Occurrence, Distribution, and Risk Assessment of Antibiotics in a Subtropical River-Reservoir System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites and Sampling
2.2. Chemicals and Standards
2.3. Sample Preparation
2.4. Chemical Analysis
2.5. Ecological Risk Assessment
2.6. Data Analysis
3. Results and Discussion
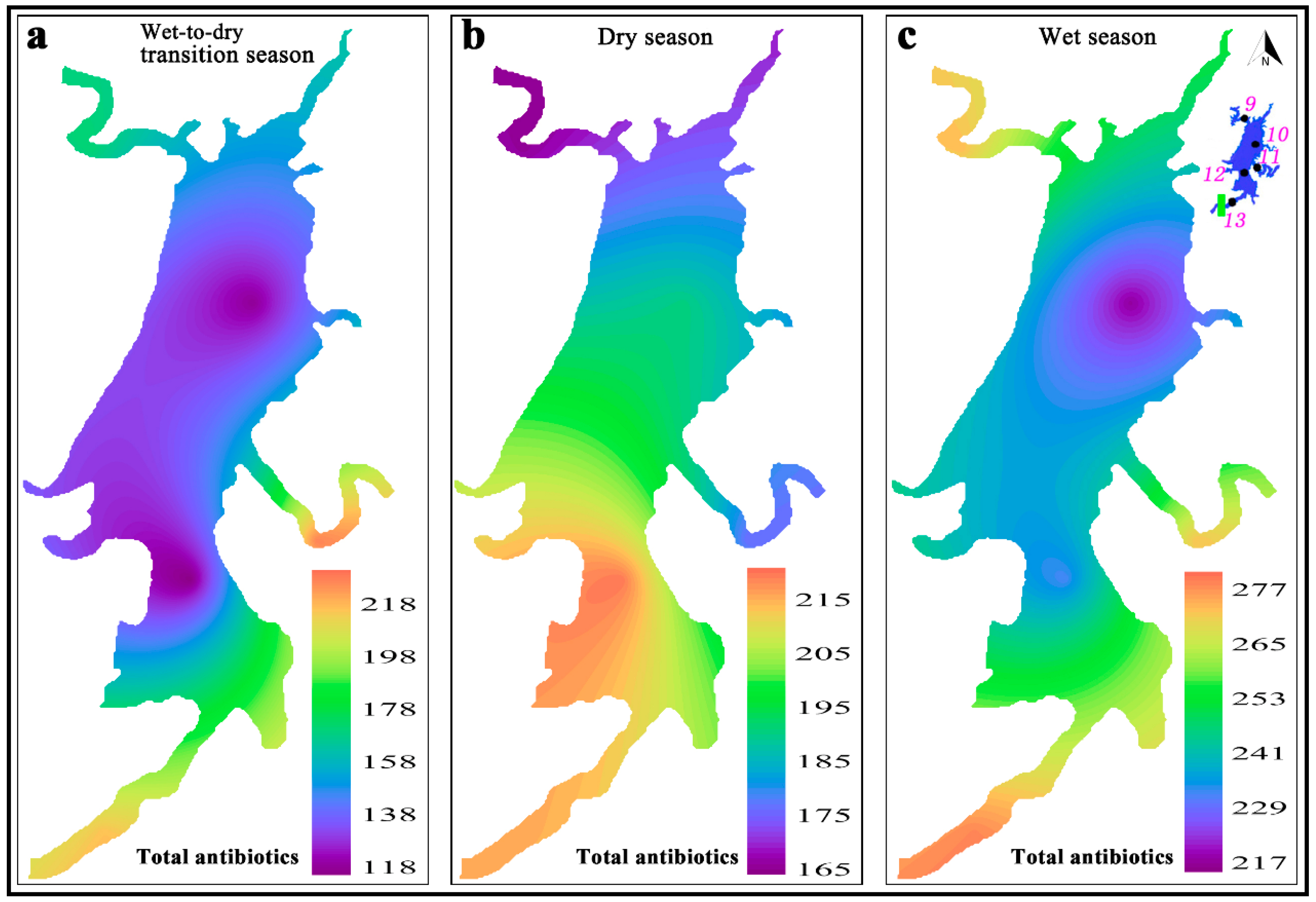
3.1. Antibiotics in the Water Phase of the HRDR
3.2. Antibiotics in the Sediment Phase of the FSBR
3.3. Relationships between Basic Parameters and Antibiotic Concentrations
3.4. Pseudo-Partitioning Coefficients of Antibiotics in the FSBR
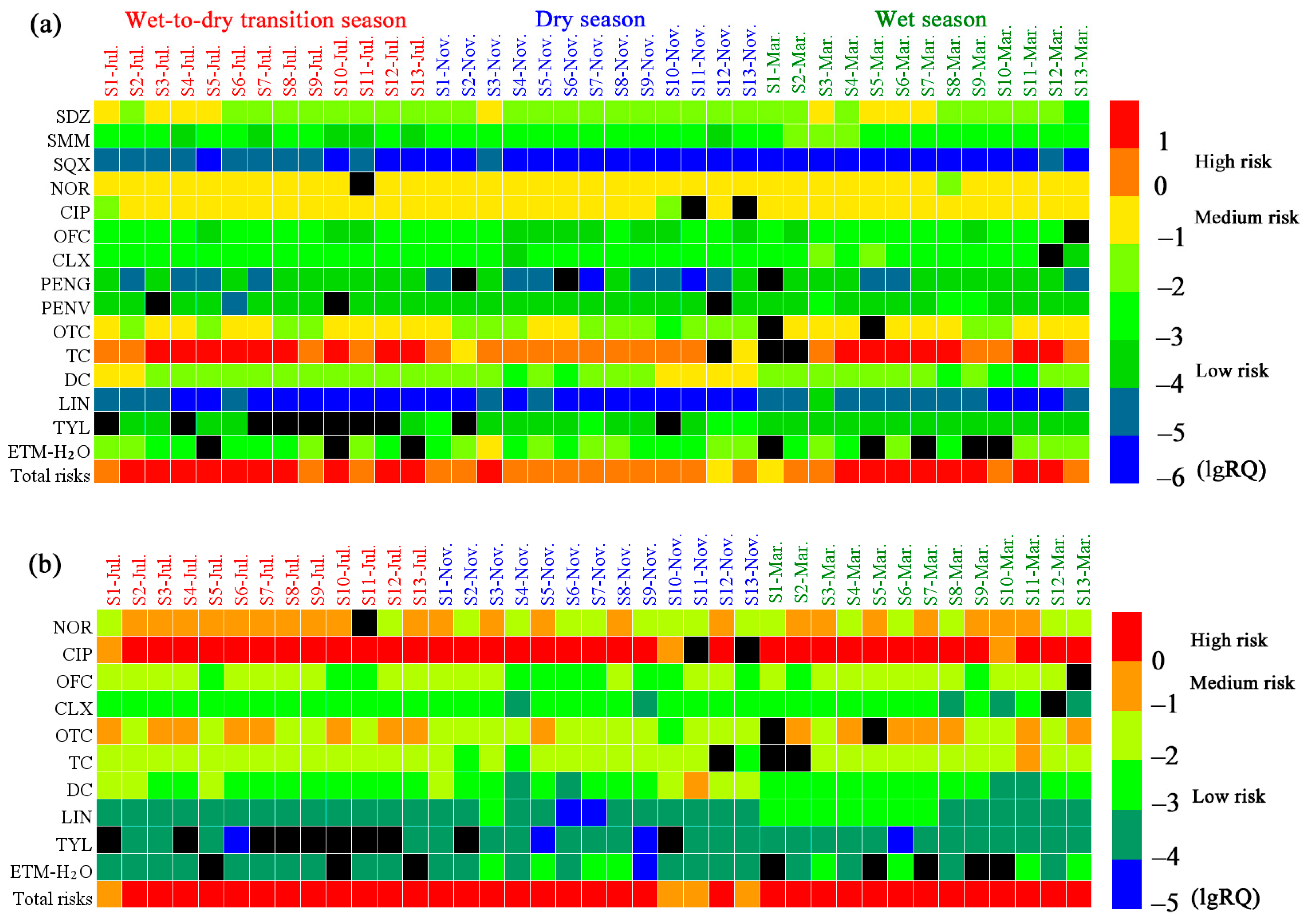
3.5. Ecological Risk Assessment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Le Page, G.; Gunnarsson, L.; Snape, J.; Tyler, C.R. Integrating human and environmental health in antibiotic risk assessment: A critical analysis of protection goals, species sensitivity and antimicrobial resistance. Environ. Int. 2017, 109, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Gbylik-Sikorska, M.; Posyniak, A.; Mitrowska, K.; Gajda, A.; Błądek, T.; Śniegocki, T.; Żmudzki, J. Occurrence of veterinary antibiotics and chemotherapeutics in fresh water, sediment, and fish of the rivers and lakes in Poland. Bull. Vet. Inst. Pulawy 2014, 58, 399–404. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, L.; Zhu, S.; Lu, H.; Liu, W. Occurrence, abundance, and distribution of sulfonamide and tetracycline resistance genes in agricultural soils across China. Sci. Total Environ. 2017, 599–600, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, W.; Xu, L.; Strong, P.J.; Chen, H. Antibiotic-resistant genes and antibiotic-resistant bacteria in the effluent of urban residential areas, hospitals, and a municipal wastewater treatment plant system. Environ. Sci. Pollut. Res. Int. 2015, 22, 4587–4596. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Su, H.C.; Pan, C.G.; Ying, G.G.; Zhao, J.L.; Zhou, L.J.; Liu, Y.S.; Tao, R.; Zhang, R.Q.; He, L.Y. Contamination profiles of antibiotic resistance genes in the sediments at a catchment scale. Sci. Total Environ. 2014, 490, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Y.; Lu, Y.; Johnson, A.C.; Sarvajayakesavalu, S.; Liu, Z.; Su, C.; Zhang, Y.; Juergens, M.D.; Jin, X. The relative risk and its distribution of endocrine disrupting chemicals, pharmaceuticals and personal care products to freshwater organisms in the Bohai Rim, China. Sci. Total Environ. 2017, 590–591, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, I.S.; Oh, J.E. Human and veterinary pharmaceuticals in the marine environment including fish farms in Korea. Sci. Total Environ. 