Considering a Threshold Energy in Reactive Transport Modeling of Microbially Mediated Redox Reactions in an Arsenic-Affected Aquifer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Hydrogeochemical Conceptual Model
2.3. Modeling Strategy
- Model 1—this model considered a threshold energy for TEAPs (i.e., Fe-oxide reduction, sulfate reduction, and methanogenesis) on a unidirectional reaction using the “extended partial equilibrium” approach [29].
- Model 2—this model implemented a bidirectional threshold energy for the Fe(III)/Fe(II) redox pair, which is the “extended partial equilibrium” approach with the “energy gap” for this reaction pair, in a similar manner to what Jakobsen [28] considered for the redox reaction pair methanogenesis/methane oxidation; sulfate reduction and methanogenesis were modeled using a unidirectional threshold energy.
- Model 3—this model implemented a bidirectional threshold energy for the redox pair methanogenesis/methane oxidation; Fe-oxide reduction and sulfate reduction were modeled using a unidirectional threshold energy.
2.4. Model Settings and Calibration
2.4.1. Model 0
- Irreversible reactions: (a) oxidation of OM sourced by peat as (CH2O)106(NH3)4.5(H3PO4), producing inorganic C, NH4, and inorganic P; and (b) the reductive dissolution of Mn-oxide driven by adding Mn-oxide to the reduced system.
- Equilibrium reactions: (a) the reductive dissolution of Fe-oxide with trace As(V); (b) the precipitation of calcite, dolomite, siderite, and rhodochrosite; and (c) the precipitation of FeS with trace As(III).
2.4.2. Model 1
2.4.3. Models 2 and 3
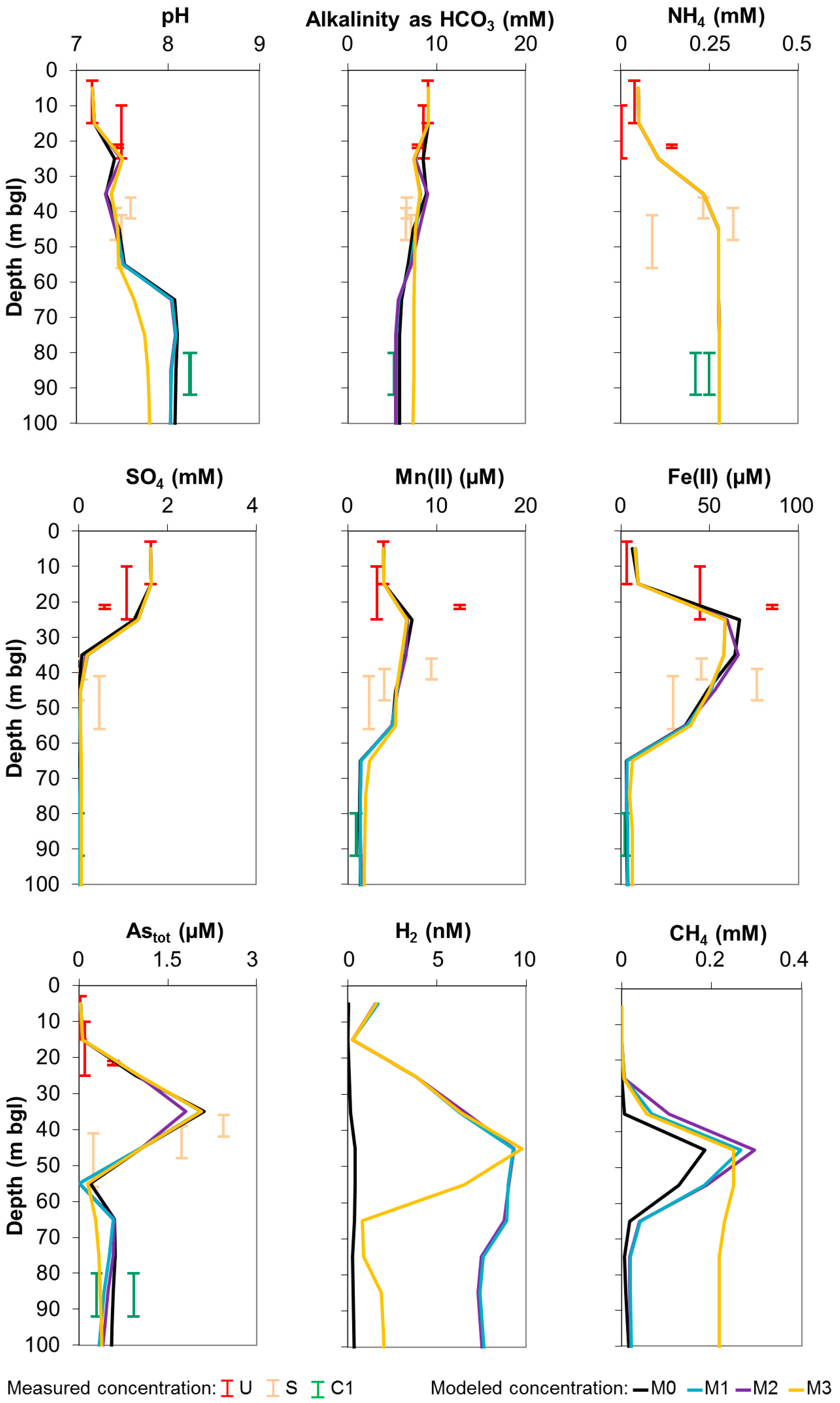
3. Results and Discussion
4. Conclusions
- The “partial equilibrium” approach can be extended to comply more closely to the energy requirements of the microorganisms mediating TEAP processes with the aim of better understanding the hydro-bio-geochemistry of the aquifer system under analysis.
