Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Experimental Methodology
2.2.1. Preparation of Solutions
2.2.2. Batch Experiments
2.2.3. Jar Test
2.2.4. Analytical Procedures
3. Results and Discussion
3.1. Effect of Solution pH on Fe Solubility and Antimony Removal
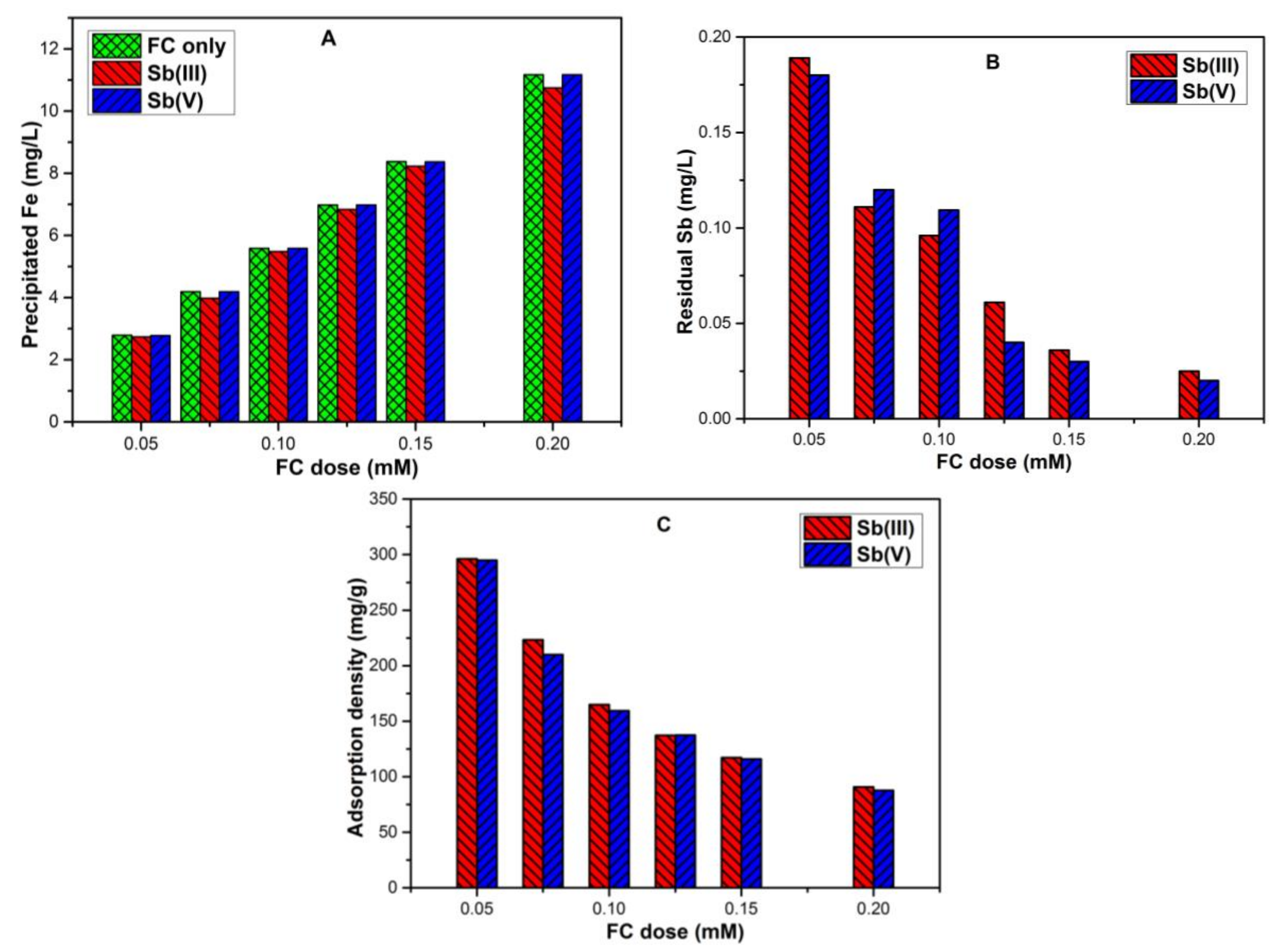
3.2. Effect of Different Ferric Chloride Coagulation (FC) Dose on Fe Solubility and Antimony Removal
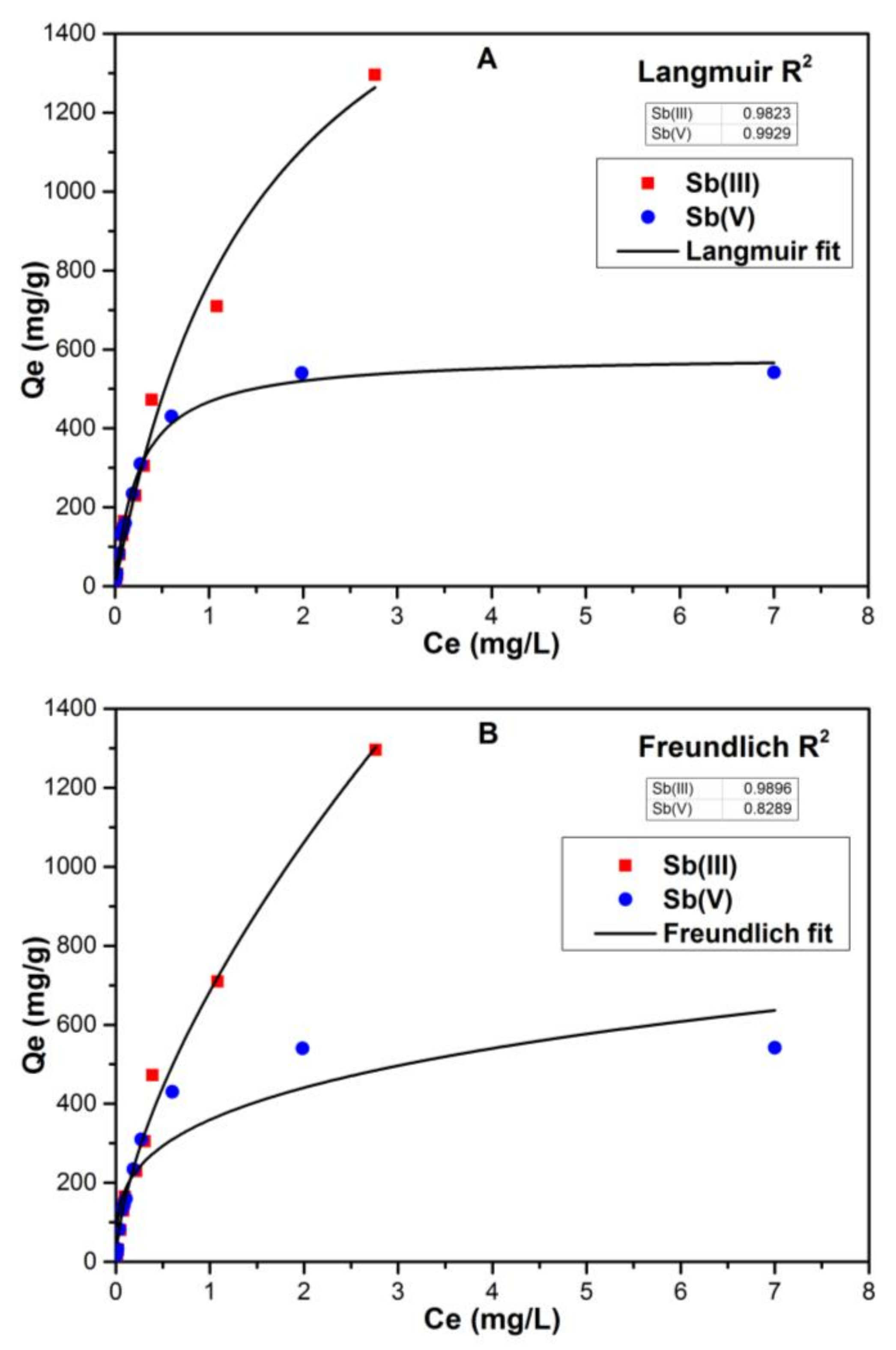
3.3. Modelling Coagulation Data by Isotherm Studies
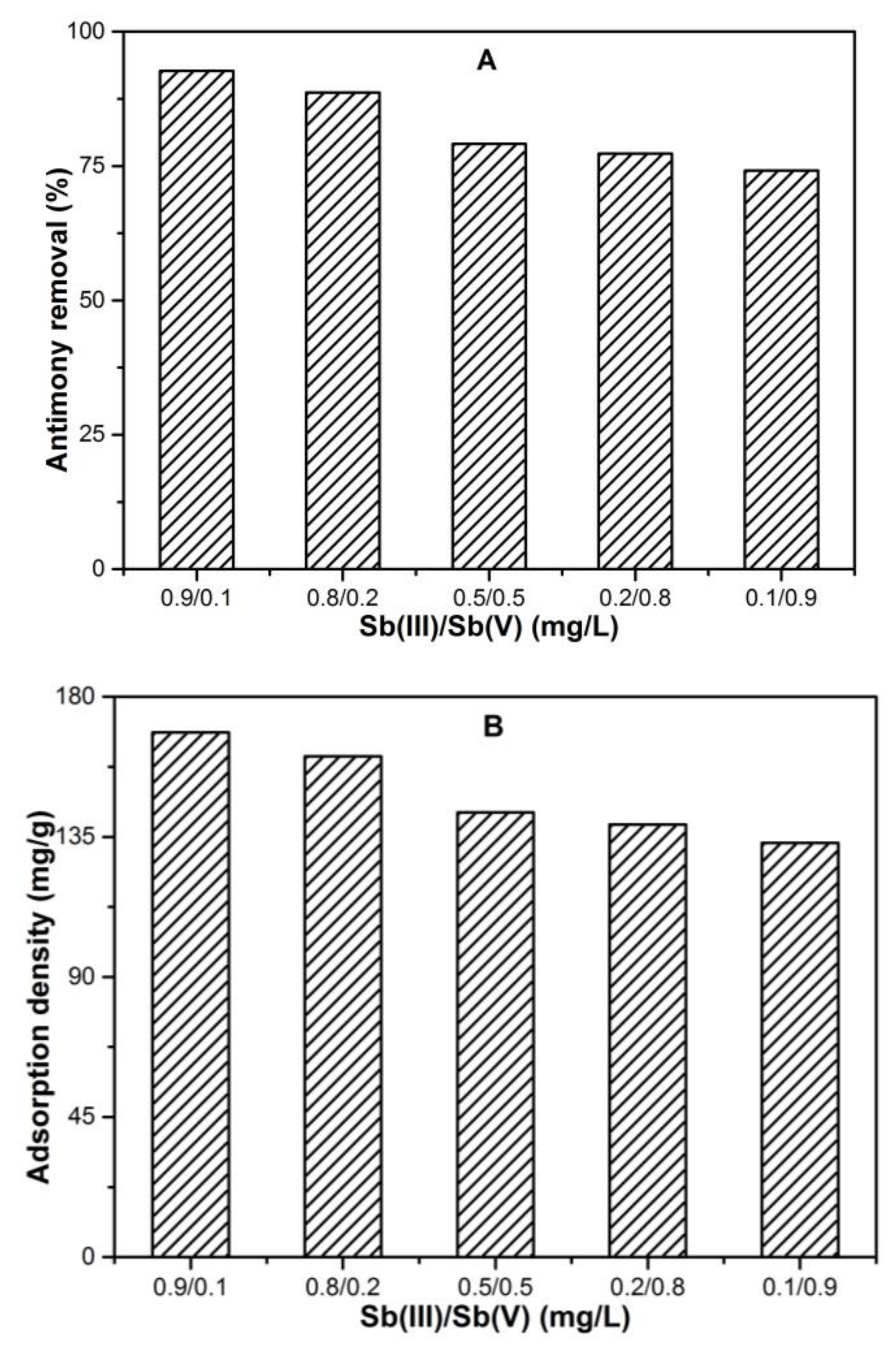
3.4. Effect of Coexisting Sb(III)-Sb(V) Species Ratio on Fe Solubility and Total Sb Removal
3.4.1. Antimony Adsorption Performance
3.4.2. Fourier Transform Infrared (FT–IR) Spectra Analysis
3.4.3. The Origin of the Significant Effect of the Sb(III)/Sb(V) Mixed Fractions on Antimony Removal
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miao, Y.; Han, F.; Pan, B.; Niu, Y.; Nie, G.; Lv, L. Antimony (V) removal from water by hydrated ferric oxides supported by calcite sand and polymeric anion exchanger. J. Environ. Sci. 2014, 26, 307–314. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters II. Relevant solution chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M. Removal of antimony (V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, X.; Wu, F.; Fu, Z. Antimony pollution in China. Sci. Total Environ. 2012, 421–422, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, H.; Chai, L.-Y.; Mirza, N.; Yang, Z.-H.; Pervez, A.; Tariq, M.; Shaheen, S.; Mahmood, Q. Antimony (Sb)—Pollution and removal techniques—Critical assessment of technologies. Toxicol. Environ. Chem. 2015, 97, 1296–1318. [Google Scholar] [CrossRef]