2017, 579, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.J.; Blanchard, M.; Chevreuil, M. Occurrence of antibiotics in rural catchments. Chemosphere 2017, 168, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Zabalza-Martínez, J.; Borràs, G.; López-Moreno, J.I.; Pla, E.; Pascual, D.; Savé, R.; Biel, C.; Funes, I.; Martín-Hernández, N.; et al. Effect of reservoirs on streamflow and river regimes in a heavily regulated river basin of Northeast Spain. Catena 2017, 149, 727–741. [Google Scholar] [CrossRef]
- Mwanamoki, P.M.; Devarajan, N.; Niane, B.; Ngelinkoto, P.; Thevenon, F.; Nlandu, J.W.; Mpiana, P.T.; Prabakar, K.; Mubedi, J.I.; Kabele, C.G.; et al. Trace metal distributions in the sediments from river-reservoir systems: Case of the Congo River and Lake Ma Vallee, Kinshasa (Democratic Republic of Congo). Environ. Sci. Pollut. Res. Int. 2015, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, J.; Zhang, A.; Liu, W.; Cheng, W. Occurrence of phthalate esters in sediments in Qiantang River, China and inference with urbanization and river flow regime. J. Hazard. Mater. 2013, 248–249, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jiang, J.; Zheng, Y.; Wang, F.; Wu, C.; Xie, R.R. Effect of a dam on the optical properties of different-sized fractions of dissolved organic matter in a mid-subtropical drinking water source reservoir. Sci. Total Environ. 2017, 598, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Temoka, C.; Wang, J.X.; Bi, Y.H.; Deyerling, D.; Pfister, G.; Henkelmann, B.; Schramm, K.W. Concentrations and mass fluxes estimation of organochlorine pesticides in Three Gorges Reservoir with virtual organisms using in situ prc-based sampling rate. Chemosphere 2016, 144, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J. Investigating the effects of point source and nonpoint source pollution on the water quality of the East River (Dongjiang) in South China. Ecol. Indic. 2013, 32, 294–304. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Do, T.V.; Reinhard, M.; Ngo, H.H.; He, Y.; Gin, K.Y. Simultaneous analysis of multiple classes of antimicrobials in environmental water samples using spe coupleSd with uhplc-esi-ms/ms and isotope dilution. Talanta 2016, 159, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhao, J.L.; Chen, F.; Zhang, R.Q.; Peng, F.Q.; Zhang, Q.Q. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2012, 1244, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Administration, S.E.P. Water and Wastewater Monitoring Analysis Method of Editorial Committee: Water and Wastewater Monitoring Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Papadakis, E.N.; Tsaboula, A.; Kotopoulou, A.; Kintzikoglou, K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Pesticides in the surface waters of lake vistonis basin, greece: Occurrence and environmental risk assessment. Sci. Total Environ. 2015, 536, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, Y.; Zhang, W.; Zhong, J.; Lou, Q.; Yang, P.; Fang, Y. Occurrence, distribution, and risk assessment of antibiotics in the surface water of poyang lake, the largest freshwater lake in china. Chemosphere 2017, 184, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. Hemi: A toolkit for illustrating heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the south yellow sea in china: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; Tong, L.; Li, Y.; Deng, Y.; Guo, W.; Gan, Y. Seasonal variation of antibiotics concentration in the aquatic environment: A case study at jianghan plain, central china. Sci. Total Environ. 2015, 527–528, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, B.; Yin, L.; Zhang, Y.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Characterization of pharmaceutically active compounds in beijing, china: Occurrence pattern, spatiotemporal distribution and its environmental implication. J. Hazard. Mater. 2017, 323, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.S.; Liu, Y.S.; Van den Brink, P.J.; Price, O.R.; Ying, G.G. Ecological risks of home and personal care products in the riverine environment of a rural region in south china without domestic wastewater treatment facilities. Ecotoxicol. Environ. Saf. 2015, 122, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, Z.; Yang, K.; Graham, D.; Xie, B. Relationships between antibiotics and antibiotic resistance gene levels in municipal solid waste leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. CLEAN Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, M.; Guo, C.; An, D.; Xu, J.; Zhang, Y.; Xi, B. Distribution and ecological risk of antibiotics in a typical effluent-receiving river (Wangyang River) in North China. Chemosphere 2014, 112, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Mokh, S.; El Khatib, M.; Koubar, M.; Daher, Z.; Al Iskandarani, M. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: A case study natural water sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.E.; Fick, J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities—A study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar] [CrossRef] [PubMed]
