOQ | |
| 13 ± 0.5 | 8 ± 0.3 | ||
| 8 ± 0.4 | Abruzzo | 7 ± 0.3 | |
| Veneto | 7 ± 0.4 | Basilicata | 6 ± 0.3 |
| 8 ± 0.3 | 8 ± 0.3 | ||
| <LOQ | 8 ± 0.2 | ||
| <LOQ | 8 ± 0.2 | ||
| Friuli V. G. | 14 ± 0.4 | 75 ± 0.5 | |
| 21 ± 0.4 | Calabria | 9 ± 0.3 | |
| <LOQ | 9 ± 0.5 | ||
| Toscana | 9 ± 0.2 | 5 ± 0.4 | |
| 8 ± 0.3 | 5 ± 0.3 | ||
| <LOQ | 13 ± 0.3 | ||
| <LOQ | 8 ± 0.3 | ||
| Campania | 25 ± 0.4 | 8 ± 0.2 | |
| <LOQ | 13 ± 0.4 | ||
| 26 ± 0.2 | Sicilia | <LOQ | |
| <LOQ | 8 ± 0.3 | ||
| <LOQ | 9 ± 0.2 | ||
| 7 ± 0.2 | <LOQ | ||
| 11 ± 0.3 | 17 ± 0.5 |
| Standard | Perchlorate (ng/L) | Recovery a (%) |
|---|---|---|
| 1 | 5 | 96 |
| 2 | 10 | 101 |
| 3 | 25 | 98 |
| 4 | 100 | 102 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Greer, M.A.; Goodman, G.; Pleus, R.C.; Greer, S.E. Health effects assessment for environmental perchlorate contamination: The dose response for inhibition of thyroidal radioiodine uptake in humans. Environ. Health Perspect. 2002, 110, 927–937. [Google Scholar] [CrossRef]
- Smith, P.N.; Theodorakis, C.W.; Anderson, T.A.; Kendall, R.J. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnak, Texas. Ecotoxicology 2001, 10, 305–313. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Martinelango, P.K.; Jackson, W.A.; Anderson, T.A.; Tian, K.; Tock, R.W.; Rajacopalan, S. The origin of naturally occurring perchlorate: The role of atmospheric processes. Environ. Sci. Technol. 2005, 39, 1569–1575. [Google Scholar] [CrossRef]
- Parker, D.R.; Seyfferth, A.L.; Reese, B.K. Perchlorate in groundwater: A synoptic survey of “Pristine” sites in the coterminous United States. Environ. Sci. Technol. 2008, 42, 1465–1471. [Google Scholar] [CrossRef]
- Rao, B.; Anderson, T.A.; Orris, G.J.; Rainwater, K.A.; Rajagopalan, S.; Sandvig, R.M.; Scanlon, B.R.; Stonestrom, D.A.; Walvoord, M.A.; Jackson, W.A. Widespread natural perchlorate in unsaturated zones of the Southwest United States. Environ. Sci. Technol. 2007, 41, 4522–4528. [Google Scholar] [CrossRef]
- Saito, K.; Yamamoto, K.; Takay, T.; Yoshida, S. Inhibition of iodide accumulation by perchlorate and thiocyanate in a model of the thyroid iodide transport system. Acta Endocionol. 1983, 104, 456–461. [Google Scholar]
- Urbansky, E.T.; Schock, M.R. Issues in managing the risks associated with perchlorate in drinking water. J. Environ. Manag. 1999, 56, 79–95. [Google Scholar] [CrossRef]
- Wolff, J. Perchlorate and tyroid gland. Pharmacol. Rev. 1998, 50, 89–105. [Google Scholar]
- Xu, J.; Song, Y.; Min, B.; Steinberg, L.; Logan, B.E. Microbial degradation of perchlorate: Principles and applications. Environ. Eng. Sci. 2003, 20, 405–422. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA), Announcement of the Draft Drinking Water Contaminant Candidate List Notice; EPA Number 815Z97003; October 1997; p. 52193.
- California Department of Health Services. Perchlorate in Drinking Water. 7 December 2012. Available online: http://www.cdph.ca.gov/certlic/drinkingwater/pages/Perchlorate.aspx (accessed on 11 March 2013).
- National Resource Council (NRC), Health Implication of Perchlorate Ingestion; National Resource Council National Academic Press: Washington, DC, USA, 2005.