- Wang, L.; Wan, C.; Zhang, Y.; Lee, D.-J.; Liu, X.; Chen, X.; Tay, J.-H. Mechanism of enhanced Sb(V) removal from aqueous solution using chemically modified aerobic granules. J. Hazard. Mater. 2015, 284, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, M.; Lin, C.; Gao, Y.; Zheng, L. Antimony(III) oxidation and antimony(V) adsorption reactions on synthetic manganite. Chem. Erde Geochem. 2012, 72, 41–47. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb (III) and Sb (V) to goethite: Influence on Sb (III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Flynn, H.C.; Meharg, A.A.; Bowyer, P.K.; Paton, G.I. Antimony bioavailability in mine soils. Environ. Pollut. 2003, 124, 93–100. [Google Scholar] [CrossRef]
- He, M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ. Geochem. Health 2007, 29, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.K.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Hiller, E.; Lalinská, B.; Chovan, M.; Jurkovič, Ľ.; Klimko, T.; Jankulár, M.; Hovorič, R.; Šottník, P.; Fľaková, R.; Ženišová, Z.; et al. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl. Geochem. 2012, 27, 598–614. [Google Scholar] [CrossRef]
- Ritchie, V.J.; Ilgen, A.G.; Mueller, S.H.; Trainor, T.P.; Goldfarb, R.J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem. Geol. 2013, 335, 172–188. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Xi, J.; Lu, X. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Salmon, K.; DuBow, M.S. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology 1998, 144, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.J.; Zika, R.J.; Petasne, R.G.; Fischer, A.M. Sunlight-Induced Photochemistry of Humic Substances in Natural Waters: Major Reactive Species. Adv. Chem. 1989, 219, 333–362. [Google Scholar] [CrossRef]
- Rapant, S.; Dietzová, Z.; Cicmanová, S. Environmental and health risk assessment in abandoned mining area, Zlata Idka, Slovakia. Environ. Geol. 2006, 51, 387–397. [Google Scholar] [CrossRef]
