Groundwater Chemistry Regulated by Hydrochemical Processes and Geological Structures: A Case Study in Tongchuan, China
Abstract
:1. Introduction
2. Study Area
3. Geology and Hydrogeology
4. Methodology
4.1. Sampling and Analysis
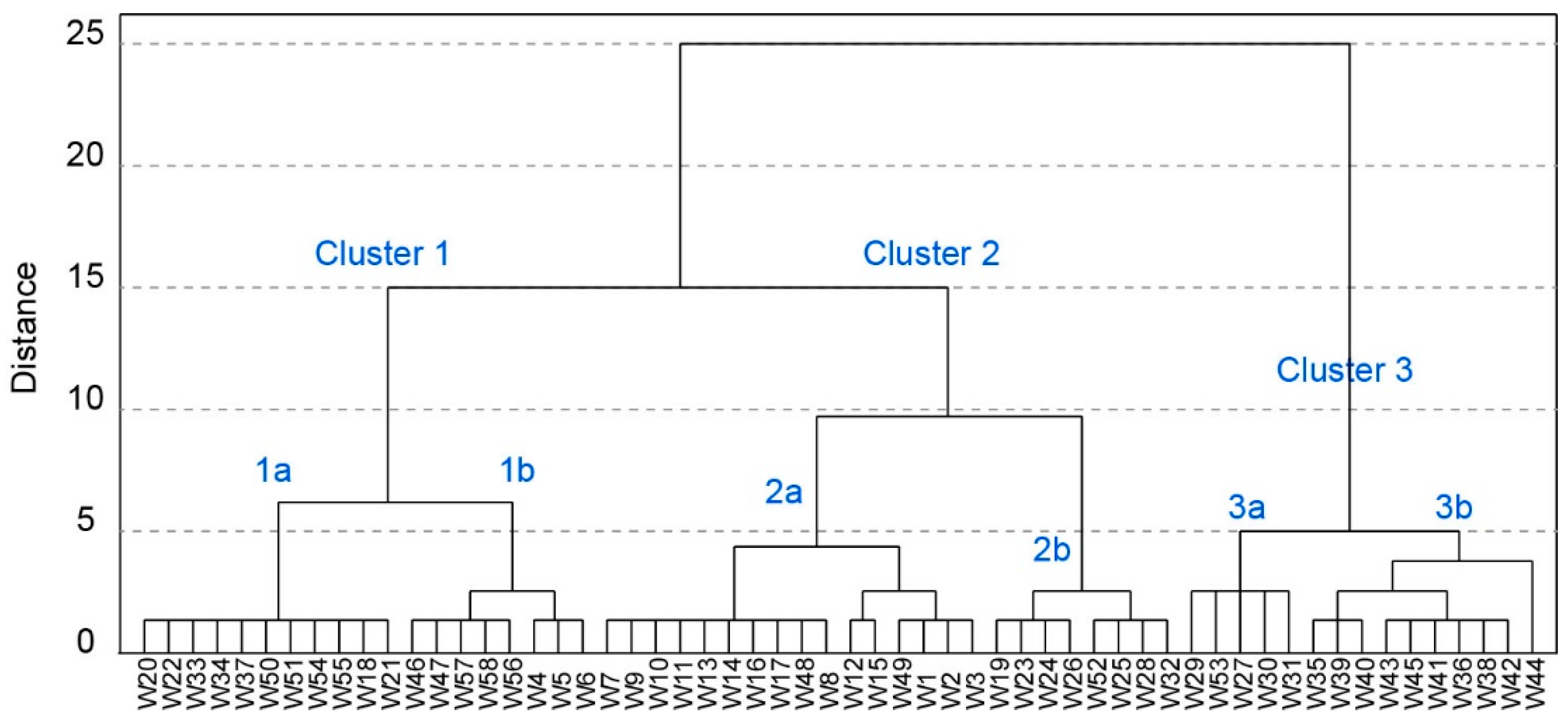
4.2. Hierarchical Cluster Analysis
4.3. Hydrochemical Processes
4.4. Stable Isotopic Analysis
5. Result and Discussion
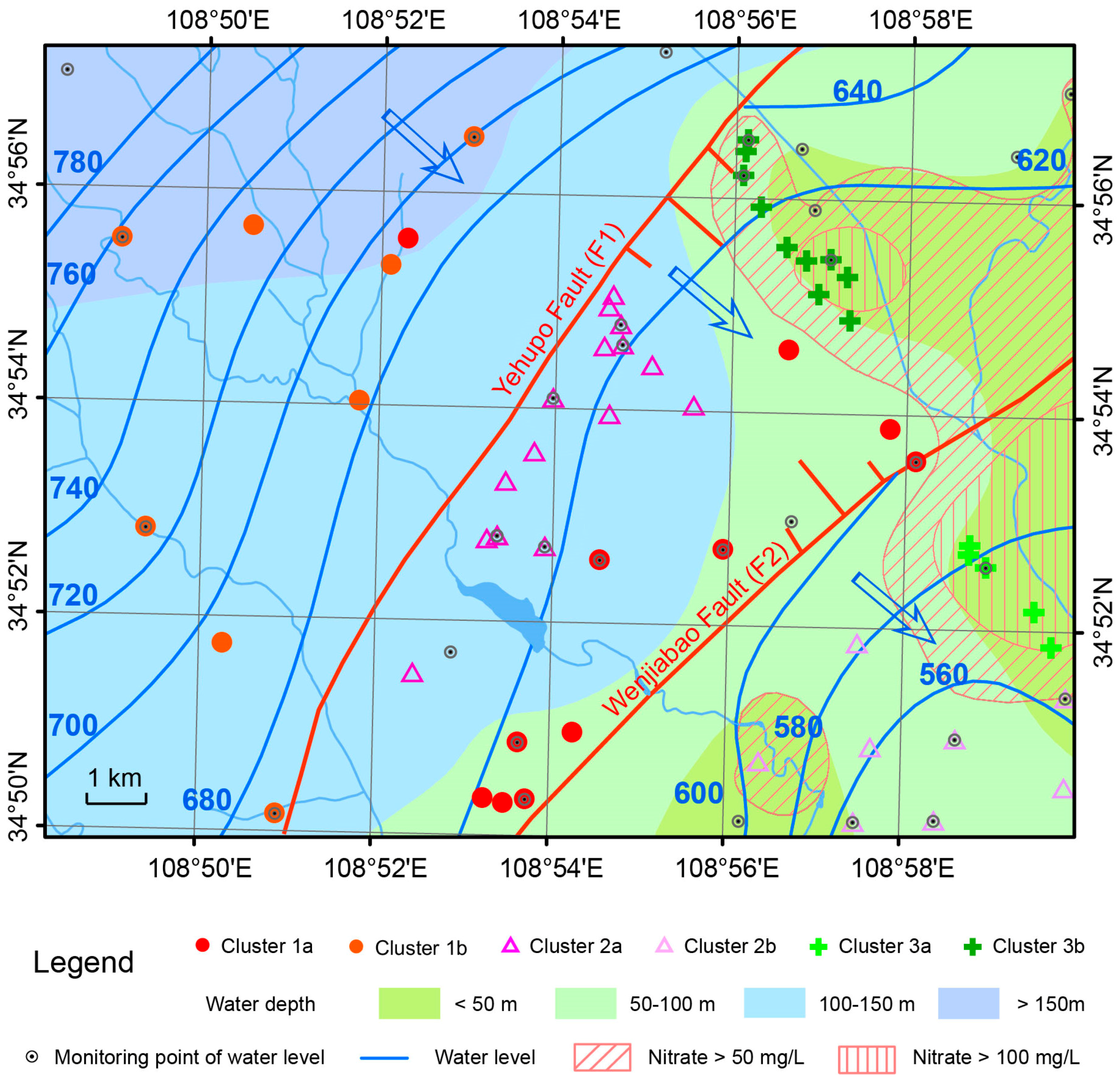
