Beyond Eutrophication: Vancouver Lake, WA, USA as a Model System for Assessing Multiple, Interacting Biotic and Abiotic Drivers of Harmful Cyanobacterial Blooms
Abstract
:1. Introduction
2. Materials and Methods
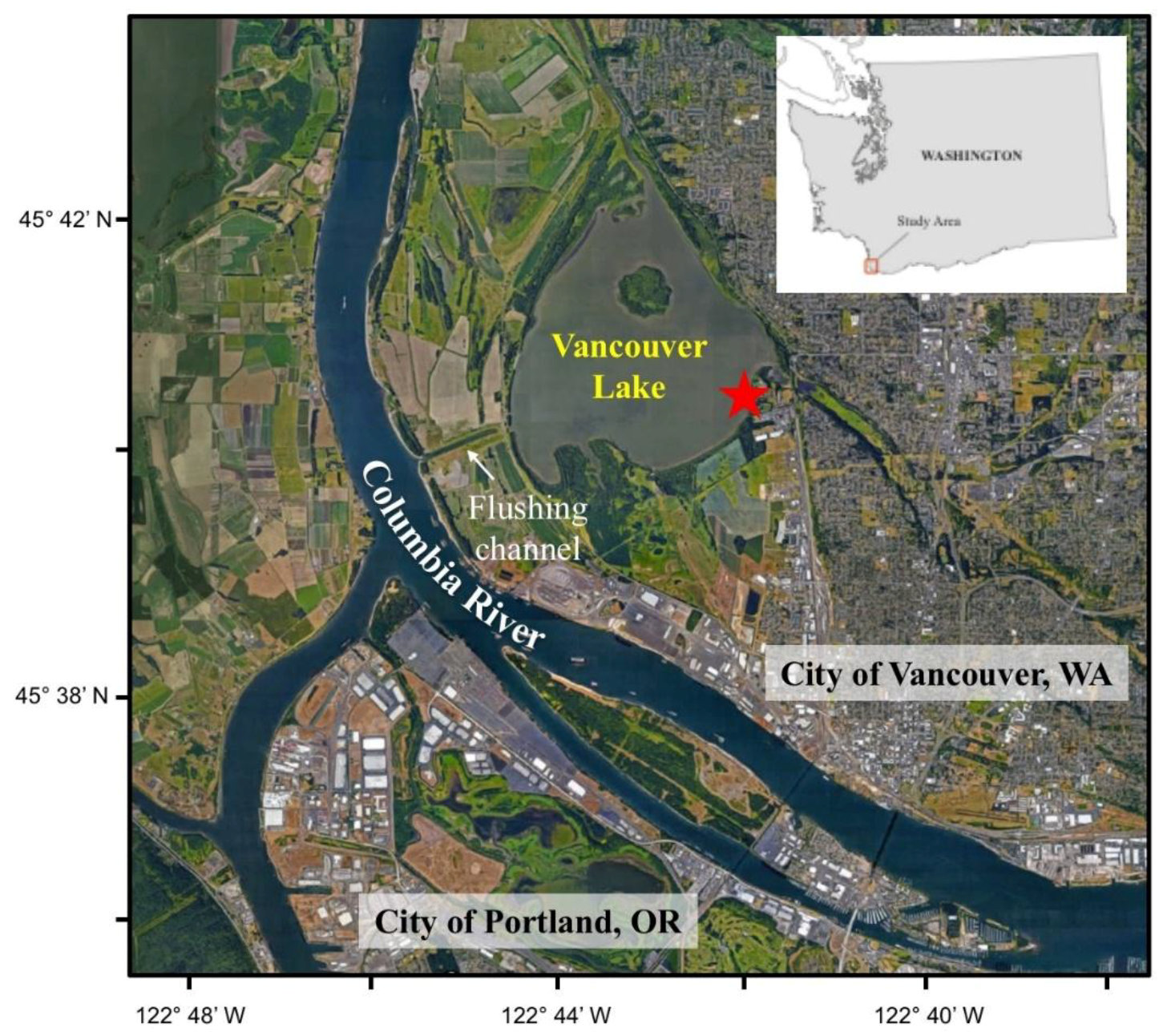
2.1. Study Area
2.1.1. Geography of Vancouver Lake
2.1.2. Lake Management Context
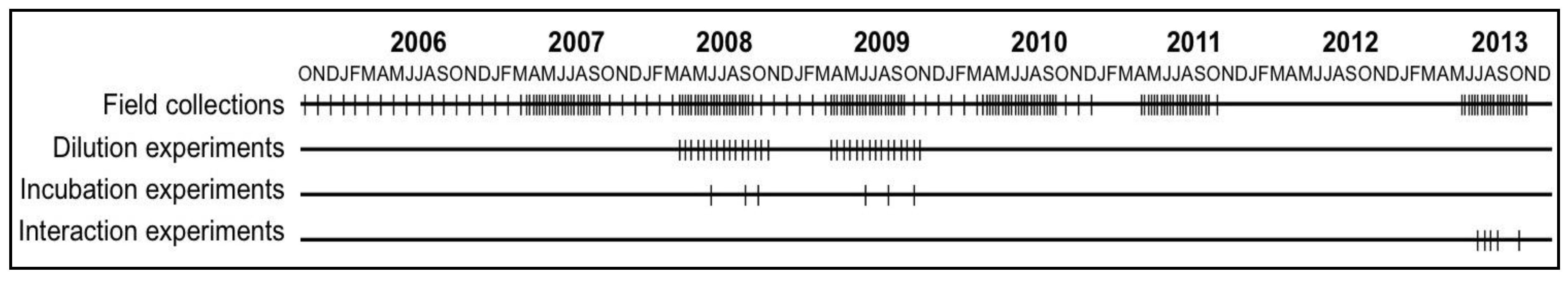
2.2. Field and Experimental Programs to Study Cyanobacterial Bloom Dynamics in Vancouver Lake
2.3. Field Sampling
2.3.1. Water quality
2.3.2. Plankton Community Composition
2.4. Statistical Analyses of Field Data
2.4.1. Analysis of Plankton Community Composition
2.4.2. Analysis of Environmental Factors Associated with Plankton Community Structure
2.5. Experimental Program
2.5.1. Microzooplankton Grazing (Dilution) Experiments
2.5.2. Mesozooplankton Feeding (Incubation) Experiments
2.5.3. Experiments to Measure the Interaction of Nutrients and Copepod Grazing on Phytoplankton Growth
3. Results
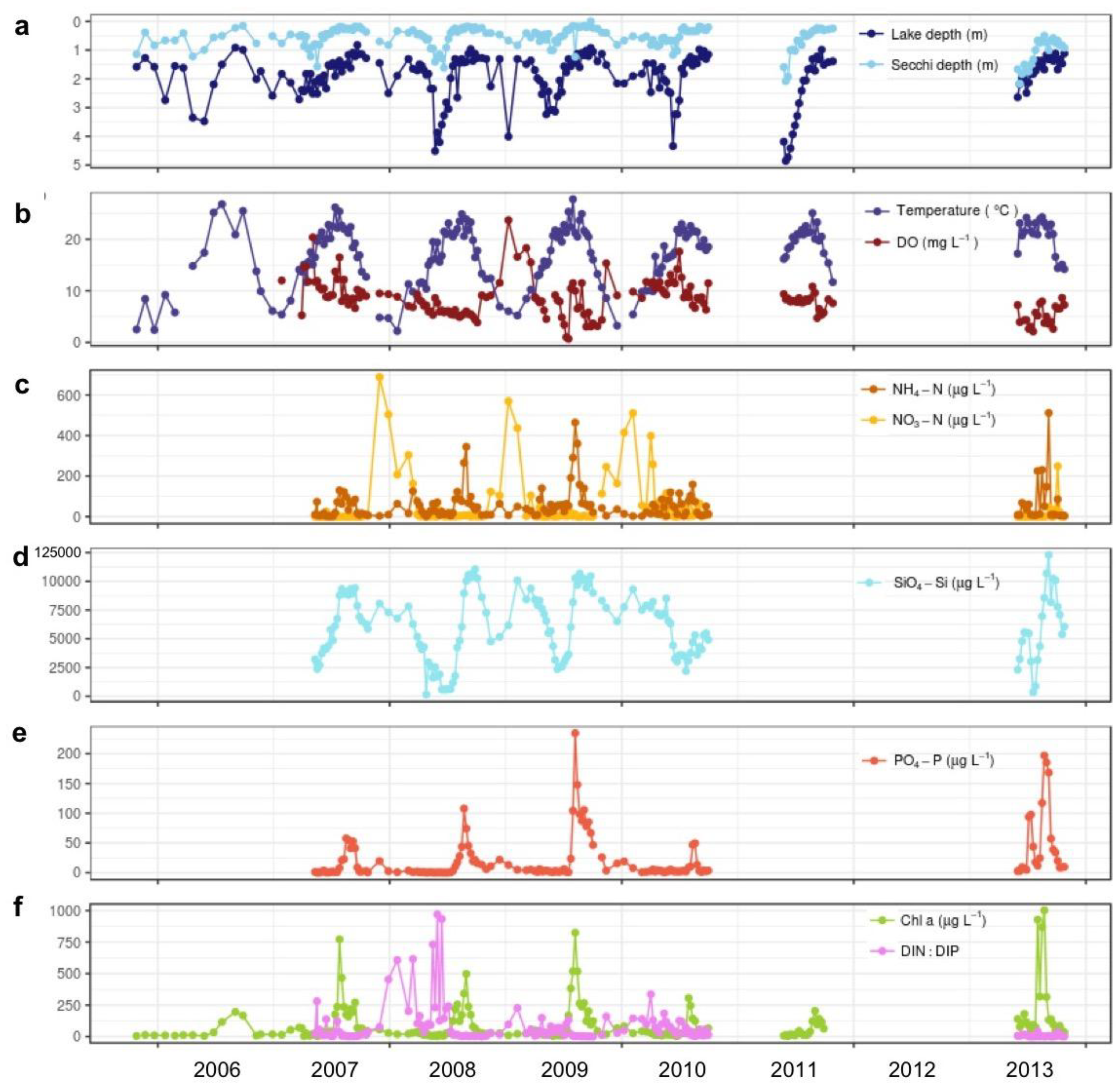
3.1. Temporal Variability of Environmental Conditions and Phytoplankton Blooms in Vancouver Lake, 2005–2013
3.1.1. Water Quality
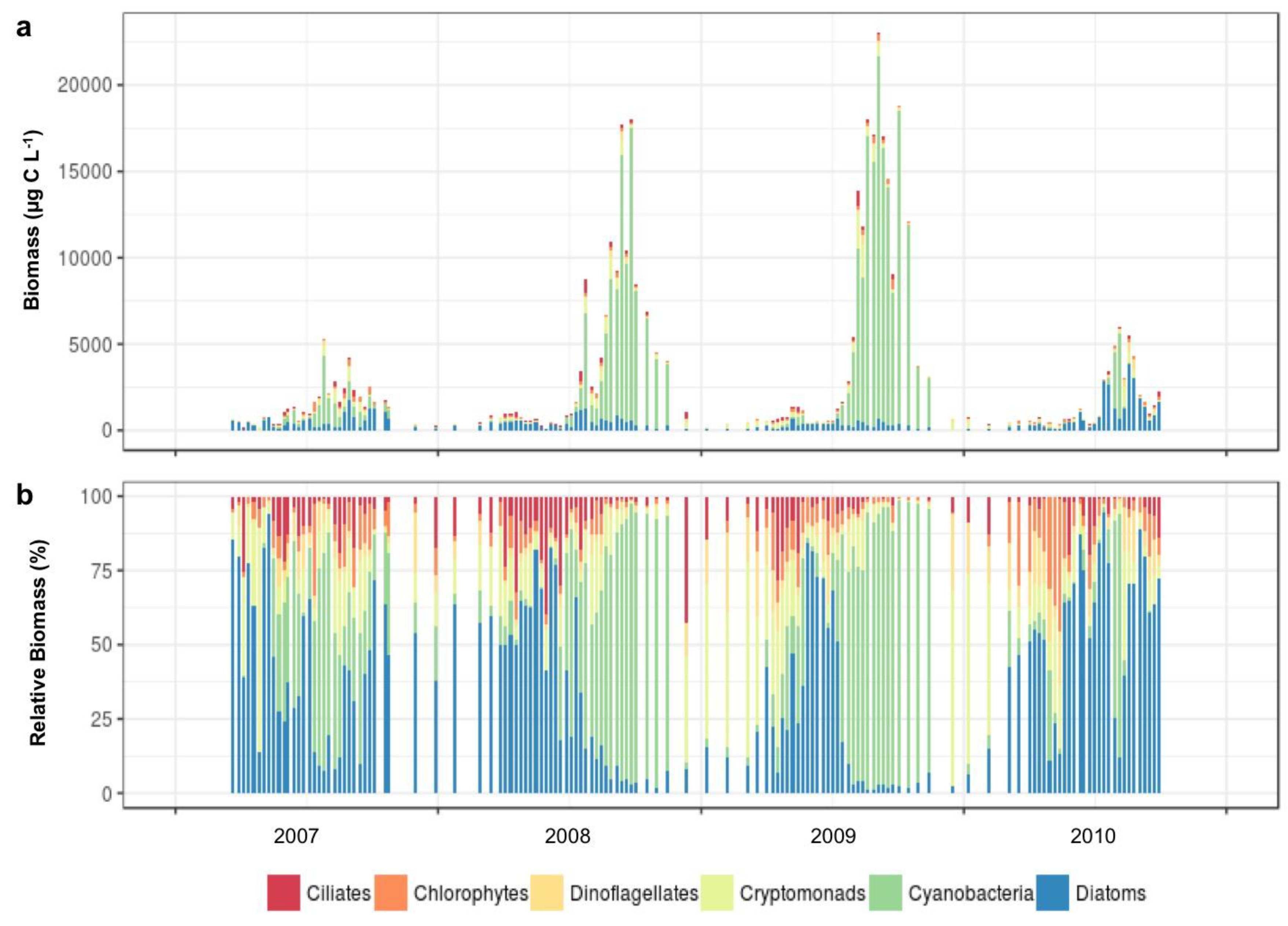
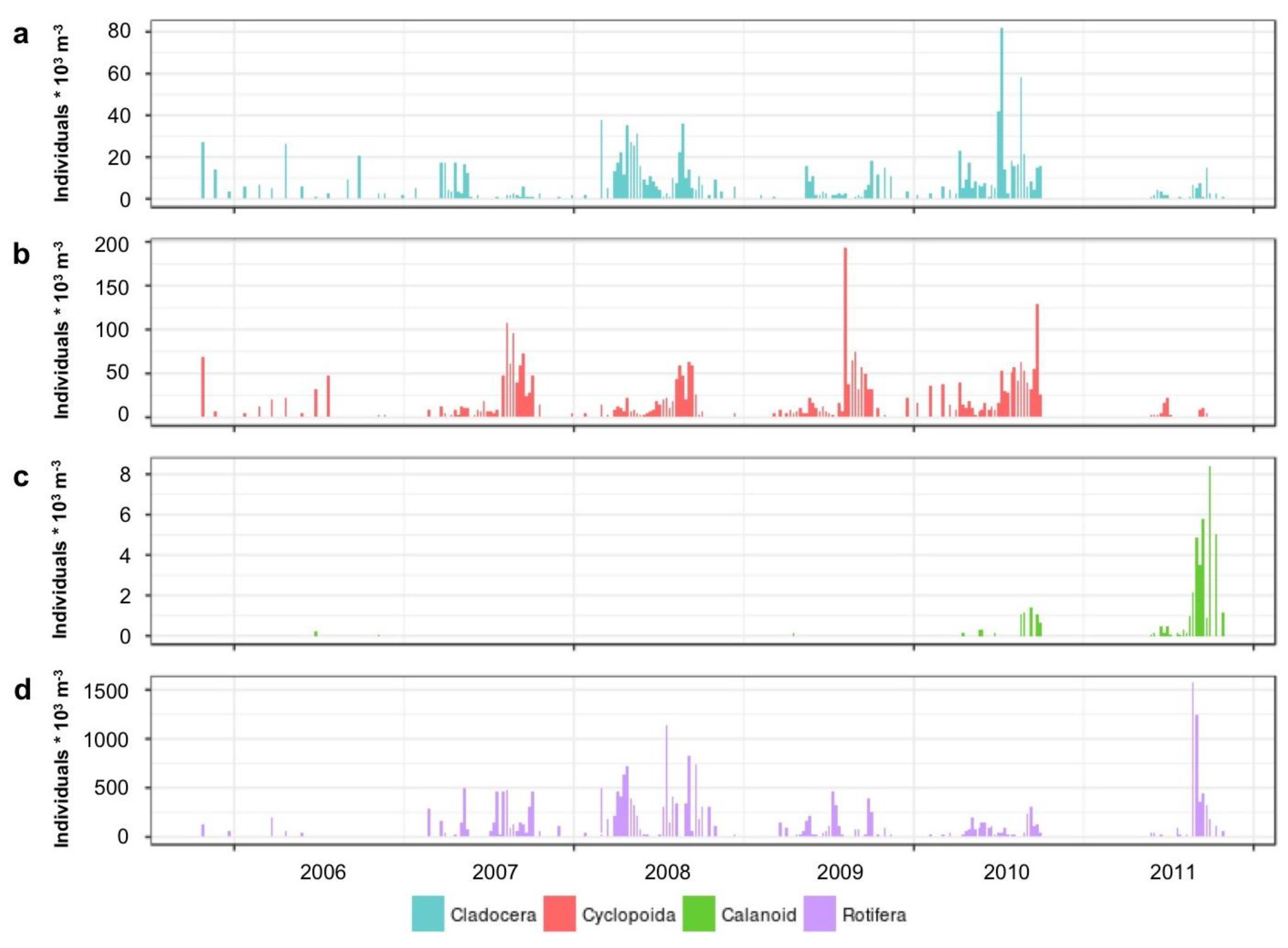
3.1.2. Microplankton and Mesozooplankton Abundance, Biomass, and Composition
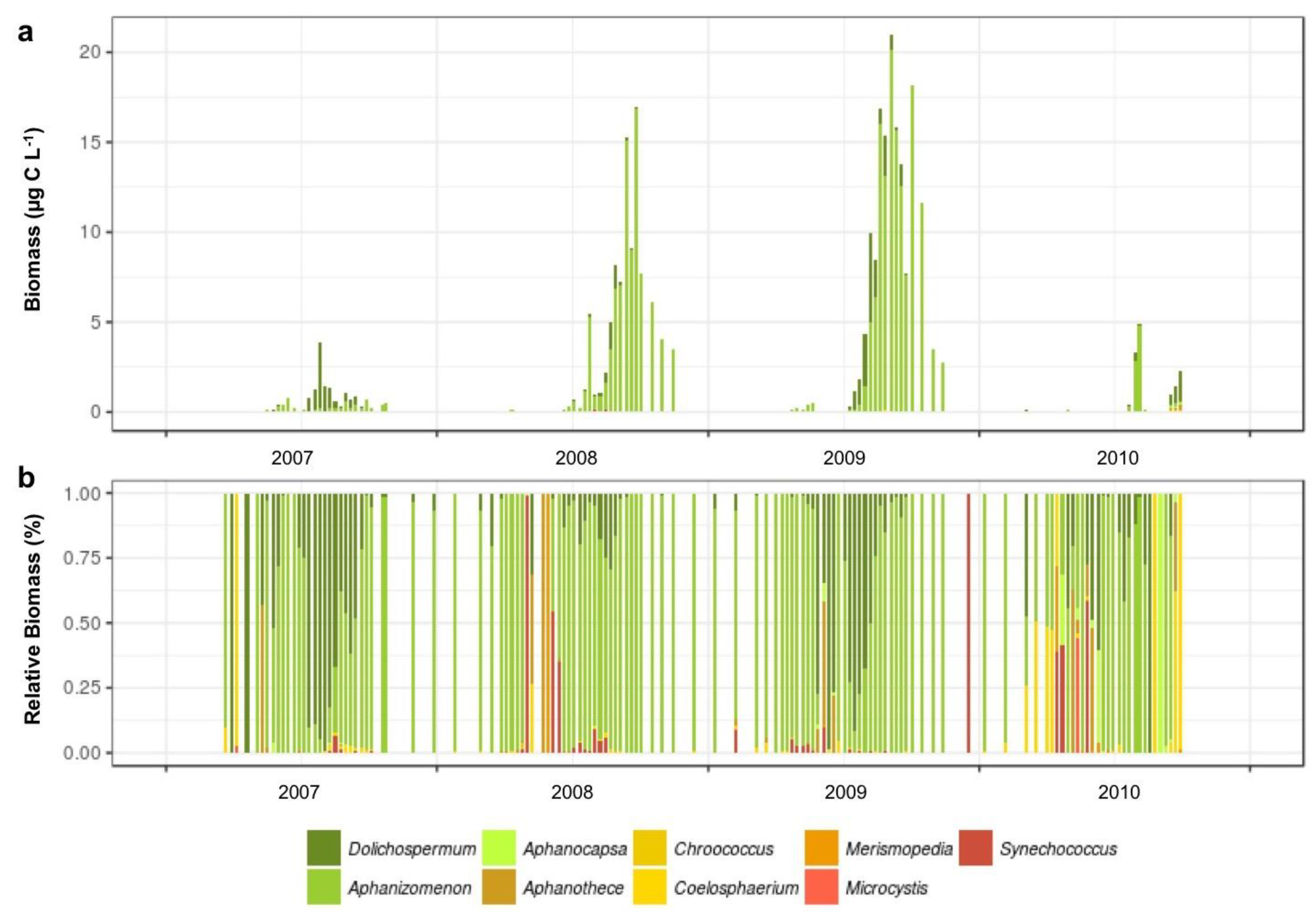
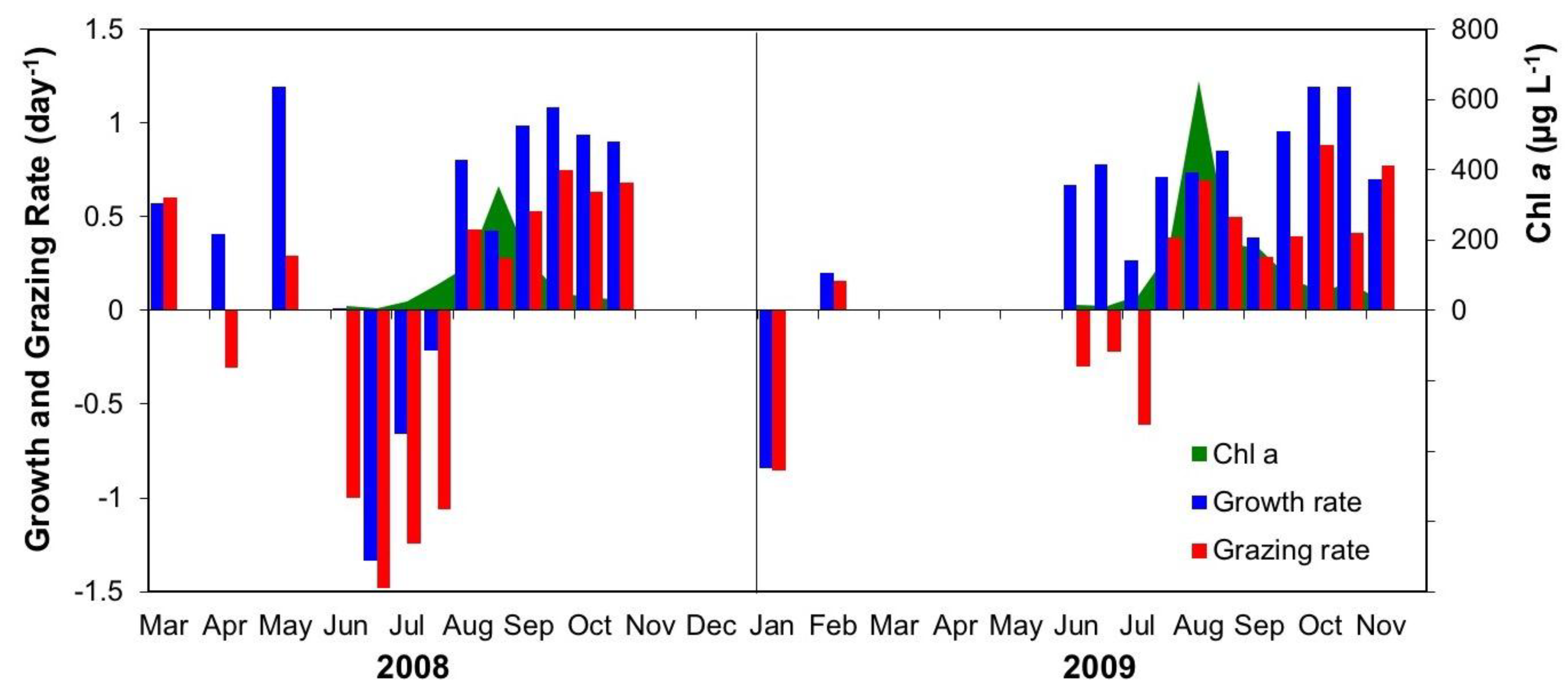
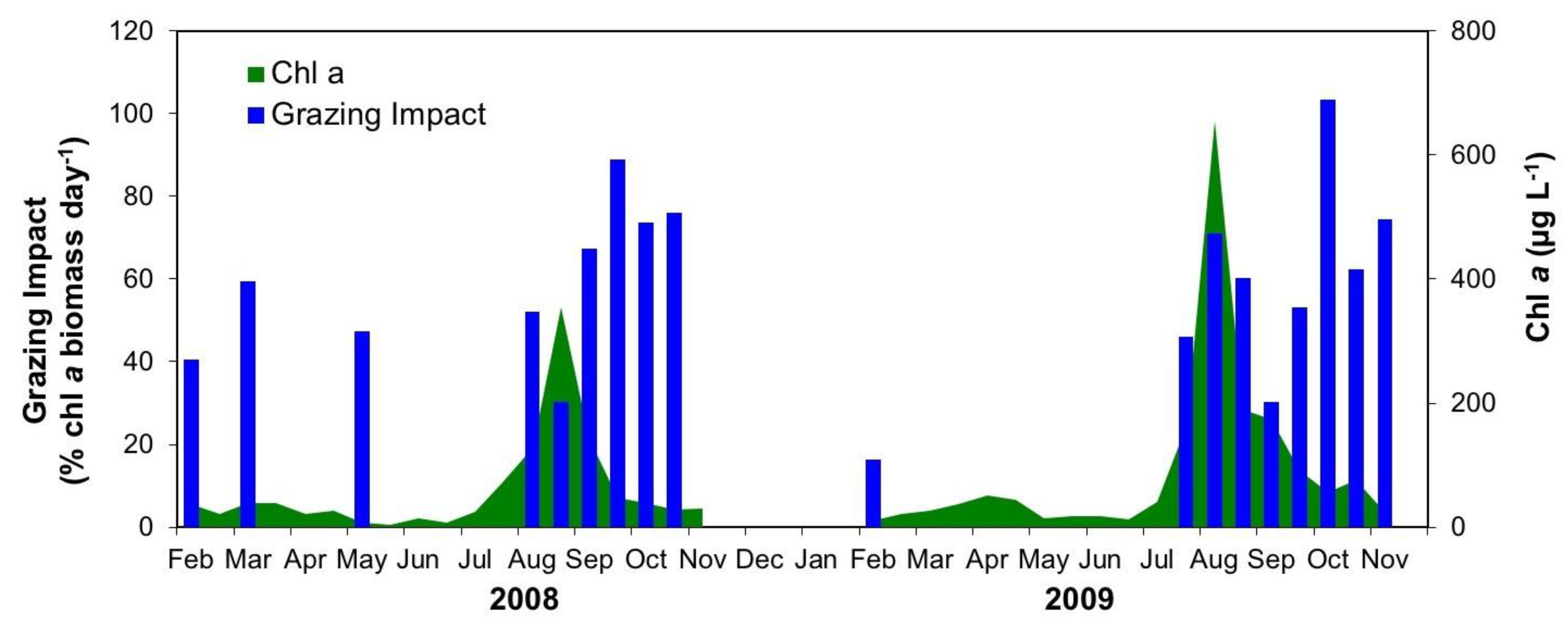
3.1.3. Interannual Pattern of Cyanobacterial Blooms in Vancouver Lake
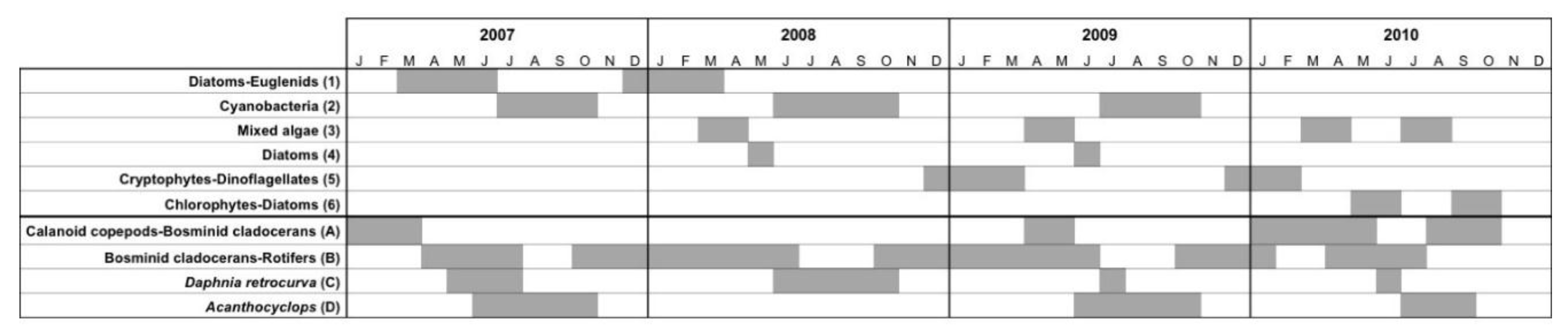
3.1.4. Seasonal Succession in the Phytoplankton and Zooplankton Assemblages of Vancouver Lake