- Gebel, T. Aresnic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- Jo, M.; Kim, T.; Choi, S.; Jung, J.; Song, H.; Lee, H.; Park, G.; Lim, S.; Sung, Y.; Oh, J. Investigation of Antimony in Natural Water and Leaching from Polyethylene Terephthalate (PET) Bottled Water. In Proceedings of the 3rd World Congress on New Technologies (NewTech’17), Rome, Italy, 6–8 June 2017. [Google Scholar]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W.; Yang, Q.; Chen, W. Chemical coagulation process for the removal of heavy metals from water: A review. Desalination Water Treat. 2014, 57, 1733–1748. [Google Scholar] [CrossRef]
- Kang, M.; Kamei, T.; Magara, Y. Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res. 2003, 37, 4171–4179. [Google Scholar] [CrossRef]
- Deng, R.-J.; Jin, C.-S.; Ren, B.-Z.; Hou, B.-L.; Hursthouse, A.S. The potential for the treatment of antimony-containing wastewater by iron-based adsorbents. Water 2017, 9, 794. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dou, X.; Li, J. Antimony(V) removal from water by iron-zirconium bimetal oxide: Performance and mechanism. J. Environ. Sci. 2012, 24, 1197–1203. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, R.; Zhao, X.; Qu, J. The mechanism of antimony(III) removal and its reactions on the surfaces of Fe-Mn Binary Oxide. J. Colloid Interface Sci. 2011, 363, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Kang, J.; Shen, J.; Chen, Z.; Fan, L. Influence of humic acid on the removal of arsenate and arsenic by ferric chloride: Effects of pH, As/Fe ratio, initial As concentration, and co-existing solutes. Environ. Sci. Pollut. Res. 2017, 24, 2381–2393. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jiang, Z.; Sun, B.; Sun, Y.; Wang, Q.; Guan, X. Arsenate and arsenite removal by FeCl 3: Effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Sep. Purif. Technol. 2012, 92, 106–114. [Google Scholar] [CrossRef]
- Hering, J.G.; Chen, P.Y.; Wilkie, J.A.; Elimelech, M.; Liang, S. Arsenic removal by ferric chloride. J. Am. Water Work. Assoc. 1996, 88, 155–167. [Google Scholar] [CrossRef]