5.1. Hydraulic Head Patterns and Flow Directions
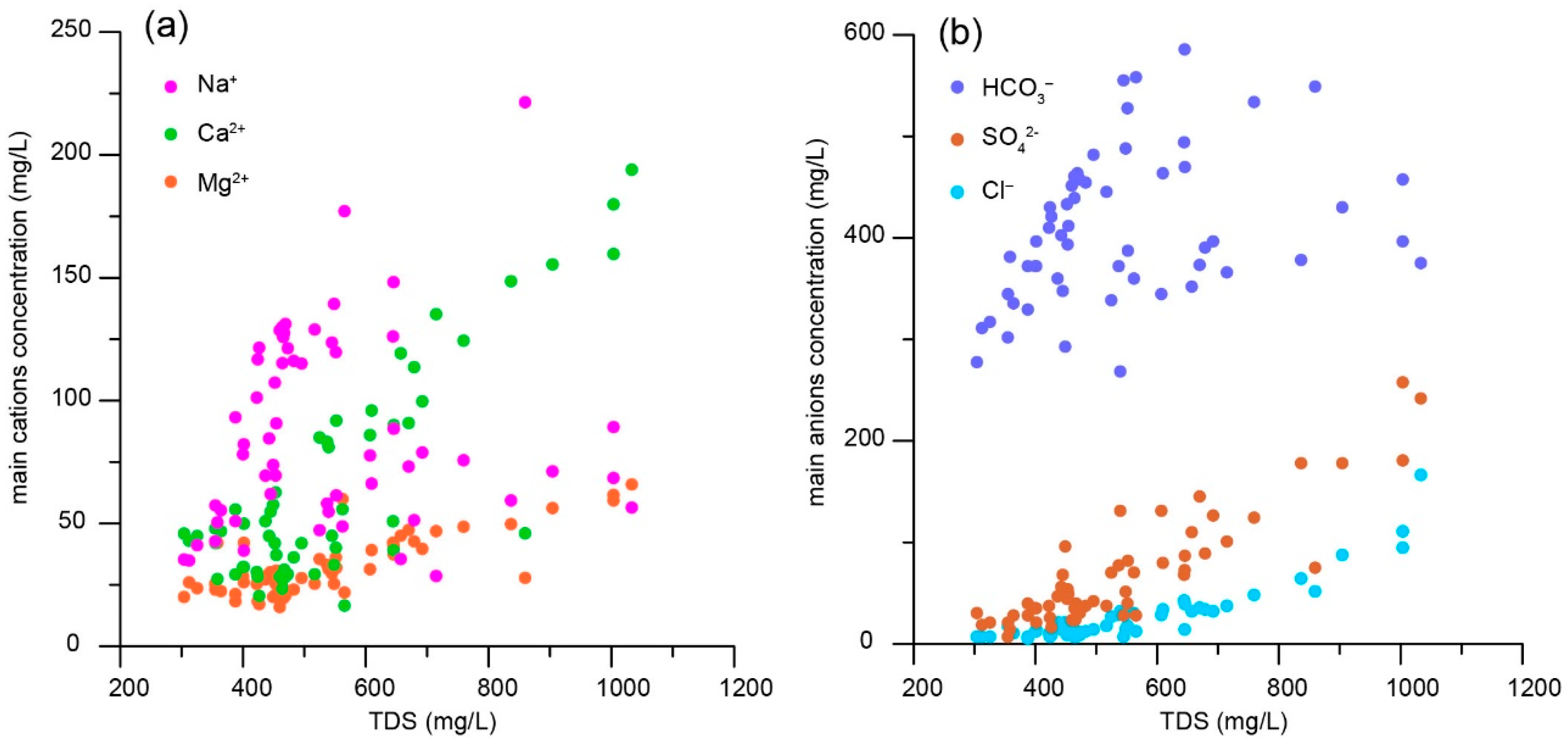
5.2. Hydrochemical Characteristics
5.3. Hierarchical Cluster Analysis
5.4. Hydrochemical Processes
5.4.1. Silicate Weathering
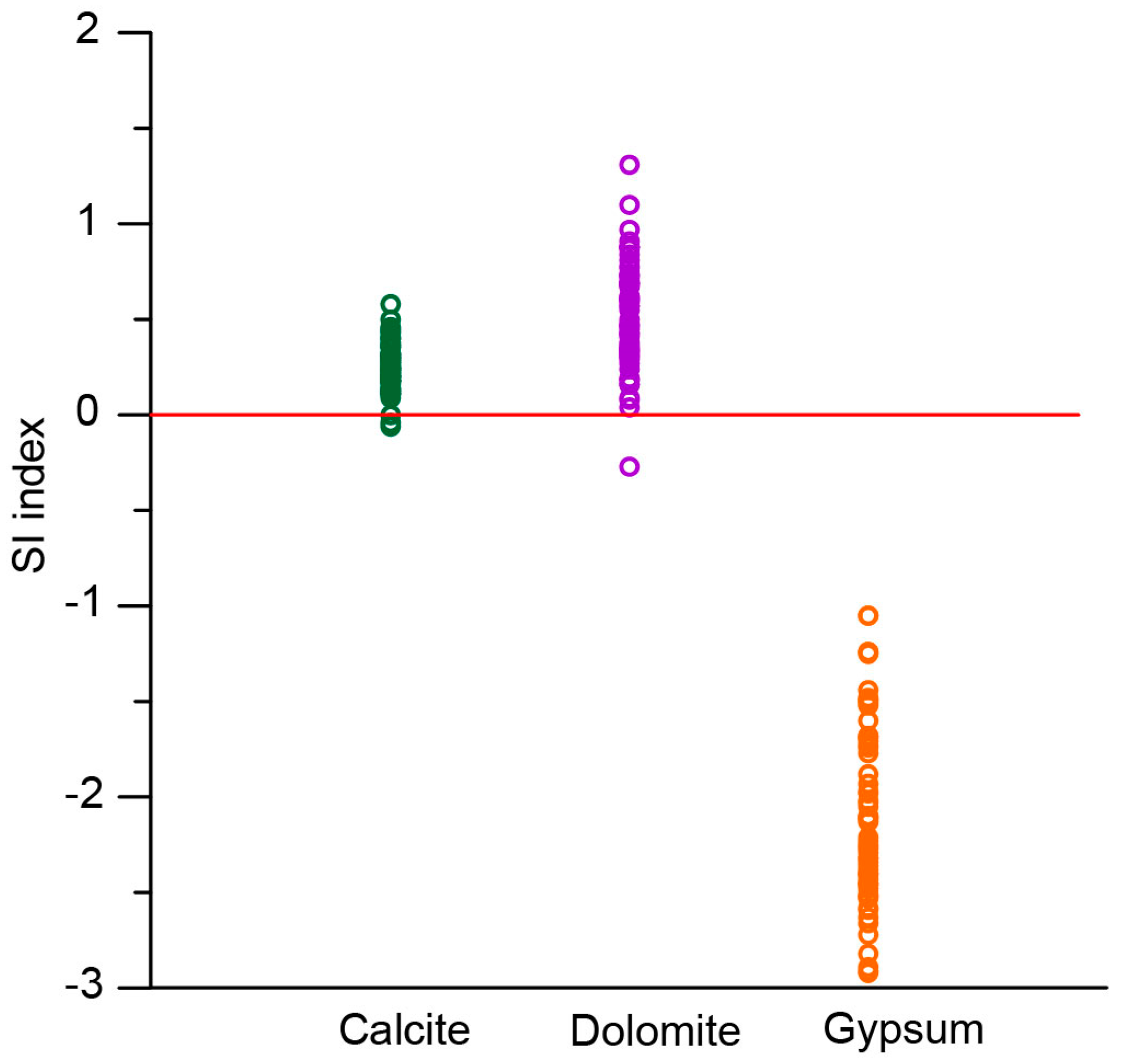
5.4.2. Carbonate Weathering and Dissolution
5.4.3. Ion Exchange
5.5. Hydrochemical Difference among All Clusters
5.6. Discussion of Cluster 2a
6. Conclusions
- Statistical analysis shows that the relative abundance of major cations in the groundwater is Na+ > Ca2+ > Mg2+, whereas the relative abundance of anions is HCO3− > SO42− > Cl−. The groundwater is polluted with NO3− in shallow water depth areas, which is attributed to anthropogenic factors, especially agriculture.
- Three clusters with different characteristics were identified using HCA in the study area, which is in accordance with the different water types. Clusters 1 and 2 exhibit hydrochemical facies of mixed-HCO3 and Na-HCO3, respectively. Cluster 3 exhibits mixed facies of Ca-HCO3 or Ca-SO4.
- Hydrochemical processes show the predominance of carbonate and silicate weathering in cluster 1, silicate weathering and ionic exchange in cluster 2, carbonate weathering in cluster 3, and ionic exchange in all clusters.