3.2. Associations among Environmental Conditions, Community Composition and Cyanobacterial Blooms
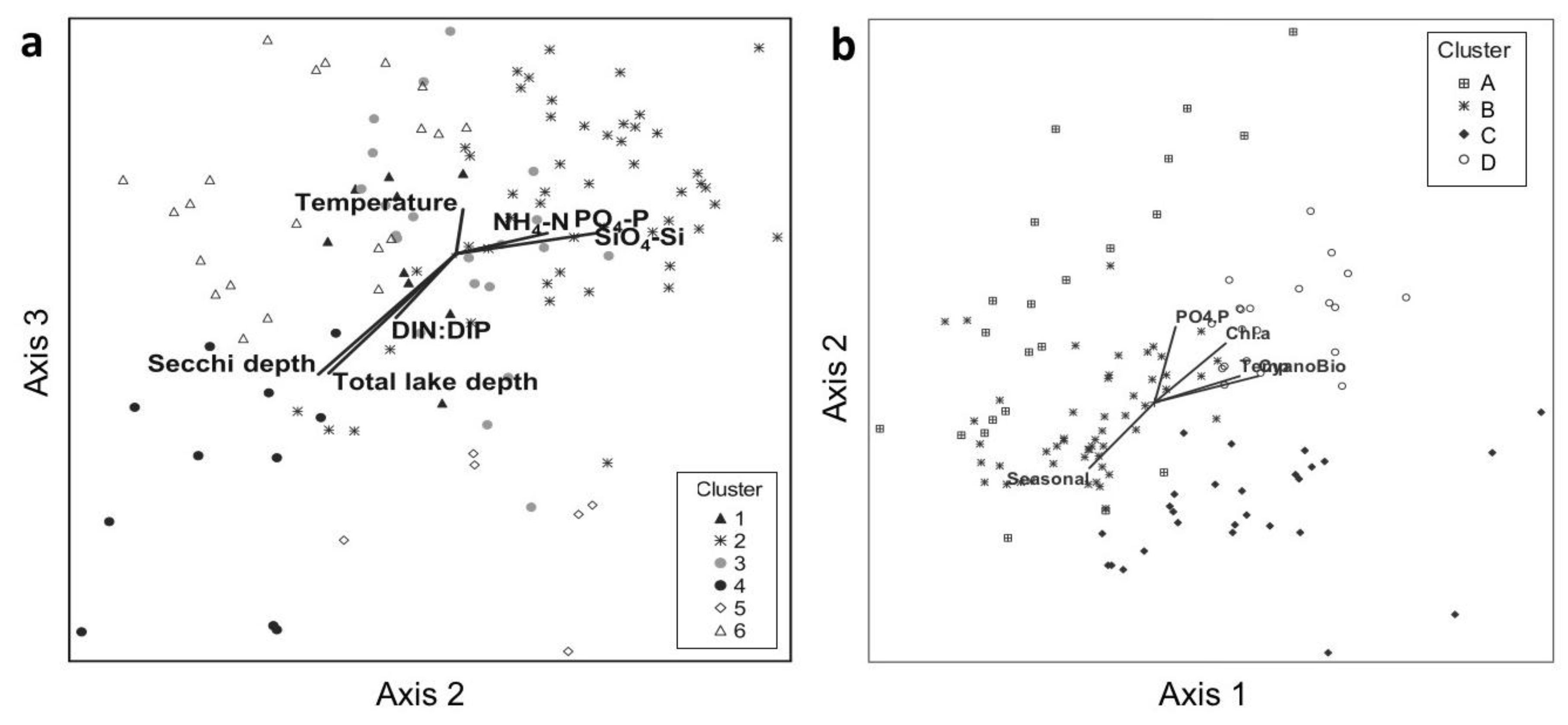
3.2.1. Associations with Abiotic Factors
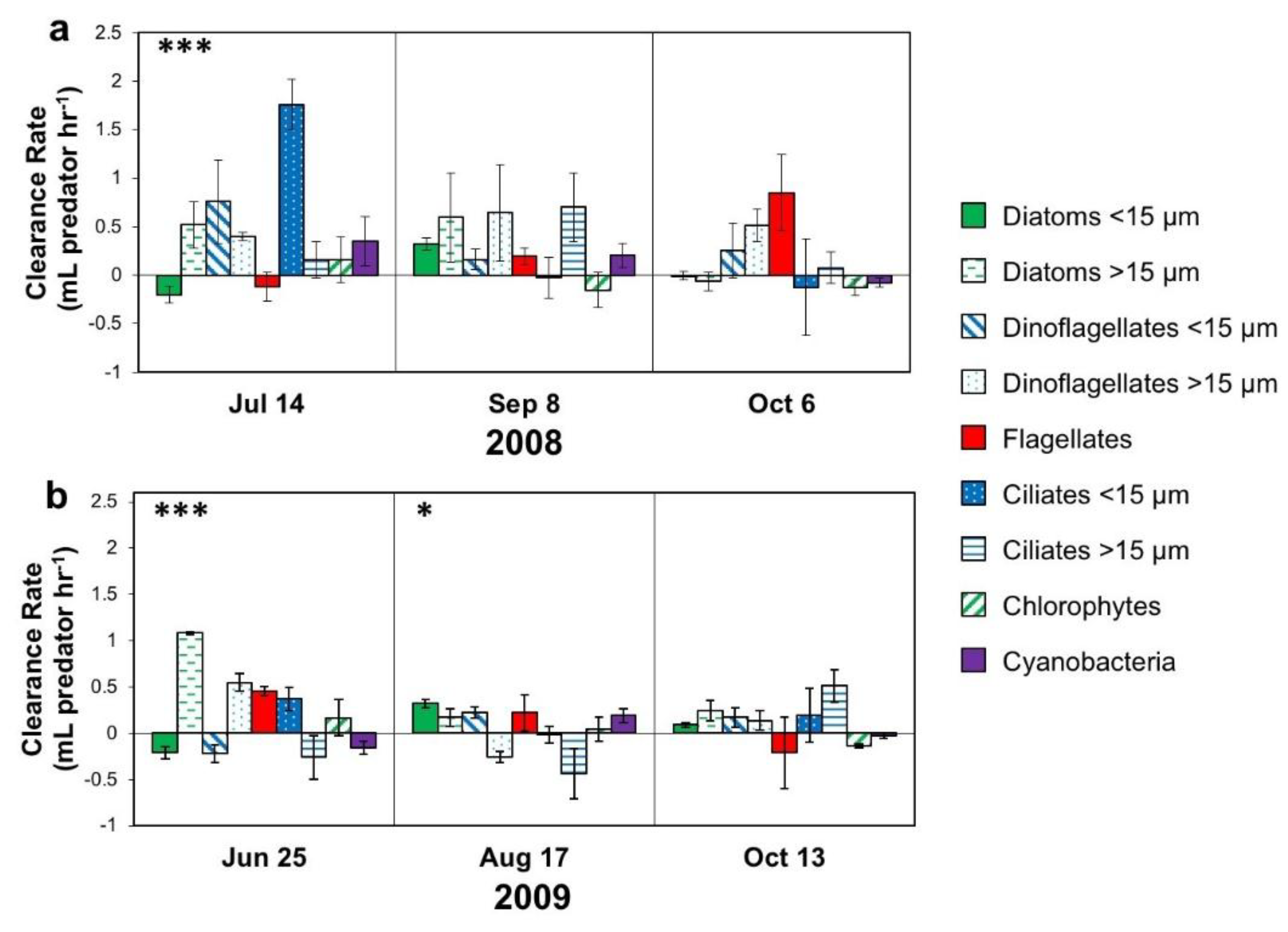
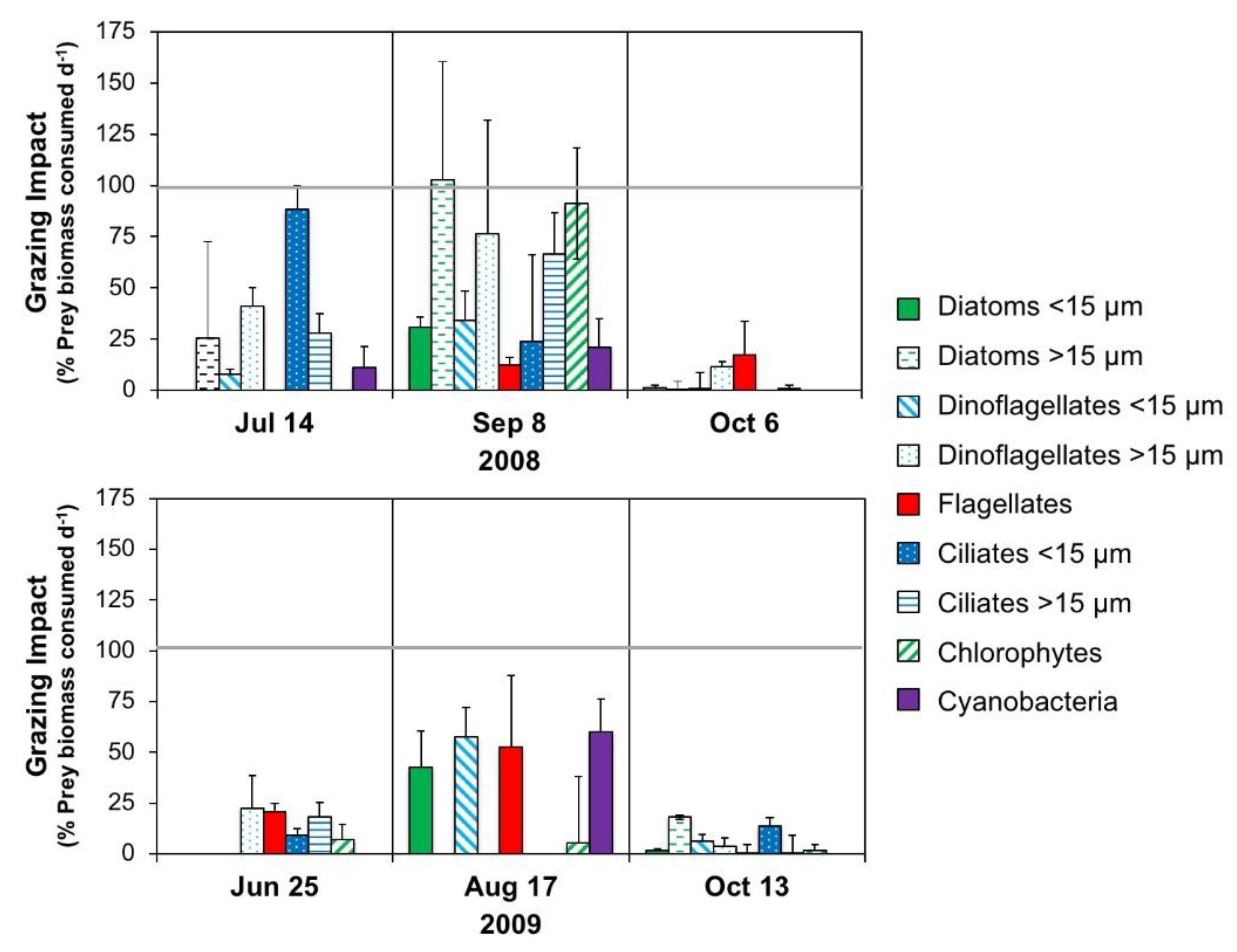
3.2.2. Associations with Biotic Factors
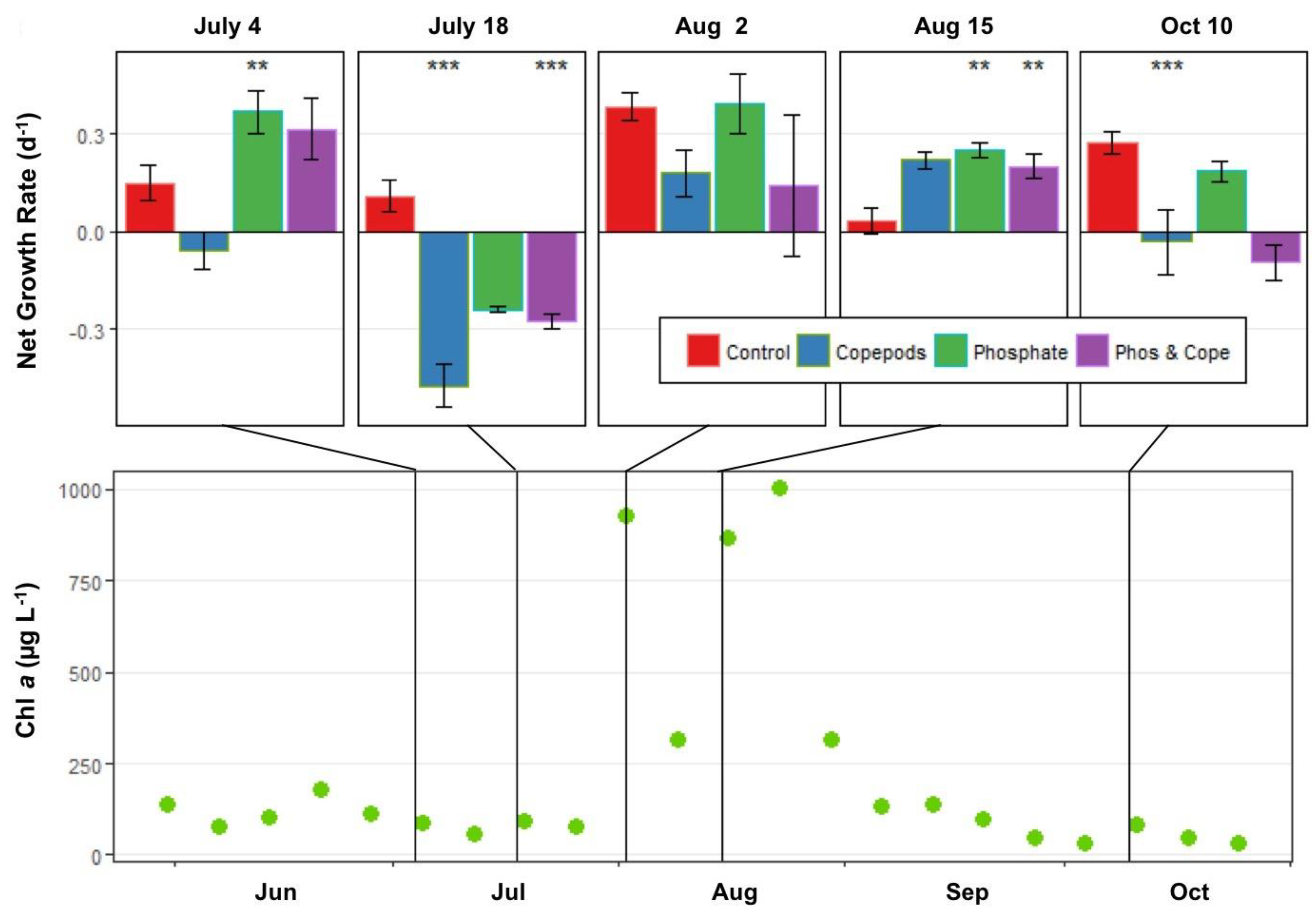
3.2.3. Interactive Effects of Copepod Grazing and Orthophosphate on Cyanobacterial Bloom Dynamics
4. Discussion
4.1. Patterns of Plankton Community Change in Vancouver Lake
4.2. Role of Eutrophication on Cyanobacterial Blooms in Vancouver Lake
4.3. The Role of Zooplankton Grazing and Predation on Cyanobacterial Bloom Dynamics in Vancouver Lake
4.4. Interactive Effects of Eutrophication and Zooplankton Trophic Cascades on Cyanobacterial Blooms
4.5. Vancouver Lake as a Model System for Investigating Multiple, Interacting Drivers of Cyanobacterial Bloom Dynamics in Shallow, Temperate Lakes
4.6. Implications of Results for Management of Future Blooms in Vancouver Lake and Elsewhere
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- The Ecology of Cyanobacteria: Their Diversity in Time and Space; Whitton, B.A.; Potts, M. (Eds.) Springer: Dordrecht, The Netherlands, 2000; ISBN 978-0-7923-4735-4. [Google Scholar]
- Havens, K.E. Cyanobacteria blooms: Effects on aquatic ecosystems. Adv. Exp. Med. Biol. 2008, 619, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I.; Bartram, J. (Eds.) E & FN Spon: London, UK; New York, NY, USA, 1999; ISBN 978-0-419-23930-7. [Google Scholar]