- Hering, B.J.G.; Member, A.; Chen, P.; Wilkie, J.A.; Elimelech, M. Arsenic removal from drinking water during coagulation. J. Environ. Eng. 1997, 123, 800–807. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, J.; Liu, S.; Li, W.; Van Leeuwen, J.; Mulcahy, D. Removal of As(III) and As(V) by ferric salts coagulation - Implications of particle size and zeta potential of precipitates. Sep. Purif. Technol. 2014, 135, 64–71. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; APHA: Washington, DC, USA, 2005; ISBN 0875530478. [Google Scholar]
- Stumm, W. The Inner-Sphere Surface Complex. Aquat. Chem. 1995, 244, 1–32. [Google Scholar] [CrossRef]
- Zinder, B.; Furrer, G.; Stumm, W. The coordination chemistry of weathering: II. Dissolution of Fe(III) oxides. Geochim. Cosmochim. Acta 1986, 50, 1861–1869. [Google Scholar] [CrossRef]
- Stone, A.T.; Torrents, A.; Smolen, J.; Vasudevan, D.; Hadley, J. Adsorption of Organic Compounds Possessing Ligand Donor Groups at the Oxide/Water Interface. Environ. Sci. Technol. 1993, 27, 895–909. [Google Scholar] [CrossRef]
- Stumm, W. Reactivity at the mineral-water interface: Dissolution and inhibition. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 143–166. [Google Scholar] [CrossRef]
- Stone, T.A. Reaction of extracellular organic ligands with dissolved mental ions and mineral surfaces. Geomicrobiol. Interact. Microbes Miner. 1997, 309–344. [Google Scholar]
- Martin, S. Precipitation and Dissolution of Iron and Manganese Oxides. Environ. Catal. 2005, 61–82. [Google Scholar] [CrossRef]
- Kang, M.; Kawasaki, M.; Tamada, S.; Kamei, T.; Magara, Y. Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 2000, 131, 293–298. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Competitive adsorption of As(III), As(V), Sb(III) and Sb(V) onto ferrihydrite in multi-component systems: Implications for mobility and distribution. J. Hazard. Mater. 