Treatment of Dairy Wastewater by Oxygen Injection: Occurrence and Removal Efficiency of a Benzotriazole Based Anticorrosive
Abstract
:1. Introduction
2. Materials and Methods
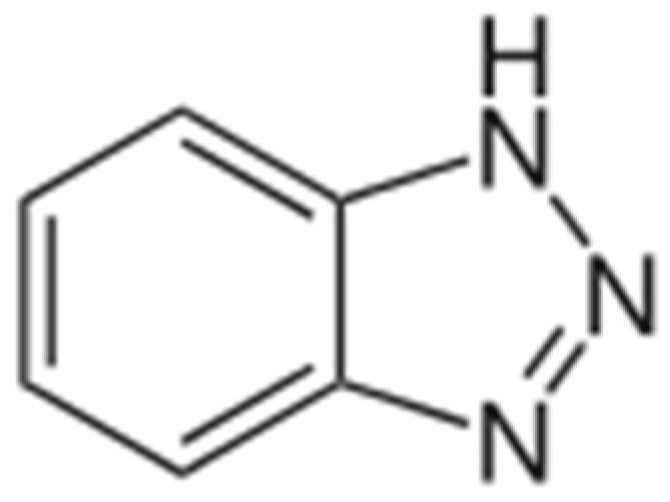
2.1. Dairy Wastewater Treatment and Anticorrosive Agent Utilization
2.2. Laboratory-Scale Experiments
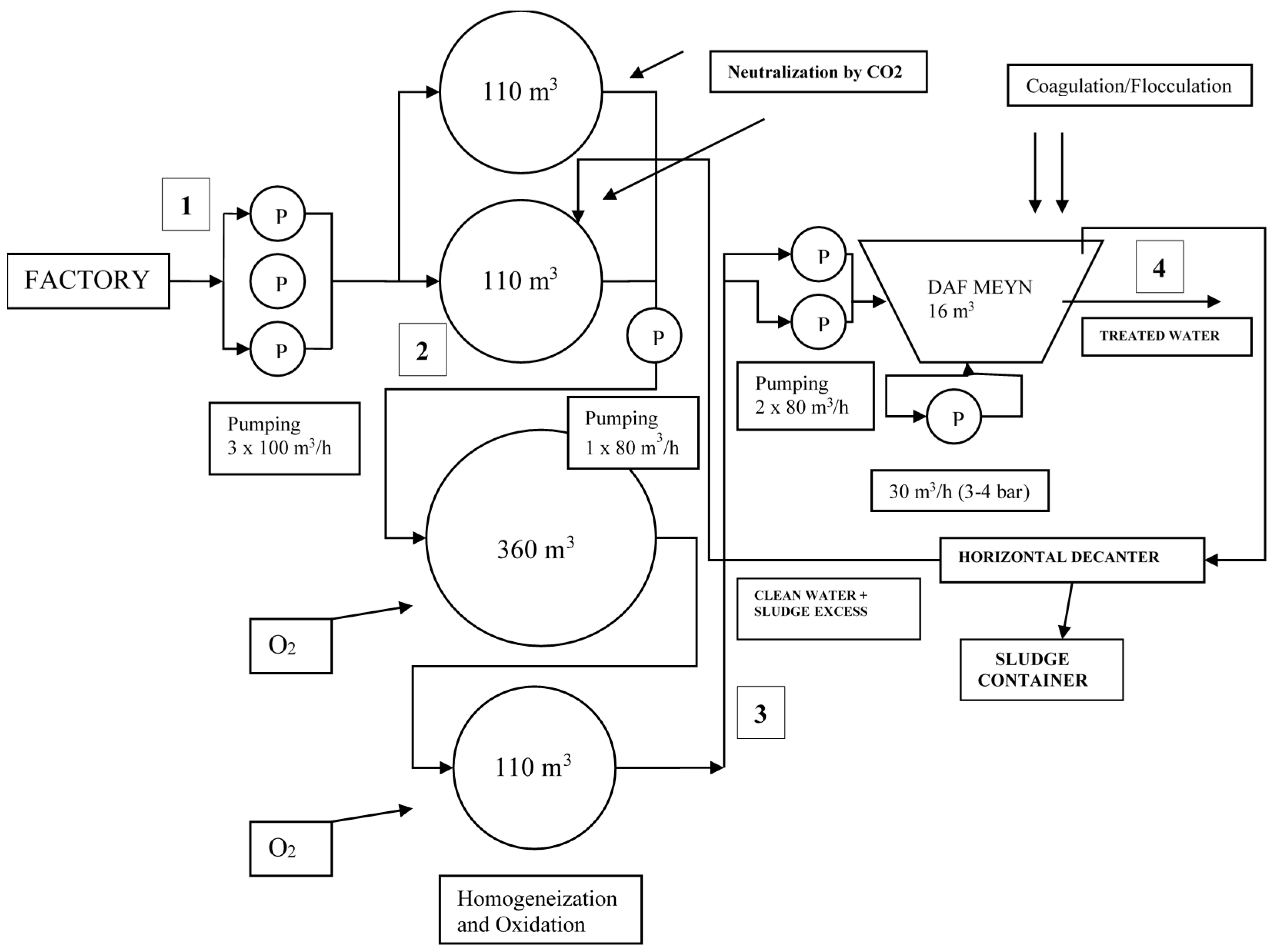
2.3. Plant Study at a Real Scale
3. Results and Discussion
3.1. Laboratory-Scale Experiments
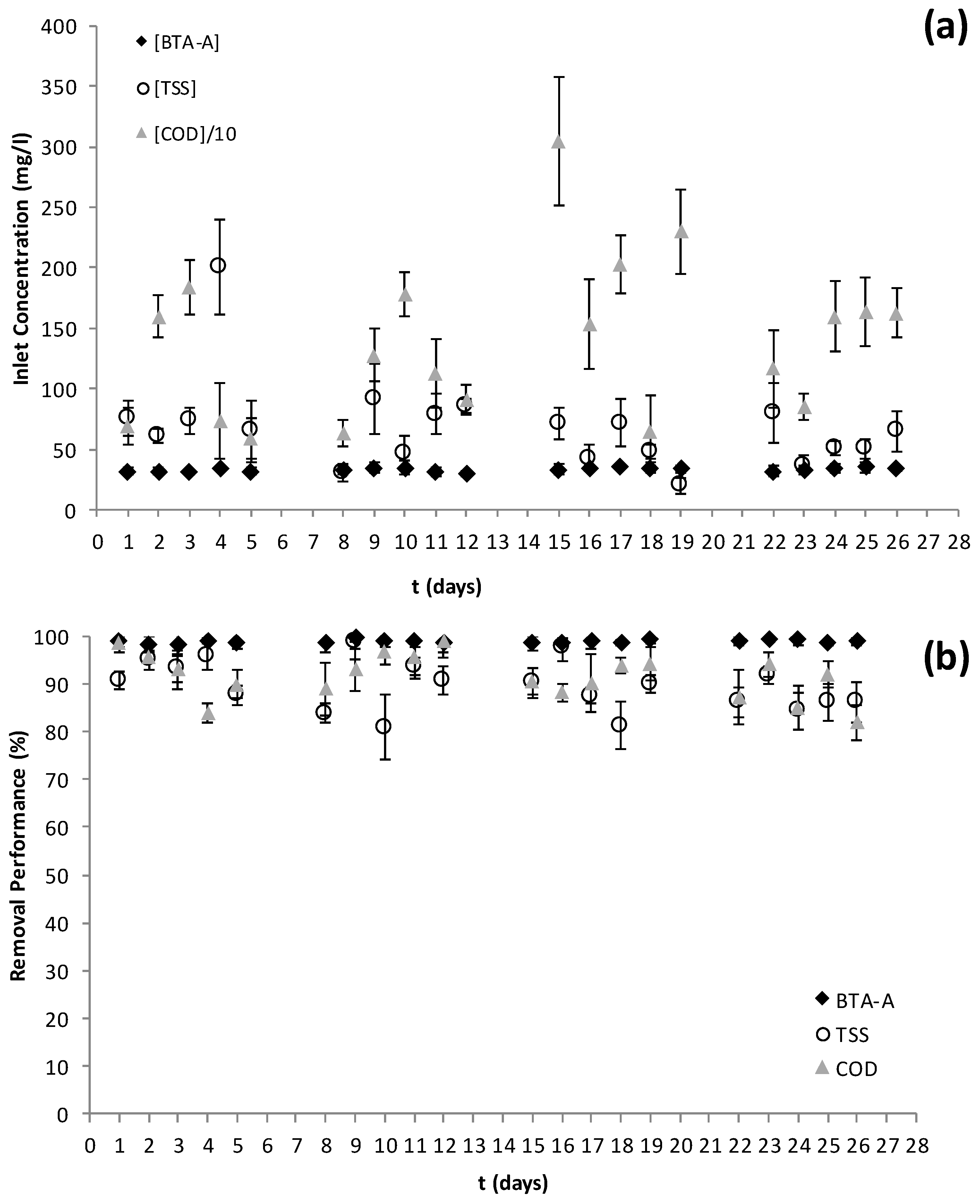
3.2. Plant Study at a Real Scale
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Voutsa, D.; Hartmann, P.; Schaffner, C.; Giger, W. Benzotriazoles, alkylphenols and bisphenol A in municipal wastewaters and in the Glatt River, Switzerland. Environ. Sci. Pollut. Res. 2006, 13, 333–341. [Google Scholar] [CrossRef]
- Seeland, A.; Oetken, M.; Kiss, A.; Fries, E.; Oehlmann, J. Acute and chronic toxicity of benzotriazoles to aquatic organisms. Environ. Sci. Pollut. Res. 2012, 19, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, R.; Ceretti, E.; Zerbini, I.; Casale, R.; Gozio, E.; Bertanza, G.; Gelatti, U.; Donato, F.; Feretti, D. Biodegradability, toxicity