- Sigee, D.C.; Glenn, R.; Andrews, M.J.; Bellinger, E.G.; Butler, R.D.; Epton, H.A.S.; Hendry, R.D. Biological control of cyanobacteria: Principles and possibilities. Hydrobiologia 1999, 395, 161–172. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glibert, P.; Seitzinger, S.; Heil, C.; Burkholder, J.; Parrow, M.; Codispoti, L.; Kelly, V. The Role of Eutrophication in the Global Proliferation of Harmful Algal Blooms. Oceanography 2005, 18, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, J.M.; Kann, J. The occurrence and response to toxic cyanobacteria in the Pacific Northwest, North America. Lake Reserv. Manag. 2007, 23, 123–143. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It Takes Two to Tango: When and Where Dual Nutrient (N & P) Reductions Are Needed to Protect Lakes and Downstream Ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for Control of Anthropogenic Nitrogen and Phosphorus to Reduce Eutrophication of Inland Waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Mitrovic, S.M. Phytoplankton co-limitation by nitrogen and phosphorus in a shallow reservoir: Progressing from the phosphorus limitation paradigm. Hydrobiologia 2015, 744, 255–269. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, I.; Bordet, F.; Chaparro, G. Bloom forming cyanobacterial complexes co-occurring in a subtropical large reservoir: Validation of dominant eco-strategies. Hydrobiologia 2012, 698, 175–190. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; Meester, L.D.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; Donk, E.; Hansson, L.-A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Trolle, D.; Søndergaard, M.; Lauridsen, T.L.; Beklioglu, M.; Brucet, S.; Volta, P.; González-Bergonzoni, I.; et al. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limnol. 2014, 73. [Google Scholar] [CrossRef] [Green Version]