- Geological factors play an important role in groundwater chemistry. The chemistry of samples near the F1 fault is different from that in the other samples in the same aquifer along the flow path, even though F1 is a permeable fault. Oxygen and hydrogen isotopes indicate mixing between Quaternary pore water and clastic rock fissure water causing abnormal groundwater chemistry.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.; Qian, H.; Gao, Y. Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo. Health 2017, 9, 183–195. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Li, X. Challenges and prospects of sustainable groundwater management in an arid region along the Silk Road economic belt, Northwest China. Int. J. Water Resour. Dev. 2016. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.L.; Rao, M.S.; Kumar, B. Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ. Geol. 2006, 50, 1025–1039. [Google Scholar] [CrossRef]
- Sonkamble, S.; Sahya, A.; Mondal, N.C.; Harikumar, P. Appraisal and evolution of hydrochemical processes from proximity basalt and granite areas of Deccan Volcanic Province (DVP) in India. J. Hydrol. 2012, 438–439, 181–193. [Google Scholar] [CrossRef]
- Moya, C.E.; Raiber, M.; Taulis, M.; Cox, M.E. Hydrochemical evolution and groundwater flow processes in the Galilee and Eromanga basins, Great Artesian Basin, Australia: A multivariate statistical approach. Sci. Total Environ. 2015, 508, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Brkić, Ž.; Briški, M.; Marković, T. Use of hydrochemistry and isotopes for improving the knowledge of groundwater flow in a semiconfined aquifer system of the Eastern Slavonia (Croatia). Catena 2016, 142, 153–165. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, K.-H.; Thao, N.T.; Batsaikhan, B.; Yun, S.-T. Hydrochemical assessment of freshening saline groundwater using multiple end-members mixing modeling: A study of Red River delta aquifer, Vietnam. J. Hydrol. 2017, 549, 703–714. [Google Scholar] [CrossRef]
- Wei, Y.; Fan, W.; Wang, W.; Deng, L. Identification of nitrate pollution sources of groundwater and analysis of potential pollution paths in loess regions: A case study in Tongchuan region, China. Environ. Earth Sci. 2017, 76, 423. [Google Scholar] [CrossRef]
- Li, X. Research of Groundwater Chemical Characteristics and Nitrate Pollution Source of Yaozhou Map-Area. Master’s Thesis, Chang’an University, Xi’an, China, 2015. (In Chinese). [Google Scholar]
- Chen, J.; Qian, H.; Wu, H. Nitrogen contamination in groundwater in an agricultural region along the New Silk Road, northwest China: Distribution and factors controlling its fate. Environ. Sci. Pollut. Res. 2017, 24, 13154–13167. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, H.; Wu, H.; Gao, Y.; Li, X. Assessment of arsenic and fluoride pollution in groundwater in Dawukou area, Northwest China and the associated health risk for inhabitants. Environ. Earth Sci. 2017, 76, 314. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Peng, J. ArcGIS-based evaluation of geo-hazards at Yaozhou County, Shaanxi, China. J. Rock Mech. Geotech. Eng. 2013, 5, 330–334. [Google Scholar] [CrossRef]
- Wei, Y.; Fan, W.; Cao, Y. Experimental study on the vertical deformation of aquifer soils under conditions of withdrawing and recharging of groundwater in Tongchuan region, China. Hydrogeol. J. 2017, 25, 297–309. [Google Scholar] [CrossRef]
- Furi, W.; Razack, M.; Abiye, T.A.; Kebede, S.; Legesse, D. Hydrochemical characterization of complex volcanic aquifers in a continental rifted zone: The Middle Awash basin, Ethiopia. Hydrogeol. J. 2012, 20, 385–400. [Google Scholar] [CrossRef]
- Rajabpour, H.; Vaezihir, A.; Sedghi, M.H. The North Tabriz Fault, a barrier to groundwater flow in an alluvial aquifer northwest of Tabriz, Iran. Environ. Earth Sci. 2016, 75, 849. [Google Scholar] [CrossRef]