- The use of a threshold energy, with the aim of incorporating the role of reaction mediation played by bacteria into reactive transport modeling, has some influence on the fitted rates of the modeled processes and on modeled H2 concentrations. So, implementing a threshold energy in reactive transport modeling could be useful when rate measurements and/or measured threshold values of H2 from in situ or batch experiments of microbially mediated processes are available. Imposing a threshold energy and using observations of rates and H2 concentrations would help to constrain the model fit.
- The implementation of a threshold energy for only the reduction processes may lead to obviously faulty reverse reactions occurring with a loss of energy. This would occur in systems with a changing condition, e.g., anoxic water that subsequently seeped into oxic sediments, and implementing 2D or 3D models that involve more complex groundwater flow paths, facilitating a mixing of different redox conditions. However, our 1D modeling indicates that in simpler flow systems and under stable anoxic conditions, the problem of reverse oxidation reactions occurring with a loss of energy is negligible for some redox reaction pairs (e.g., Fe(III) reduction/Fe(II) oxidation), so the use of a unidirectional threshold energy simulated through the logK shifting method could be suitable in these cases.
- By implementing a description of the redox process pairs that has the “energy gap”, the artefact of faulty reverse reactions occurring with a loss of energy can be avoided. The use of the bidirectional energy gap description is required in 1D modeling for those reaction pairs that alternate their preferential reaction direction, as occurred in our modeling for methanogenesis/methane oxidation, and is recommended for all the important TEAPs in 2D or 3D models involving more complex groundwater flow paths which can facilitate a mixing of anoxic and oxic conditions where, for instance, both methane and Fe2+ would be oxidized.
- The main advantages and disadvantages of implementing a threshold energy in reactive transport modeling that emerge from this study are: (a) including threshold energies makes it possible to include H2 data when calibrating; (b) models implementing a bidirectional threshold energy take much longer to run and often show convergence problems; (c) unidirectional threshold energies cannot be used when reoxdation occurs within the model.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar]
- Nickson, R.; McArthur, J.; Burgess, W.; Ahmed, K.M.; Ravenscroft, P.; Rahmann, M. Arsenic poisoning of Bangladesh groundwater. Nature 1998, 395, 338. [Google Scholar] [CrossRef] [PubMed]
- Burgess, W.G.; Pinto, L. Preliminary observations on the release of arsenic to groundwater in the presence of hydrocarbon contaminants in UK aquifers. Mineral. Mag. 2005, 69, 887–896. [Google Scholar] [CrossRef]
- McArthur, J.M.; Ravenscroft, P.; Safiulla, S.; Thirlwall, M.F. Arsenic in groundwater: Testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour. Res. 2001, 37, 109–117. [Google Scholar] [CrossRef]
- Ravenscroft, P.; McArthur, J.M.; Hoque, B.A. Geochemical and palaeohydrological controls on pollution of groundwater by arsenic. In Arsenic Exposure and Health Effects IV; Chappell, W.R., Abernathy, C.O., Calderon, R., Eds.; Elsevier Science: Oxford, UK, 2001; pp. 53–78. [Google Scholar]
- Lawson, M.; Polya, D.A.; Boyce, A.J.; Bryant, C.; Ballentine, C.J. Tracing organic matter composition and distribution and its role on arsenic release in shallow Cambodian groundwaters. Geochim. Cosmochim. Acta 2016, 178, 160–177. [Google Scholar] [CrossRef]
- McArthur, J.M.; Ravenscroft, P.; Sracek, O. Aquifer arsenic source. Nat. Geosci. 2011, 4, 655–656. [Google Scholar] [CrossRef]