- Coles, J.F.; Jones, R.C. Effect of temperature on photosynthesis-light response and growth of four phytoplankton species isolated from a tidal freshwater river. J. Phycol. 2000, 36, 7–16. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton. Available online: https://www.cambridge.org/core/books/the-ecology-of-phytoplankton/7E14FD43792ECC717C9E90E3519A1803 (accessed on 17 March 2018).
- Paerl, H.W.; Huisman, J. Climate. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling cyanobacterial harmful blooms in freshwater ecosystems. Microb. Biotechnol. 2017, 10, 1106–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Lampert, W. Laboratory studies on zooplankton-cyanobacteria interactions. N. Z. J. Mar. Freshw. Res. 1987, 21, 483–490. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Gustafsson, S.; Rengefors, K.; Bomark, L. Cyanobacterial chemical warfare affects zooplankton community composition. Freshw. Biol. 2007, 52, 1290–1301. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Sarnelle, O. Initial conditions mediate the interaction between Daphnia and bloom-forming cyanobacteria. Limnol. Oceanogr. 2007, 52, 2120–2127. [Google Scholar] [CrossRef] [Green Version]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) Model: Mechanisms Driving Plankton Succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Ekvall, M.K.; Urrutia-Cordero, P.; Hansson, L.-A. Linking Cascading Effects of Fish Predation and Zooplankton Grazing to Reduced Cyanobacterial Biomass and Toxin Levels Following Biomanipulation. PLoS ONE 2014, 9, e112956. [Google Scholar] [CrossRef] [PubMed]