- Dasgupta, P.K.; Dyke, J.V.; Kirk, A.B.; Jackson, W.A. Perchlorate in the United States. Analysis of relative source contributions to the food chain. Environ. Sci. Technol. 2006, 40, 6608–6614. [Google Scholar] [CrossRef]
- Dyke, J.V.; Kirk, A.B.; Martinelango, P.K.; Dasgupta, P.K. Sample processing method for the determination of perchlorate in milk. Anal. Chim. Acta 2006, 567, 73–78. [Google Scholar] [CrossRef]
- Kirk, A.B.; Smith, E.E.; Tian, K.; Anderson, T.A.; Dasgupta, P.K. Perchlorate in milk. Environ. Sci. Technol. 2003, 37, 4979–4981. [Google Scholar] [CrossRef]
- Krynitsky, A.J.; Niemann, R.A.; Nortrup, D.A. Determination of perchlorate anion in foods by ion chromatography-tandem mass spectrometry. Anal. Chem. 2004, 76, 5518–5522. [Google Scholar] [CrossRef]
- Sanchez, C.A.; Crump, K.S.; Krieger, R.I.; Khandaker, N.R.; Gibbs, J.P. Perchlorate and nitrate in leafy vegetables of North America. Environ. Sci. Technol. 2005, 39, 9391–9397. [Google Scholar] [CrossRef]
- Stetson, S.J.; Wanty, R.B.; Helsel, D.R.; Kalkhoff, S.J.; Mcalady, D.L. Stability of low levels of perchlorate in drinking water and natural water samples. Anal. Chim. Acta 2006, 567, 108–113. [Google Scholar] [CrossRef]
- Kosaka, K.; Asami, M.; Matsuoka, Y.; Kamoshita, M.; Kunikane, S. Occurrence of perchlorate in drinking water sources of metropolitan area in Japan. Water Res. 2007, 41, 3473–3482. [Google Scholar]
- Quinones, O.; Oh, J.; Vanderford, B.; Kim, J.H.; Cho, J.; Snyder, S.A. Perchlorate assessment of the Nakdong and Yeongsan watersheds, Republic of Korea. Environ. Toxicol. Chem. 2007, 26, 1349–1354. [Google Scholar] [CrossRef]
- Backus, S.M.; Klawuun, P.; Brown, S.; D’sa, I.; Sharp, S.; Surette, C.; Williams, D.J. Determination of perchlorate in selected surface waters in the Great Lakes Basin by HPLC/MS/MS. Chemosphere 2005, 61, 834–843. [Google Scholar] [CrossRef]
- Vigliotta, G.; Motta, O.; Guarino, F.; Iannece, P.; Proto, A. Assessment of perchlorate-reducing bacteria in a highly polluted river. Int. J. Hyg. Environ. Health 2010, 213, 437–443. [Google Scholar] [CrossRef]
- Urbansky, E.T. Quantitation of perchlorate ion: Practices and advances applied to the analysis of common matrices. Crit. Rev. Anal. Chem. 2000, 30, 311–343. [Google Scholar] [CrossRef]
- Heinnickel, M.; Smith, S.C.; Koo, J.; O’Connor, S.M.; Coates, J.D. A bioassay for the detection of perchlorate in the ppb range. Environ. Sci. Technol. 2011, 45, 2958–2964. [Google Scholar] [CrossRef]
- Anderson, T.A.; Wu, T.H. Extraction, clean-up, and analysis of the perchlorate anion in tissue samples. Bull. Environ. Contam. Toxicol. 2002, 68, 684–691. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, S.; Heberling, S. Determination of trace level bromate and perchlorate in drinking water by ion chromatography with an evaporative preconcentration technique. J. Chromatogr. A 2002, 956, 85–91. [Google Scholar] [CrossRef]
- Magnuson, M.L.; Ubansky, E.T.; Kelty, C.A. Microscale extraction of perchlorate in drinking water with low level detection by electrospray-mass spectrometry. Talanta 2000, 52, 285–291. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Gu, B.; Magnuson, M.L.; Brown, G.M.; Kelly, C.A. Survey of bottled waters for perchlorate by electrospray ionization mass spectrometry (ESI-MS) and ion chromatography (IC). J. Sci. Food Agric. 2000, 80, 1798–1804. [Google Scholar] [CrossRef]
- Johnson, J.; Grimshaw, D.; Richman, K. Analysis for Perchlorate by Ion Chromatography: Significant Recent Findings; American Pacific Corp.: Las Vegas, NV, USA, 2003. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA), Method 314.0 Determination of Perchlorate in Drinking Water Using Ion Chromatography, Revision 1.0.; USEPA: Cincinnati, OH, USA, 1999.