- Neumann, R.B.; Ashfaque, K.N.; Badruzzaman, A.B.M.; Ali, M.A.; Shoemaker, J.K.; Harvey, C.F. Reply to ‘Aquifer arsenic source’. Nat. Geosci. 2011, 4, 656. [Google Scholar] [CrossRef]
- Carraro, A.; Fabbri, P.; Giaretta, A.; Peruzzo, L.; Tateo, F.; Tellini, F. Effects of redox conditions on the control of arsenic mobility in shallow alluvial aquifers on the Venetian Plain (Italy). Sci. Total Environ. 2015, 532, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Dalla Libera, N.; Fabbri, P.; Mason, L.; Piccinini, L.; Pola, M. Geostatistics as a tool to improve the natural background level definition: An application in groundwater. Sci. Total Environ. 2017, 598, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Rotiroti, M.; Jakobsen, R.; Fumagalli, L.; Bonomi, T. Arsenic release and attenuation in a multilayer aquifer in the Po Plain (northern Italy): Reactive transport modeling. Appl. Geochem. 2015, 63, 599–609. [Google Scholar] [CrossRef]
- Rotiroti, M.; Sacchi, E.; Fumagalli, L.; Bonomi, T. Origin of arsenic in groundwater from the multi-layer aquifer in Cremona (northern Italy). Environ. Sci. Technol. 2014, 48, 5395–5403. [Google Scholar] [CrossRef] [PubMed]
- Zavatti, A.; Attramini, D.; Bonazzi, A.; Boraldi, V.; Malagò, R.; Martinelli, G.; Naldi, S.; Patrizi, G.; Pezzera, G.; Vandini, W.; et al. La presenza di arsenico nelle acque sotterranee della Pianura Padana: Evidenze ambientali e ipotesi geochimiche “Occurrence of groundwater arsenic in the Po Plain: Environmental evidences and geochemical hypothesis”. Quad. Geol. Appl. 1995, S2, 2.301–2.326. [Google Scholar]
- Banfield, J.F.; Nealson, K.H. Geomicrobiology: Interactions between Microbes and Minerals; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Washington, DC, USA, 1997; Volume 35. [Google Scholar]
- Chapelle, H.F. The significance of microbial processes in hydrogeology and geochemistry. Hydrogeol. J. 2000, 8, 41–46. [Google Scholar] [CrossRef]
- Islam, F.S.; Gault, A.G.; Boothman, C.; Polya, D.A.; Charnock, J.M.; Chatterjee, D.; Lloyd, J.R. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 2004, 430, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, Y.; Jia, Y.; Tang, X.; Li, X.; Shen, M.; Lu, H.; Han, S.; Wei, C.; Norra, S.; et al. Sulfur cycling-related biogeochemical processes of arsenic mobilization in the western Hetao Basin, China: Evidence from multiple isotope approaches. Environ. Sci. Technol. 2016, 50, 12650–12659. [Google Scholar] [CrossRef] [PubMed]
- Cavalca, L.; Corsini, A.; Zaccheo, P.; Andreoni, V.; Muyzer, G. Microbial transformations of arsenic: Perspectives for biological removal of arsenic from water. Future Microbiol. 2013, 8, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Corsini, A.; Zaccheo, P.; Muyzer, G.; Andreoni, V.; Cavalca, L. Arsenic transforming abilities of groundwater bacteria and the combined use of Aliihoeflea sp. strain 2WW and goethite in metalloid removal. J. Hazard. Mater. 2014, 269, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bethke, C.M.; Sanford, R.A.; Kirk, M.F.; Jin, Q.; Flynn, T.M. The thermodynamic ladder in geomicrobiology. Am. J. Sci. 2011, 311, 183–210. [Google Scholar] [CrossRef]
- Jin, Q.; Bethke, C.M. A new rate law describing microbial respiration. Appl. Environ. Microbiol. 2003, 69, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Bethke, C.M. The thermodynamics and kinetics of microbial metabolism. Am. J. Sci. 2007, 307, 643–677. [Google Scholar] [CrossRef]
- Colombani, N.; Mastrocicco, M.; Prommer, H.; Sbarbati, C.; Petitta, M. Fate of arsenic, phosphate and ammonium plumes in a coastal aquifer affected by saltwater intrusion. J. Contam. Hydrol. 2015, 179, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Kocar, B.D.; Benner, S.G.; Fendorf, S. Deciphering and predicting spatial and temporal concentrations of arsenic within the Mekong Delta aquifer. Environ. Chem. 2014, 11, 579–594. [Google Scholar] [CrossRef]