- Hagedorn, B.; Whittier, R.B. Solute sources and water mixing in a flashy mountainous stream (Pahsimeroi River, U.S. Rocky Mountains): Implications on chemical weathering rate and groundwater–surface water interaction. Chem. Geol. 2015, 391, 123–137. [Google Scholar] [CrossRef]
- Zhuang, J.; Peng, J.; Wang, G.; Iqbal, J.; Wang, Y.; Li, W.; Xu, Q.; Zhu, X. Prediction of rainfall-induced shallow landslides in the Loess Plateau, Yan’an, China, using the TRIGRS model. Earth Surf. Process. Landf. 2017, 42, 915–927. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Qian, H. Detection of anomalies and changes of rainfall in the Yellow River Basin, China, through two graphical methods. Water 2018, 10, 15. [Google Scholar] [CrossRef]
- Wu, H.; Qian, H. Innovative trend analysis of annual and seasonal rainfall and extreme values in Shaanxi, China, since the 1950s. Int. J. Climatol. 2017, 37, 2582–2592. [Google Scholar] [CrossRef]
- Peng, J.; Chen, L.; Huang, Q.; Men, Y.; Fan, W.; Yan, J. Physical simulation of ground fissures triggered by underground fault activity. Eng. Geol. 2013, 155, 19–30. [Google Scholar] [CrossRef]
- Liu, B.K.; Chen, Y.W.; Zhou, C.K. Hydrogeology Survey Report of Valley Region in Yaoxian County, Shaanxi; Department of Geology and Mineral Resources of Shaanxi Province: Xi’an, China, 1985. (In Chinese) [Google Scholar]
- Si, P.Z.; Yu, J.X.; Ge, X.W.; Xue, Y.B. Summary of Geology and Hydrogeology Mapping in Yaoxian County, Shaanxi; Shaanxi Coal Hydrogeology Team: Xi’an, China, 1997. (In Chinese) [Google Scholar]
- Ministry of Health of PRC; Standardization Administration of PRC. Standard Examination Methods for Drinking Water (GB/T5750-2006); Standards Press of China: Beijing, China. (In Chinese)
- Lambrakis, N.; Antonakos, A.; Panagopoulos, G. The use of multicomponent statistical analysis in hydrogeological environmental research. Water Res. 2004, 38, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Belkhiri, L.; Boudoukha, A.; Mouni, L.; Baouz, T. Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—A case study: Ain Azel plain (Algeria). Geoderma 2010, 159, 390–398. [Google Scholar] [CrossRef]
- Suk, H.; Lee, K.-K. Characterization of a ground water hydrochemical system through multivariate analysis: Clustering into ground water zones. Groundwater 1999, 37, 358–366. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H.; Duan, Z.; Zhang, X. Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: A case study in Laoheba phosphorite mine in Sichuan, China. Arab. J. Geosci. 2014, 7, 3973–3982. [Google Scholar] [CrossRef]
- Askri, B. Hydrochemical processes regulating groundwater quality in the coastal plain of Al Musanaah, Sultanate of Oman. J. Afr. Earth Sci. 2015, 106, 87–98. [Google Scholar] [CrossRef]
- Parkhurst, D.; Appelo, C. User’s Guide to PHREEQC (Version 2); US Geological Survey: Reston, VA, USA, 1999.
- Qian, H.; Wu, J.; Zhou, Y.; Li, P. Stable oxygen and hydrogen isotopes as indicators of lake water recharge and evaporation in the lakes of the Yinchuan Plain. Hydrol. Process. 2014, 28, 3554–3562. [Google Scholar] [CrossRef]
- Qian, H.; Li, P.; Wu, J.; Zhou, Y. Isotopic characteristics of precipitation, surface and ground waters in the Yinchuan plain, Northwest China. Environ. Earth Sci. 2013, 70, 57–70. [Google Scholar] [CrossRef]
- Somaratne, N.; Mustafa, S.; Lawson, J. Use of hydrochemistry, stable isotope, radiocarbon, 222Rn and terrigenic 4He to study the geochemical processes and the mode of vertical leakage to the Gambier Basin tertiary confined sand aquifer, South Australia. Water 2016, 8, 180. [Google Scholar] [CrossRef]
- Yin, G.; Ni, S. Deuterium excess parameter evolution in ground water bulletin of mineralogy. Petrol. Geochem. 2001, 20, 409–411. (In Chinese) [Google Scholar]
- Vengosh, A.; Hening, S.; Ganor, J.; Mayer, B.; Weyhenmeyer, C.E.; Bullen, T.D.; Paytan, A. New isotopic evidence for the origin of groundwater from the Nubian Sandstone Aquifer in the Negev, Israel. Appl. Geochem. 2007, 22, 1052–1073. [Google Scholar] [CrossRef]