and mutagenicity of detergents: Integrated experimental evaluations. Ecotoxicol. Environ. Saf. 2012, 84, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhou, P.; Chen, Y.; Ou, H.; Liu, J.; Li, C.; Li, Q. Degradation of 1H-benzotriazole using ultraviolet activating persulfate: Mechanisms, products and toxicological analysis. Chem. Eng. J. 2018, 334, 1493–1501. [Google Scholar] [CrossRef]
- Xu, J.; Li, L.; Guo, C.; Zhang, Y.; Wang, S. Removal of benzotriazole from solution by BiOBr photocatalysis under simulated solar irradiation. Chem. Eng. J. 2013, 221, 230–237. [Google Scholar] [CrossRef]
- Farré, M.L.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Alotaibi, M.D.; McKinley, A.J.; Patterson, B.M.; Reeder, A.Y. Benzotriazoles in the aquatic environment: A review of their occurrence, toxicity, degradation and analysis. Water Air Soil Pollut. 2015, 226, 226. [Google Scholar] [CrossRef]
- Barrington, D.J.; Prior, A.; Ho, G. The role of water auditing in achieving water conservation in the process industry. J. Clean. Prod. 2013, 52, 356–361. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Foteinis, S.; Mantzavinos, D.; Tsoutsos, T. Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J. Clean. Prod. 2013, 54, 229–234. [Google Scholar] [CrossRef]
- Chong, M.N.; Sharma, A.K.; Burn, S.; Saint, C.P. Feasibility study on the application of advanced oxidation technologies for decentralised wastewater treatment. J. Clean. Prod. 2012, 35, 230–238. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Gallardo, M.; Molina, R.; López-Muñoz, M.J. Life cycle assessment and techno-economic evaluation of alternatives for the treatment of wastewater in a chrome-plating industry. J. Clean. Prod. 2018, 172, 2351–2362. [Google Scholar] [CrossRef]
- Martín-Rilo, S.; Coimbra, R.N.; Martín-Villacorta, J.; Otero, M. Treatment of dairy industry wastewater by oxygen injection: Performance and outlay parameters from the full scale implementation. J. Clean. Prod. 2015, 86, 15–23. [Google Scholar] [CrossRef]
- Asplund, S. The Biogas Production Plant at Umeå Dairy. Evaluation of Design and Start Up. Master’s Thesis, Linköpings Universitet, Sweden, 2005. Available online: http://www.diva-portal.org/smash/get/diva2:21340/FULLTEXT01.pdf (accessed on 15 November 2017).