- Postma, D.; Larsen, F.; Minh Hue, N.T.; Duc, M.T.; Viet, P.H.; Nhan, P.Q.; Jessen, S. Arsenic in groundwater of the Red River floodplain, Vietnam: Controlling geochemical processes and reactive transport modeling. Geochim. Cosmochim. Acta 2007, 71, 5054–5071. [Google Scholar] [CrossRef]
- Postma, D.; Pham, T.K.T.; Sø, H.U.; Hoang, V.H.; Vi, M.L.; Nguyen, T.T.; Larsen, F.; Pham, H.V.; Jakobsen, R. A model for the evolution in water chemistry of an arsenic contaminated aquifer over the last 6000 years, Red River floodplain, Vietnam. Geochim. Cosmochim. Acta 2016, 195, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.; Siade, A.; Sun, J.; Neidhardt, H.; Berg, M.; Prommer, H. Quantifying reactive transport processes governing arsenic mobility after injection of reactive organic carbon into a Bengal Delta aquifer. Environ. Sci. Technol. 2017, 51, 8471–8480. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, R. Redox microniches in groundwater: A model study on the geometric and kinetic conditions required for concomitant Fe oxide reduction, sulfate reduction, and methanogenesis. Water Resour. Res. 2007, 43, W12S12. [Google Scholar] [CrossRef]
- Jakobsen, R.; Cold, L. Geochemistry at the sulfate reduction–methanogenesis transition zone in an anoxic aquifer—A partial equilibrium interpretation using 2D reactive transport modeling. Geochim. Cosmochim. Acta 2007, 71, 1949–1966. [Google Scholar] [CrossRef]
- Jin, Q.; Bethke, C.M. Cellular energy conservation and the rate of microbial sulfate reduction. Geology 2009, 37, 1027–1030. [Google Scholar] [CrossRef]
- Hoehler, T.M.; Alperin, M.J.; Albert, D.B.; Martens, C.S. Apparent minimum free energy requirements for methanogenic Archaea and sulfate-reducing bacteria in an anoxic marine sediment. FEMS Microbiol. Ecol. 2001, 38, 33–41. [Google Scholar] [CrossRef]
- West, J.M.; McKinley, I.G.; Palumbo-Roe, B.; Rochelle, C.A. Potential impact of CO2 storage on subsurface microbial ecosystems and implications for groundwater quality. Energy Procedia 2011, 4, 3163–3170. [Google Scholar] [CrossRef]
- Rotiroti, M.; Di Mauro, B.; Fumagalli, L.; Bonomi, T. COMPSEC, a new tool to derive natural background levels by the component separation approach: Application in two different hydrogeological contexts in northern Italy. J. Geochem. Explor. 2015, 158, 44–54. [Google Scholar] [CrossRef]
- Rotiroti, M.; McArthur, J.; Fumagalli, L.; Stefania, G.A.; Sacchi, E.; Bonomi, T. Pollutant sources in an arsenic-affected multilayer aquifer in the Po Plain of Italy: Implications for drinking-water supply. Sci. Total Environ. 2017, 578, 502–512. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.M.; Banerjee, D.M.; Hudson-Edwards, K.A.; Mishra, R.; Purohit, R.; Ravenscroft, P.; Cronin, A.; Howarth, R.J.; Chatterjee, A.; Talukder, T.; et al. Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: The example of West Bengal and its worldwide implications. Appl. Geochem. 2004, 19, 1255–1293. [Google Scholar] [CrossRef]
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Lowers, H.A.; Breit, G.N.; Foster, A.L.; Whitney, J.; Yount, J.; Uddin, M.N.; Muneem, A.A. Arsenic incorporation into authigenic pyrite, Bengal Basin sediment, Bangladesh. Geochim. Cosmochim. Acta 2007, 71, 2699–2717. [Google Scholar] [CrossRef]
- Postma, D.; Jakobsen, R. Redox zonation: Equilibrium constraints on the Fe(III)/SO4-reduction interface. Geochim. Cosmochim. Acta 1996, 60, 3169–3175. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods, Book 6, Modeling Techniques; USGS: Reston, VA, USA, 2013; Chapter 43. [Google Scholar]
- Ball, J.W.; Nordstrom, D.K. Wateq4f—User’s Manual with Revised Thermodynamic Data Base and Test Cases for Calculating Speciation of Major, Trace and Redox Elements in Natural Waters; USGS Open-File Report 90-129; USGS: Reston, VA, USA, 1991.