- Tamtam, F.; Mercier, F.; Le Bot, B.; Eurin, J.; Tuc Dinh, Q.; Clement, M.; Chevreuil, M. Occurrence and fate of antibiotics in the Seine river in various hydrological conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yan, W.; Li, X.; Zou, Y.; Chen, X.; Huang, W.; Miao, L.; Zhang, R.; Zhang, G.; Zou, S. Antibiotics in riverine runoff of the Pearl River delta and Pearl River Estuary, China: Concentrations, mass loading and ecological risks. Environ. Pollut. 2013, 182, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Zhang, L.; Liu, S.; Guo, Z.; Hua, X. Antibiotics in water and sediments from Liao river in Jilin province, China: Occurrence, distribution, and risk assessment. Environ. Earth Sci. 2016, 75, 1202. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pang, S.; Wang, P.; Wang, C.; Han, N.; Liu, B.; Han, B.; Li, Y.; Anim-Larbi, K. Antibiotic concentration and antibiotic-resistant bacteria in two shallow urban lakes after stormwater event. Environ. Sci. Pollut. Res. Int. 2016, 23, 9984–9992. [Google Scholar] [CrossRef] [PubMed]
- Stoob, K.; Singer, H.P.; Mueller, S.R.; Schwarzenbach, R.P.; Stamm, C.H. Dissipation and transport of veterinary sulfonamide antibiotics after manure application to grassland in a small catchment. Environ. Sci. Technol. 2007, 41, 7349–7355. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.L.; Borch, T.; Furlong, E.T.; Davis, J.G.; Yager, T.J.; Yang, Y.Y.; Kolpin, D.W. Rainfall-runoff of anthropogenic waste indicators from agricultural fields applied with municipal biosolids. Sci. Total Environ. 2017, 580, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, X.; Huang, X.; Zhang, T.; Li, X. Differentiating anthropogenic impacts on args in the Pearl River Estuary by using suitable gene indicators. Water Res. 2013, 47, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Paiga, P.; Santos, L.H.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis River (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Meng, W.; Xu, J.; Zhang, Y.; Guo, C. Occurrence, distribution and bioaccumulation of antibiotics in the Liao River basin in China. Environ. Sci. Process. Impacts 2014, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Zhao, Y.; Li, B.; Huang, C.L.; Zhang, S.Y.; Yu, S.; Chen, Y.S.; Zhang, T.; Gillings, M.R.; Su, J.Q. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Feng, C.; Zhang, J.; Tian, C.; Liu, J. Role of dams in the phase transfer of antibiotics in an urban river receiving wastewater treatment plant effluent. Sci. Total Environ. 2017, 607–608, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River basin, China. Environ. Sci. Technol 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, X.; Cheng, D.; Liu, G.; Liang, B.; Cui, B.; Bai, J. Temporal-spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River delta, China. Sci. Total Environ. 