- Passeggi, M.; López, I.; Borzacconi, L. Modified UASB reactor for dairy industry wastewater: Performance indicators and comparison with the traditional approach. J. Clean. Prod. 2012, 26, 90–94. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association (APHA): Washington, DC, USA, 1995; ISBN 0-87553-223-3. [Google Scholar]
- Herzog, B.; Lemmer, H.; Huber, B.; Horn, H.; Müller, E. Xenobiotic benzotriazoles-biodegradation under meso- and oligotrophic conditions as well as denitrifying, sulfate-reducing, and anaerobic conditions. Environ. Sci. Pollut. Res. 2014, 21, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ying, G.; Shareef, A.; Kookana, R.S. Biodegradation of three selected benzotriazoles under aerobic and anaerobic conditions. Water Res. 2011, 45, 5005–5014. [Google Scholar] [CrossRef] [PubMed]
- Mazioti, A.A.; Stasinakis, A.S.; Pantazi, Y.; Andersen, H.R. Biodegradation of benzotriazoles and hydroxy-benzothiazole in wastewater by activated sludge and moving bed biofilm reactor systems. Bioresour. Technol. 2015, 192, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reemtsma, T.; Miehe, U.; Duennbier, U.; Jekel, M. Polar pollutants in municipal wastewater and the water cycle: Occurrence and removal of benzotriazoles. Water Res. 2010, 44, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S.; Thomaidis, N.S.; Arvaniti, O.S.; Asimakopoulos, A.G.; Samaras, V.G.; Ajibola, A.; Mamais, D.; Lekkas, T.D. Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant. Sci. Total Environ. 2013, 463–464, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Behera, C.R.; Srinivasan, B. Simulation and experimental studies to enhance water reuse and reclamation in India’s largest dairy industry. J. Environ. Eng. Chem. 2016, 4, 601–616. [Google Scholar] [CrossRef]
- Löwenberg, J.; Zenker, A.; Baggenstos, M.; Koch, G.; Kazner, C.; Wintgens, T. Comparison of two PAC/UF processes for the removal of micropollutants from wastewater treatment plant effluent: Process performance and removal efficiency. Water Res. 2014, 56, 26–36. [Google Scholar] [CrossRef] [PubMed]


| Contaminant Removed | O2 Flowrate | Parameters of the Pseudo First-Order Kinetic Model | Goodness of Fitting | ||
|---|---|---|---|---|---|
| (m3/h) | k1 (min−1) | %max | R2 | Sxy | |
| TSS | 25 | 0.0207 ± 0.0021 a | 77.24 ± 2.76 a | 0.9818 | 3.74 |
| 20 | 0.0158 ± 0.0017 b | 71.02 ± 3.05 a | 0.9814 | 3.37 | |
| 15 | 0.0057 ± 0.0013 c | 80.05 ± 12.65 a | 0.9818 | 3.50 | |
| COD | 25 | 0.0077 ± 0.0026 a | 80.72 ± 15.07 a | 0.9394 | 6.73 |
| 20 | 0.0085 ± 0.0023 a | 85.93 ± 12.47 a | 0.9491 | 6.46 | |
| 15 | 0.0074 ± 0.0010 a | 80.62 ± 6.27 a | 0.9894 | 2.62 | |
| BTA-A | 25 | 0.0175 ± 0.0010 a | 83.40 ± 1.82 a | 0.9947 | 2.19 |
| 20 | 0.0124 ± 0.0017 b | 85.59 ± 5.45 a | 0.9789 | 4.67 | |
| 15 | 0.0102 ± 0.0021 b | 85.15 ± 8.39 a | 0.9640 | 5.62 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Rilo, S.; Coimbra, R.N.; Escapa, C.; Otero, M. Treatment of Dairy Wastewater by Oxygen Injection: Occurrence and Removal Efficiency of a Benzotriazole Based Anticorrosive. Water 2018, 10, 155. https://doi.org/10.3390/w10020155
Martín-Rilo S, Coimbra RN, Escapa C, Otero M. Treatment of Dairy Wastewater by Oxygen Injection: Occurrence and Removal Efficiency of a Benzotriazole Based Anticorrosive. Water. 2018; 10(2):155. https://doi.org/10.3390/w10020155
Chicago/Turabian StyleMartín-Rilo, Santiago, Ricardo N. Coimbra, Carla Escapa, and Marta Otero. 2018. "Treatment of Dairy Wastewater by Oxygen Injection: Occurrence and Removal Efficiency of a Benzotriazole Based Anticorrosive" Water 10, no. 2: 155. https://doi.org/10.3390/w10020155