- Knisely, K.; Geller, W. Selective Feeding of Four Zooplankton Species on Natural Lake Phytoplankton. Oecologia 1986, 69, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Panosso, R.; Lurling, M. Daphnia magna feeding on Cylindrospermopsis raciborskii: The role of food composition, filament length and body size. J. Plankton Res. 2010, 32, 1393–1404. [Google Scholar] [CrossRef]
- Epp, G.T. Grazing on filamentous cyanobacteria by Daphnia pulicaria. Limnol. Oceanogr. 1996, 41, 560–567. [Google Scholar] [CrossRef] [Green Version]
- Ghadouani, A.; Pinel-Alloul, B.; Prepas, E.E. Effects of experimentally induced cyanobacterial blooms on crustacean zooplankton communities. Freshw. Biol. 2003, 48, 363–381. [Google Scholar] [CrossRef]
- Hong, Y.; Burford, M.A.; Ralph, P.J.; Udy, J.W.; Doblin, M.A. The cyanobacterium Cylindrospermopsis raciborskii is facilitated by copepod selective grazing. Harmful Algae 2013, 29, 14–21. [Google Scholar] [CrossRef]
- Cowles, T.J.; Olson, R.J.; Chisholm, S.W. Food selection by copepods: Discrimination on the basis of food quality. Mar. Biol. 1988, 100, 41–49. [Google Scholar] [CrossRef]
- Olson, M.; Lessard, E.; Wong, C.; Bernhardt, M. Copepod feeding selectivity on microplankton, including the toxigenic diatoms Pseudo-nitzschia spp., in the coastal Pacific Northwest. Mar. Ecol. Prog. Ser. 2006, 326, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Rollwagen-Bollens, G.; Bollens, S.; Gonzalez, A.; Zimmerman, J.; Lee, T.; Emerson, J. Feeding dynamics of the copepod Diacyclops thomasi before, during and following filamentous cyanobacteria blooms in a large, shallow temperate lake. Hydrobiologia 2013, 705, 101–118. [Google Scholar] [CrossRef]