2016, 569–570, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhou, C.; Guo, C.; Wang, D.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci. Total Environ. 2014, 497–498, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Siedlewicz, G.; Bialk-Bielinska, A.; Borecka, M.; Winogradow, A.; Stepnowski, P.; Pazdro, K. Presence, concentrations and risk assessment of selected antibiotic residues in sediments and near-bottom waters collected from the polish coastal zone in the Southern Baltic Sea—Summary of 3 years of studies. Mar. Pollut. Bull. 2017. [Google Scholar] [CrossRef] [PubMed]
- Osorio, V.; Larranaga, A.; Acena, J.; Perez, S.; Barcelo, D. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Sci. Total Environ. 2016, 540, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Zhou, K.; Sun, X.L.; Zhao, L.R.; Zhang, Y.B. Occurrence and distribution of the environmental pollutant antibiotics in Gaoqiao mangrove area, China. Chemosphere 2016, 147, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, V.G.; MacKay, A.A. Ph-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Tamtam, F.; van Oort, F.; Le Bot, B.; Dinh, T.; Mompelat, S.; Chevreuil, M.; Lamy, I.; Thiry, M. Assessing the fate of antibiotic contaminants in metal contaminated soils four years after cessation of long-term waste water irrigation. Sci. Total Environ. 2011, 409, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Ge, G.; Sun, W.; Liang, Y.; Wu, L. Impacts of soil organic matter, ph and exogenous copper on sorption behavior of norfloxacin in three soils. J. Environ. Sci. China 2009, 21, 632–640. (In Chinese) [Google Scholar] [CrossRef]
- Cheng, D.; Liu, X.; Wang, L.; Gong, W.; Liu, G.; Fu, W.; Cheng, M. Seasonal variation and sediment-water exchange of antibiotics in a shallower large lake in north China. Sci. Total Environ. 2014, 476–477, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Cao, X.H.; Lin, H.; Wang, J. Antibiotics and antibiotic resistance genes in sediment of Honghu Lake and east Dongting Lake, China. Microb. Ecol. 2016, 72, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Larsson, D.G. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G. Antibiotics in the environment. Upsala J. Med. Sci. 2014, 119, 108–112. [Google Scholar] [CrossRef] [PubMed]



| Classification | Compound | Range | Mean | Median | Detection Rate (%) |
|---|---|---|---|---|---|
| Sulfonamides | sulfadiazine | 1.7–83.8 | 15.9 | 10.3 | 100 (39/39) |
| sulfamonomethoxine | 2.2–66.0 | 18.6 | 14.7 | 100 (39/39) | |