- Christensen, T.H.; Bjerg, P.L.; Banwart, S.A.; Jakobsen, R.; Heron, G.; Albrechtsen, H.-J. Characterization of redox conditions in groundwater contaminant plumes. J. Contam. Hydrol. 2000, 45, 165–241. [Google Scholar] [CrossRef]
- Chapelle, F.H.; McMahon, P.B.; Dubrovsky, N.M.; Fujii, R.F.; Oaksford, E.T.; Vroblesky, D.A. Deducing the distribution of terminal electron-accepting processes in hydrologically diverse groundwater systems. Water Resour. Res. 1995, 31, 359–371. [Google Scholar] [CrossRef]
- Jakobsen, R.; Postma, D. In situ rates of sulfate reduction in an aquifer (Rømø, Denmark) and implications for the reactivity of organic matter. Geology 1994, 22, 1101–1106. [Google Scholar] [CrossRef]
- McMahon, P.B.; Chapelle, F.H. Redox processes and water quality of selected principal aquifer systems. Ground Water 2008, 46, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.R.; Donahoe, R.J.; Roden, E.E.; Ferris, F.G. Effects of microbial iron oxide reduction on pH and alkalinity in anaerobic bicarbonate-buffered media: Implications for metal mobility. Mineral. Mag. 1998, 62A, 657–658. [Google Scholar] [CrossRef]
- Osborne, T.H.; McArthur, J.M.; Sikdar, P.K.; Santini, J.M. Isolation of an arsenate-respiring bacterium from a redox front in an arsenic-polluted aquifer in West Bengal, Bengal basin. Environ. Sci. Technol. 2015, 49, 4193–4199. [Google Scholar] [CrossRef] [PubMed]

| Cell | Depth (m bgl) | Hydrogeological Unit |
|---|---|---|
| 1 | 0–10 | Aquifer U |
| 2 | 10–20 | Aquifer U |
| 3 | 20–30 | Aquitard U/S |
| 4 | 30–40 | Aquifer S |
| 5 | 40–50 | Aquifer S |
| 6 | 50–60 | Aquifer S |
| 7 | 60–70 | Aquitard S/C1 |
| 8 | 70–80 | Aquitard S/C1 |
| 9 | 80–90 | Aquifer C1 |
| 10 | 90–100 | Aquifer C1 |
| 11 | 100–110 | Aquifer C1 |
| 12 | 110–120 | Aquitard C1/C2 |
| Name | Reaction | logK |
|---|---|---|
| Fe-oxide reduction | Fe3+ + e− ↔ Fe2+ | 13.02 |
| Sulfate reduction | SO42− + 10H+ + 8e− ↔ H2S + 4H2O | 40.64 |
| Methanogenesis | CO32− + 10H+ + 8e− ↔ CH4 + 3H2O | 41.07 |
| Name | Reaction | logK | Shifted logK 1 | Threshold Energy (kJ/mol e−) |
|---|---|---|---|---|
| Fe-oxide reduction | Fe3+ + 0.5H2 ↔ Fe2+ + H+ | 14.55 | 13.87 | 3.76 2 |
| Sulfate reduction | SO42− + 2H+ + 4H2 ↔ H2S + 4H2O | 52.96 | 46.44 | 4.50 |
| Methanogenesis | HCO3− + H+ + 4H2 ↔ CH4 + 3H2O | 40.18 | 37.86 | 1.60 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotiroti, M.; Jakobsen, R.; Fumagalli, L.; Bonomi, T. Considering a Threshold Energy in Reactive Transport Modeling of Microbially Mediated Redox Reactions in an Arsenic-Affected Aquifer. Water 2018, 10, 90. https://doi.org/10.3390/w10010090
Rotiroti M, Jakobsen R, Fumagalli L, Bonomi T. Considering a Threshold Energy in Reactive Transport Modeling of Microbially Mediated Redox Reactions in an Arsenic-Affected Aquifer. Water. 2018; 10(1):90. https://doi.org/10.3390/w10010090
Chicago/Turabian StyleRotiroti, Marco, Rasmus Jakobsen, Letizia Fumagalli, and Tullia Bonomi. 2018. "Considering a Threshold Energy in Reactive Transport Modeling of Microbially Mediated Redox Reactions in an Arsenic-Affected Aquifer" Water 10, no. 1: 90. https://doi.org/10.3390/w10010090