- Bowen, A.; Rollwagen-Bollens, G.; Bollens, S.M.; Zimmerman, J. Feeding of the invasive copepod Pseudodiaptomus forbesi on natural microplankton assemblages within the lower Columbia River. J. Plankton Res. 2015, fbv078. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Ekvall, M.K.; Hansson, L.-A. Responses of cyanobacteria to herbivorous zooplankton across predator regimes: Who mows the bloom? Freshw. Biol. 2015, 60, 960–972. [Google Scholar] [CrossRef]
- Rose, V.; Rollwagen-Bollens, G.; Bollens, S.M. Interactive effects of phosphorus and zooplankton grazing on cyanobacterial blooms in a shallow temperate lake. Hydrobiologia 2017, 788, 345–359. [Google Scholar] [CrossRef]
- Jeppesen, E.; Sondergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake responses to reduced nutrient loading—An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T. Throwing Fuel on the Fire: Synergistic Effects of Excessive Nitrogen Inputs and Global Warming on Harmful Algal Blooms. Environ. Sci. Technol. 2010, 44, 7756–7758. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Orsborn, J. A Summary Report on Water Quantity and Quality Studies of Vancouver Lake; Washington State University: Pullman, WA, USA, 1971. [Google Scholar]
- Gorini, R. Lake restoration by dredging. In Proceedings of the Management of Bottom Sediments Containing Toxic Substances: Proceedings of the U.S./Japan Experts Meeting (13th), Baltimore, MD, USA, 3–5 November 1987; pp. 355–360. [Google Scholar]
- Sheibley, R.; Foreman, J.; Marshall, C.; Welch, W. Water and Nutrient Budgets for Vancouver Lake, Vancouver, Washington, October 2010–October 2012; U.S. Geological Survey Scientific Investigations Report 2014-5201; U.S. Geological Survey: Reston, VA, USA, 2014.