- Yu, L.; Cheng, Q.; Canas, J.; Valentin-Blasini, L.; Blount, B.C.; Anderson, T. Challenges in determining perchlorate in biological tissues and fluids: Implications for characterizing perchlorate exposure. Anal. Chim. Acta 2006, 567, 66–72. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA), Method 331.0 Determination of Perchlorate in Drinking Water by Liquid Chromatography Electrospray Ionization Mass Spectrometry, Revision 1.0.; USEPA: Cincinnati, OH, USA, 2005.
- El Aribi, H.; Le Blanc, Y.J.C.; Antosen, S.; Sakuma, T. Analysis of perchlorate in foods and beverages by ion chromatography coupled with tandem mass spectrometry (IC-ESI-MS/MS). Anal. Chim. Acta 2006, 567, 39–47. [Google Scholar] [CrossRef]
- Krynitsky, A.J.; Niemann, R.A.; Williams, A.D.; Hopper, M.L. Streamlined sample preparation procedure for determination of perchlorate anion in foods by ion chromatography-tandem mass spectrometry. Anal. Chim. Acta 2006, 567, 94–99. [Google Scholar] [CrossRef]
- Snyder, S.A.; Pleus, R.C.; Vanderford, B.J.; Holady, J.C. Perchlorate and chlorate in dietary supplements and flavour enhancing ingredients. Anal. Chim. Acta 2006, 567, 26–32. [Google Scholar] [CrossRef]
- Oldi, J.F.; Kannan, K. Analysis of perchlorate in human saliva by liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol. 2009, 43, 142–147. [Google Scholar] [CrossRef]
- Wang, Z.; Lau, B.P.-Y.; Tague, B.; Sparling, M.; Forsyth, D. Determination of perchlorate in infant formula by isotope dilution ion chromatography/tandem mass spectrometry. Food Addit. Contam. 2011, 28, 799–806. [Google Scholar] [CrossRef]
- Ells, E.; Barnett, D.A.; Purves, R.W.; Guevremont, R. Trace determination of perchlorate in water matrices in human urine using ESI-FAIMS-MS. J. Environ. Monitor. 2000, 2, 393–397. [Google Scholar] [CrossRef]
- Snyder, S.A.; Vanderford, B.J.; Rexing, D.J. Trace analysis of bromate, chlorate, iodate, perchlorate in natural and bottled waters. Environ. Sci. Technol. 2005, 39, 4586–4593. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Perchlorate in Water, Soils and Solid Wastes Using High Performance Liquid Chromatography/Electrospray Ionization/Mass Spectrometry (HPLC/ESI/MS or HPLC/ESI/MS/MS). 2007. Available online: http://www.epa.gov/osw/hazard/testmethods/pdfs/6850.pdf (accessed on 11 March 2013).
- Li, Y.; George, E.J. Analysis of perchlorate in water by reversed–phase LC/ESI–MS/MS using an internal standard technique. Anal. Chem. 2005, 77, 4453–4458. [Google Scholar] [CrossRef]
- Li, Y.; George, E.J. Reversed-phase liquid chromatograhy/electrospray ionization tandem mass spectrometry for analysis of perchlorate in water. J. Chromatogr. A 2006, 1133, 215–220. [Google Scholar] [CrossRef]
- Phenomenex Technical Note TN-1080. Available online: http://www.phenomenex.com/Kinetex/CoreShellTechnology (accessed on 19 March 2012).
- Niccolucci, V.; Botto, S.; Rugani, B.; Nicolardi, V.; Bastianoni, S.; Gaggi, C. The real water consumption behind drinking water: the case of Italy. J. Environ. Manag. 2011, 92, 2611–2618. [Google Scholar] [CrossRef]
- Stanford, B.D.; Pisarenko, A.N.; Snyder, S.A.; Gordon, G. Perchlorate, bromate, and chlorate in hypochlorite solutions: Guidelines for utilities. J. Am. Water Works Assoc. 2011, 103, 71–83. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iannece, P.; Motta, O.; Tedesco, R.; Carotenuto, M.; Proto, A. Determination of Perchlorate in Bottled Water from Italy. Water 2013, 5, 767-779. https://doi.org/10.3390/w5020767
Iannece P, Motta O, Tedesco R, Carotenuto M, Proto A. Determination of Perchlorate in Bottled Water from Italy. Water. 2013; 5(2):767-779. https://doi.org/10.3390/w5020767
Chicago/Turabian StyleIannece, Patrizia, Oriana Motta, Rossella Tedesco, Maurizio Carotenuto, and Antonio Proto. 2013. "Determination of Perchlorate in Bottled Water from Italy" Water 5, no. 2: 767-779. https://doi.org/10.3390/w5020767