| sulfaquinoxaline | 0.4–6.0 | 2.68 | 2.7 | 100 (39/39) | |
| Fluoroquinolones | norfloxacin | <LOQ–156.3 | 62.3 | 58.9 | 97.4 (38/39) |
| ciprofloxacin | <LOQ–442.1 | 169.2 | 156.7 | 94.9 (37/39) | |
| ofloxacin | <LOQ–17.2 | 7.1 | 6.7 | 97.4 (38/39) | |
| Beta-lactamases | cefalexin | <LOQ–25.7 | 7.4 | 5.6 | 97.4 (38/39) |
| penicillin G | <LOQ–97.0 | 19.2 | 10.8 | 92.3 (36/39) | |
| penicillin V | <LOQ–115.8 | 42.7 | 35.4 | 92.3 (36/49) | |
| Tetracyclines | oxytetracycline | <LOQ–135.5 | 49.7 | 41.0 | 94.9 (37/39) |
| tetracycline | <LOQ–111.5 | 44.9 | 43.3 | 92.3 (36/39) | |
| doxycycline | 0.8~256.4 | 20.6 | 9.1 | 100 (39/39) | |
| Others | tylosin | <LOQ–1.6 | 0.6 | 0.6 | 74.4 (29/39) |
| erythromycin-H2O | <LOQ–6.9 | 0.7 | 0.3 | 79.5 (31/38) | |
| lincomycin | 0.13–10.4 | 1.4 | 0.7 | 100 (39/39) |
| Classification | Compound | Range | Mean | Median | Detection Rate (%) |
|---|---|---|---|---|---|
| Sulfonamides | sulfadiazine | 0.7–19.5 | 7.6 | 3.8 | 100 (15/15) |
| sulfamonomethoxine | 1.5–20.1 | 7.8 | 5.5 | 100 (15/15) | |
| sulfaquinoxaline | 0.8–6.6 | 2.8 | 1.9 | 100 (15/15) | |
| Fluoroquinolones | norfloxacin | 9.7–132.3 | 30.3 | 22.0 | 100 (15/15) |
| ciprofloxacin | 6.1–27.4 | 16.4 | 18.4 | 100 (15/15) | |
| ofloxacin | <LOQ–13.5 | 6.9 | 5.0 | 93.3 (14/15) | |
| Beta-lactamases | cefalexin | 3.7–21.9 | 12.6 | 10.8 | 100 (15/15) |
| penicillin G | <LOQ–28.9 | 4.8 | <LOQ | 33.3 (5/15) | |
| penicillin V | <LOQ–73.2 | 16.3 | 11.3 | 80 (12/15) | |
| Tetracyclines | oxytetracycline | 12.2–102.4 | 44.6 | 44.2 | 100 (15/15) |
| tetracycline | 6.0–95.7 | 40.0 | 30.4 | 100 (15/15) | |
| doxycycline | 0.8–20.9 | 5.2 | 3.1 | 100 (15/15) | |
| Others | tylosin | <LOQ–4.8 | 0.6 | <LOQ | 46.7 (7/15) |
| erythromycin-H2O | 0.4–9.1 | 3.0 | 2.0 | 100 (15/15) | |
| lincomycin | 0.2–24.7 | 6.4 | 6.0 | 100 (15/15) | |
| vancomycin | <LOQ–7.0 | 1.4 | 1.1 | 60 (9/15) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, H.; Zhang, L.; Jiang, Y.; Gin, K.Y.-H.; He, Y. Occurrence, Distribution, and Risk Assessment of Antibiotics in a Subtropical River-Reservoir System. Water 2018, 10, 104. https://doi.org/10.3390/w10020104
Chen Y, Chen H, Zhang L, Jiang Y, Gin KY-H, He Y. Occurrence, Distribution, and Risk Assessment of Antibiotics in a Subtropical River-Reservoir System. Water. 2018; 10(2):104. https://doi.org/10.3390/w10020104
Chicago/Turabian StyleChen, Yihan, Hongjie Chen, Li Zhang, Yue Jiang, Karina Yew-Hoong Gin, and Yiliang He. 2018. "Occurrence, Distribution, and Risk Assessment of Antibiotics in a Subtropical River-Reservoir System" Water 10, no. 2: 104. https://doi.org/10.3390/w10020104