- Lee, T.A.; Rollwagen-Bollens, G.; Bollens, S.M.; Faber-Hammond, J.J. Environmental influence on cyanobacteria abundance and microcystin toxin production in a shallow temperate lake. Ecotoxicol. Environ. Saf. 2015, 114, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Vancouver Lake Watershed Partnership. Vancouver Lake Partnership Planning Process and Recommendations Report 2013. Available online: http://www.vancouverlakepartnership.org/Partnership%20Planning%20Process%20and%20Recommendations%20Report.pdf.
- Strickland, J.; Parsons, T. A practical Handbook for Seawater Analysis; Fisheries Research Board of Canada Bulletin: Ottawa, ON, Canada, 1972; Volume 167. [Google Scholar]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Academic Press: Cambridge, MA, USA, 2010; ISBN 978-0-12-374855-3. [Google Scholar]
- Lee, T.A.; Rollwagen-Bollens, G.; Bollens, S.M. The influence of water quality variables on cyanobacterial blooms and phytoplankton community composition in a shallow temperate lake. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.A.; Bollens, S.M.; Rollwagen-Bollens, G.; Emerson, J.E. The effects of eutrophication and invasive species on zooplankton community dynamics in a shallow temperate lake. Fundam. Appl. Limnol. Für Hydrobiol. 2016, 188, 215–231. [Google Scholar] [CrossRef]
- Stroud, J.T.; Bush, M.R.; Ladd, M.C.; Nowicki, R.J.; Shantz, A.A.; Sweatman, J. Is a community still a community? Reviewing definitions of key terms in community ecology. Ecol. Evol. 2015, 5, 4757–4765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Mielke, P.W.; Berry, K.J.; Brier, G.W. Application of Multi-Response Permutation Procedures for Examining Seasonal Changes in Monthly Mean Sea-Level Pressure Patterns. Mon. Weather Rev. 1981, 109, 120–126. [Google Scholar] [CrossRef] [Green Version]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; ISBN 978-0-9721290-0-8. [Google Scholar]
- Dufrene, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Landry, M.R.; Hassett, R.P. Estimating the grazing impact of marine micro-zooplankton. Mar. Biol. 1982, 67, 283–288. [Google Scholar] [CrossRef]
- Landry, M.R.; Kirshtein, J.; Constantinou, J. A refined dilution technique for measuring the community grazing impact of microzooplankton, with experimental tests in the central Equatorial Pacific. Mar. Ecol. Prog. Ser. 1995, 120, 53–63. [Google Scholar] [CrossRef]
- Gobler, C.J.; Davis, T.W.; Coyne, K.J.; Boyer, G.L. Interactive influences of nutrient loading, zooplankton grazing, and microcystin synthetase gene expression on cyanobacterial bloom dynamics in a eutrophic New York lake. Harmful Algae 2007, 6, 119–133. [Google Scholar] [CrossRef]
- Boyer, J.; Rollwagen-Bollens, G.; Bollens, S.M. Microzooplankton grazing before, during and after a cyanobacterial bloom in Vancouver Lake, Washington, USA. Aquat. Microb. Ecol. 2011, 64, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Rollwagen-Bollens, G.C.R.; Penry, D.L. Feeding dynamics of Acartia spp. copepods in a large, temperate estuary (San Francisco Bay, CA). Mar. Ecol. Prog. Ser. 2003, 257, 139–158. [Google Scholar] [CrossRef] [Green Version]
- Gifford, S.; Rollwagen-Bollens, G.; Bollens, S. Mesozooplankton omnivory in the upper San Francisco Estuary. Mar. Ecol. Prog. Ser. 2007, 348, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Marin, V.; Huntley, M.; Frost, B.W. Measuring feeding rates of pelagic herbivores: Analysis of experimental design and methods. Mar. Biol. 1986, 93, 49–58. [Google Scholar] [CrossRef]
- Zar, J. Biostatistical Analysis, 3rd ed.; Pearson: Hong Kong, China, 1996. [Google Scholar]
- Vanderploeg, H.A.; Scavia, D. Two Electivity Indices for Feeding with Special Reference to Zooplankton Grazing. J. Fish. Res. Board Can. 1979, 36, 362–365. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Scavia, D. Calculation and use of selectivity coefficients of feeding: Zooplankton grazing. Ecol. Model. 1979, 7, 135–149. [Google Scholar] [CrossRef]
- Bollens, S.M.; Breckenridge, J.K.; Cordell, J.R.; Rollwagen-Bollens, G.C.; Kalata, O. Invasive copepods in the Lower Columbia River Estuary: Seasonal abundance, co-occurrence and potential competition with native copepods. Aquat. Invasions 2012, 7, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Breckenridge, J.K.; Bollens, S.M.; Rollwagen-Bollens, G.; Roegner, G.C. Plankton Assemblage Variability in a River-Dominated Temperate Estuary During Late Spring (High-flow) and Late Summer (Low-flow) Periods. Estuar. Coasts 2015, 38, 93–103. [Google Scholar] [CrossRef]
- Emerson, J.; Bollens, S.; Counihan, T. Seasonal dynamics of zooplankton in Columbia–Snake River reservoirs, with special emphasis on the invasive copepod Pseudodiaptomus forbesi. Aquat. Invasions 2015, 10, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Dexter, E.; Bollens, S.M.; Rollwagen-Bollens, G.; Emerson, J.; Zimmerman, J. Persistent vs. ephemeral invasions: 8.5 years of zooplankton community dynamics in the Columbia River: Persistent vs. ephemeral invasions. Limnol. Oceanogr. 2015, 60, 527–539. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Kent, A.D.; Yannarell, A.C.; Rusak, J.A.; Triplett, E.W.; McMahon, K.D. Synchrony in aquatic microbial community dynamics. ISME J. 2007, 1, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadda, A.; Markova, S.; Kotlik, P.; Luglie, A.; Padedda, B.; Buscarinu, P.; Sechi, N.; Manca, M. First record of planktonic crustaceans in Sardinian reservoirs. Biologia 2011, 66, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Lemke, M.J.; Paver, S.F.; Dungey, K.E.; Velho, L.F.M.; Kent, A.D.; Rodrigues, L.C.; Kellerhals, D.M.; Randle, M.R. Diversity and succession of pelagic microorganism communities in a newly restored Illinois River floodplain lake. Hydrobiologia 2017, 804, 35–58. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Yang, J.; Liu, L.; Yu, X.; Yu, Z.; Chiang, P. Temperature and nutrients are significant drivers of seasonal shift in phytoplankton community from a drinking water reservoir, subtropical China. Environ. Sci. Pollut. Res. 2014, 21, 5917–5928. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.; Galvão, H.; Barbosa, A. Role of transient silicon limitation in the development of cyanobacteria blooms in the Guadiana estuary, south-western Iberia. Mar. Ecol. Prog. Ser. 2002, 228, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Hudnell, H.K.; Dortch, Q.; Zenick, H. An overview of the interagency, international symposium on cyanobacterial harmful algal blooms (ISOC-HAB): Advancing the scientific understanding of freshwater harmful algal blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–16. [Google Scholar]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Padedda, B.M.; Sechi, N.; Lai, G.G.; Mariani, M.A.; Pulina, S.; Sarria, M.; Satta, C.T.; Virdis, T.; Buscarinu, P.; Lugliè, A. Consequences of eutrophication in the management of water resources in Mediterranean reservoirs: A case study of Lake Cedrino (Sardinia, Italy). Glob. Ecol. Conserv. 2017, 12, 21–35. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.; Glibert, P.M.; Wang, Y.; Tong, M. Rates of nitrogen uptake by cyanobacterially-dominated assemblages in Lake Taihu, China, during late summer. Harmful Algae 2017, 65, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.A.; Padedda, B.M.; Kaštovský, J.; Buscarinu, P.; Sechi, N.; Virdis, T.; Lugliè, A. Effects of trophic status on microcystin production and the dominance of cyanobacteria in the phytoplankton assemblage of Mediterranean reservoirs. Sci. Rep. 2015, 5, 17964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, J.M.; Collier, D.C.; Welch, E.B.; Hardy, F.J.; Crayton, M. Environmental factors associated with a toxic bloom of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 2000, 57, 231–240. [Google Scholar] [CrossRef]