- Hagedorn, B.; Cartwright, I. Climatic and lithologic controls on the temporal and spatial variability of CO2 consumption via chemical weathering: An example from the Australian Victorian Alps. Chem. Geol. 2009, 260, 234–253. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Qian, H.; Zhang, X. Chemical characteristics and quality assessment of groundwater of exploited aquifers in Beijiao Water Source of Yinchuan, China: A case study for drinking, irrigation, and industrial purposes. J. Chem. N. Y. 2015, 2015, 726340. [Google Scholar] [CrossRef]
- Mackenzie, F.J.; Garrells, R.H. Silicates: Reactivity with sea water. Sci. J. 1965, 150, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Mayback, M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am. J. Sci. 1987, 287, 401–428. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo. Health 2016, 8, 331–348. [Google Scholar] [CrossRef]
- Rogers, R.J. Geochemical comparison of groundwater in areas of New England, New York, and Pennsylvania. Groundwater 1989, 27, 690–712. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, southern India. Environ. Geol. 2004, 46, 47–61. [Google Scholar] [CrossRef]
- Lakshmanan, E.; Kannan, R.; Kumar, M.S. Major ion chemistry and identification of hydrogeochemical processes of ground water in a of Kancheepuram district, Tamil Nadu, India. Environ. Geol. 2003, 10, 157–166. [Google Scholar] [CrossRef]
- Datta, P.S.; Tyagi, S.K. Major ion chemistry of groundwater in Delhi area: Chemical weathering processes and groundwater flow regime. J. Geol. Soc. India 1996, 47, 179–188. [Google Scholar]
- Fisher, R.S.; Mullican, W.F., III. Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol. J. 1997, 5, 4–16. [Google Scholar] [CrossRef]
- Maya, A.L.; Loucks, M.D. Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J. Hydrol. 1995, 172, 31–59. [Google Scholar] [CrossRef]
- Anders, R.; Mendez, G.O.; Futa, K.; Danskin, W.R. A geochemical approach to determine sources and movement of saline groundwater in a coastal aquifer. Groundwater 2014, 52, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, I.; Weaver, T.; Petrides, B. Controls on 87Sr/86Sr ratios of groundwater in silicate-dominated aquifers: SE Murray Basin, Australia. Chem. Geol. 2007, 246, 107–123. [Google Scholar] [CrossRef]
- Yidana, S.M.; Yidana, A. Assessing water quality using water quality index and multivariate analysis. Environ. Earth Sci. 2010, 59, 1461–1473. [Google Scholar] [CrossRef]
- Gat, J.R. Comments on the stable isotope method in regional groundwater investigations. Water Resour. Res. 1971, 7, 980–993. [Google Scholar] [CrossRef]




| Index | Mean | Min | Max | Acceptable Limits (WHO 2004) |
|---|---|---|---|---|
| pH | 7.5 | 6.9 | 7.9 | 6.5–9.2 |
| Total Dissolved Solids (mg/L) | 528.1 | 303.9 | 1033.2 | 1000 |
| Na+ + K+ (mg/L) | 92.1 | 28.7 | 227.7 | 200 |
| Ca2+ (mg/L) | 56.8 | 14.7 | 194.0 | 200 |
| Mg2+ (mg/L) | 31.0 | 10.7 | 66.0 | 150 |
| Cl− (mg/L) | 24.1 | 5.4 | 166.5 | 250 |
| SO42− (mg/L) | 68.9 | 7.1 | 293.6 | 250 |
| HCO3− (mg/L) | 406.1 | 268.5 | 585.8 | 240 |
| F− (mg/L) | 0.5 | 0.1 | 1.5 | 1.5 |
| NO3− (mg/L) | 37.1 | 0.5 | 173.3 | 50 |
| PO43− (mg/L) | 0 | 0 | 0.2 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wu, H.; Qian, H.; Gao, Y. Groundwater Chemistry Regulated by Hydrochemical Processes and Geological Structures: A Case Study in Tongchuan, China. Water 2018, 10, 338. https://doi.org/10.3390/w10030338
Li X, Wu H, Qian H, Gao Y. Groundwater Chemistry Regulated by Hydrochemical Processes and Geological Structures: A Case Study in Tongchuan, China. Water. 2018; 10(3):338. https://doi.org/10.3390/w10030338
Chicago/Turabian StyleLi, Xinyan, Hao Wu, Hui Qian, and Yanyan Gao. 2018. "Groundwater Chemistry Regulated by Hydrochemical Processes and Geological Structures: A Case Study in Tongchuan, China" Water 10, no. 3: 338. https://doi.org/10.3390/w10030338