2017, 330, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe-Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Luo, J.; Song, S.; Li, Y.; Li, Q. The remarkable effect of the coexisting arsenite and arsenate species ratios on arsenic removal by manganese oxide. Chem. Eng. J. 2017, 315, 159–166. [Google Scholar] [CrossRef]
- Santos, A.F.M.; Macedo, L.J.A.; Chaves, M.H.; Espinoza-Castañeda, M.; Merkoçi, A.; Limac, F.D.C.A.; Cantanhêde, W. Hybrid self-assembled materials constituted by ferromagnetic nanoparticles and tannic acid: A theoretical and experimental investigation. J. Braz. Chem. Soc. 2016, 27, 727–734. [Google Scholar] [CrossRef]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Li, G.T. Removal mechanism of As(III) by a novel Fe-Mn binary oxide adsorbent: Oxidation and sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, M.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Castor leaf mediated synthesis of iron nanoparticles for evaluating catalytic effects in transesterification of castor oil. RSC Adv. 2016, 6, 9261–9269. [Google Scholar] [CrossRef]
- Fan, J.X.; Wang, Y.J.; Fan, T.T.; Cui, X.D.; Zhou, D.M. Photo-induced oxidation of Sb(III) on goethite. Chemosphere 2014, 95, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The Formulas of Antimonic Acid and the Antimonates. J. Am. Chem. Soc. 1933, 55, 1895–1900. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, M.A.; Khan, R.; Park, D.R.; Lee, Y.-W.; Yeom, I.T. Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility. Water 2018, 10, 418. https://doi.org/10.3390/w10040418
Inam MA, Khan R, Park DR, Lee Y-W, Yeom IT. Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility. Water. 2018; 10(4):418. https://doi.org/10.3390/w10040418
Chicago/Turabian StyleInam, Muhammad Ali, Rizwan Khan, Du Ri Park, Yong-Woo Lee, and Ick Tae Yeom. 2018. "Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility" Water 10, no. 4: 418. https://doi.org/10.3390/w10040418