- Rapala, J.; Sivonen, K.; Lyra, C. Variation of Microcystins, Cyanobacterial Hepatotoxins, in Anabaena spp. as a Function of Growth Stimuli. Appl. Environ. Microbiol. 1997, 63, 2206–2212. [Google Scholar] [PubMed]
- Leonard, J.A.; Paerl, H.W. Zooplankton community structure, micro-zooplankton grazing impact, and seston energy content in the St. Johns river system, Florida as influenced by the toxic cyanobacterium Cylindrospermopsisraciborskii. Hydrobiologia 2005, 537, 89–97. [Google Scholar] [CrossRef]
- Davis, T.W.; Koch, F.; Marcoval, M.A.; Wilhelm, S.W.; Gobler, C.J. Mesozooplankton and microzooplankton grazing during cyanobacterial blooms in the western basin of Lake Erie. Harmful Algae 2012, 15, 26–35. [Google Scholar] [CrossRef]
- Landry, M.; Selph, K.; Yang, E.-J. Decoupled phytoplankton growth and microzooplankton grazing in the deep euphotic zone of the eastern equatorial Pacific. Mar. Ecol. Prog. Ser. 2011, 421, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Calbet, A.; Saiz, E.; Almeda, R.; Movilla, J.I.; Alcaraz, M. Low microzooplankton grazing rates in the Arctic Ocean during a Phaeocystis pouchetii bloom (Summer 2007): Fact or artifact of the dilution technique? J. Plankton Res. 2011, 33, 687–701. [Google Scholar] [CrossRef]
- Calbet, A.; Saiz, E. Effects of trophic cascades in dilution grazing experiments: From artificial saturated feeding responses to positive slopes. J. Plankton Res. 2013, 35, 1183–1191. [Google Scholar] [CrossRef]
- Leitão, E.; Ger, K.A.; Panosso, R. Selective Grazing by a Tropical Copepod (Notodiaptomus iheringi) Facilitates Microcystis Dominance. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Acharya, K.; Stone, M.C.; Yu, Z.; Young, M.H.; Shafer, D.S.; Zhu, J.; Gray, K.; Stone, A.; Fan, L.; et al. Spatiotemporal patterns in nutrient loads, nutrient concentrations, and algal biomass in Lake Taihu, China. Lake Reserv. Manag. 2011, 27, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Kane, D.D.; Conroy, J.D.; Richards, R.P.; Baker, D.B.; Culver, D.A. Re-eutrophication of Lake Erie: Correlations between tributary nutrient loads and phytoplankton biomass. J. Gt. Lakes Res. 2014, 40, 496–501. [Google Scholar] [CrossRef]
- Reavie, E.D.; Cai, M.; Twiss, M.R.; Carrick, H.J.; Davis, T.W.; Johengen, T.H.; Gossiaux, D.; Smith, D.E.; Palladino, D.; Burtner, A.; et al. Winter–spring diatom production in Lake Erie is an important driver of summer hypoxia. J. Great Lakes Res. 2016, 42, 608–618. [Google Scholar] [CrossRef]
- McCarthy, M.J.; James, R.T.; Chen, Y.; East, T.L.; Gardner, W.S. Nutrient ratios and phytoplankton community structure in the large, shallow, eutrophic, subtropical Lakes Okeechobee (Florida, USA) and Taihu (China). Limnology 2009, 10, 215–227. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of Phosphorus Limitation in Lakes. Sci. New Ser. 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, V.H. Low Nitrogen to Phosphorus Ratios Favor Dominance by Blue-Green Algae in Lake Phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Galford, A.E.; Chrzanowski, T.H.; Findlay, D.L.; Mills, K.H.; Paterson, M.J.; Stainton, M.P.; Schindler, D.W. Pelagic C:N:P Stoichiometry in a Eutrophied Lake: Responses to a Whole-Lake Food-Web Manipulation. Ecosystems 2000, 3, 293–307. [Google Scholar] [CrossRef]
- Bormans, M. Spatial and temporal variability in cyanobacterial populations controlled by physical processes. J. Plankton Res. 2004, 27, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Gobler, C.J. Grazing by mesozooplankton and microzooplankton on toxic and non-toxic strains of Microcystis in the Transquaking River, a tributary of Chesapeake Bay. J. Plankton Res. 2011, 33, 415–430. [Google Scholar] [CrossRef]
- Sterner, R.W. The Role of Grazers in Phytoplankton Succession. In Plankton Ecology; Brock/Springer Series in Contemporary Bioscience; Springer: Berlin/Heidelberg, Germany, 1989; pp. 107–170. ISBN 978-3-642-74892-9. [Google Scholar]
- Hambright, K.D.; Zohary, T.; Easton, J.; Azoulay, B.; Fishbein, T. Effects of zooplankton grazing and nutrients on the bloom-forming, N2-fixing cyanobacterium Aphanizomenon in Lake Kinneret. J. Plankton Res. 2001, 23, 165–174. [Google Scholar] [CrossRef]
- Smith, K.F.; Lester, P.J. Trophic interactions promote dominance by cyanobacteria (Anabaena spp.) in the pelagic zone of Lower Karori Reservoir, Wellington, New Zealand. N. Z. J. Mar. Freshw. Res. 2007, 41, 143–155. [Google Scholar] [CrossRef]
- Interagency Working Group on the Harmful Algal Bloom and Hypoxia Research and Control Act (IWG-HABHRCA). Harmful Algal Blooms and Hypoxia Comprehensive Research Plan and Action Strategy: An Interagency Report; US Office of Science and Technology Policy: Washington, DC, USA, 2016; p. 94. [Google Scholar]
- Du Plooy, S.J.; Carrasco, N.K.; Perissinotto, R. Effects of zooplankton grazing on the bloom-forming Cyanothece sp. in a subtropical estuarine lake. J. Plankton Res. 2017, 39, 826–835. [Google Scholar] [CrossRef]
- Goleski, J.A.; Koch, F.; Marcoval, M.A.; Wall, C.C.; Jochem, F.J.; Peterson, B.J.; Gobler, C.J. The Role of Zooplankton Grazing and Nutrient Loading in the Occurrence of Harmful Cyanobacterial Blooms in Florida Bay, USA. Estuar. Coasts 2010, 33, 1202–1215. [Google Scholar] [CrossRef]
- Buskey, E.J. How does eutrophication affect the role of grazers in harmful algal bloom dynamics? Harmful Algae 2008, 8, 152–157. [Google Scholar] [CrossRef]
- Elser, J.J. The pathway to noxious cyanobacteria blooms in lakes: The food web as the final turn. Freshw. Biol. 1999, 42, 537–543. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling harmful cyanobacterial blooms in a climatically more extreme world: Management options and research needs. J. Plankton Res. 2017, 39, 763–771. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Olesen, J.E.; Audet, J.; Søndergaard, M.; Hoffmann, C.C.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Larsen, S.E.; et al. Climate change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 2011, 663, 1–21. [Google Scholar] [CrossRef]
- Cooke, G.D.; Welch, E.B.; Peterson, S.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; CRC Press: Bocaton, FL, USA, 2016; ISBN 978-1-4200-3210-9. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; Özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a Restoration Tool to Combat Eutrophication. In Advances in Ecological Research; Elsevier: New York, NY, USA, 2012; Volume 47, pp. 411–488. ISBN 978-0-12-398315-2. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollwagen-Bollens, G.; Lee, T.; Rose, V.; Bollens, S.M. Beyond Eutrophication: Vancouver Lake, WA, USA as a Model System for Assessing Multiple, Interacting Biotic and Abiotic Drivers of Harmful Cyanobacterial Blooms. Water 2018, 10, 757. https://doi.org/10.3390/w10060757
Rollwagen-Bollens G, Lee T, Rose V, Bollens SM. Beyond Eutrophication: Vancouver Lake, WA, USA as a Model System for Assessing Multiple, Interacting Biotic and Abiotic Drivers of Harmful Cyanobacterial Blooms. Water. 2018; 10(6):757. https://doi.org/10.3390/w10060757
Chicago/Turabian StyleRollwagen-Bollens, Gretchen, Tammy Lee, Vanessa Rose, and Stephen M. Bollens. 2018. "Beyond Eutrophication: Vancouver Lake, WA, USA as a Model System for Assessing Multiple, Interacting Biotic and Abiotic Drivers of Harmful Cyanobacterial Blooms" Water 10, no. 6: 757. https://doi.org/10.3390/w10060757




