1. Introduction
Magnetic nanoparticles (MNPs) are materials with large surface-to-volume ratio and high surface energies with unique magnetic, optical, thermal, catalytic, and sorption properties, making them good candidates for advanced nanotechnological applications [
1]. In order to reduce their surface energies, they undergo aggregation and a typical bare magnetic nanoparticle possesses a very high chemical activity and tends to oxidize in air, thereby causing a decrease or loss in their magnetic and dispersibility characteristics. To prevent this decrease or loss in some of their properties, there is a need to coat the surface of MNPs and effectively protect it against instability. Many of the strategies adopted for the surface modification of MNPs include covalent (such as the grafting of or surface coating with organic molecules, including surfactants, small organic molecules, polymers and biomolecules) and non-covalent ones (such as coating with an inorganic layer, metal or non metal). Other strategies involve the grafting of other nanomaterial types onto MNPs to create composite materials. Most times, surface coating or functionalization not only stabilizes the magnetic core, but also affords further functionalization [
2,
3], thereby allowing the introduction of new functional groups such as -OH, -COOH, -NH
2, C=N, -NR, -SR, -F, and -Cl onto the walls or sides of the MNPs.
The use of chelating ligand, 4-{[(E)-phenylmethylidene]amino}benzoic acid (Maph-COOH) for the functionalization of MNPs so as to introduce the imine functional group CH=N onto the walls of MNPs has generated huge potential in the functionalization of MNPs for environmental applications. However the application of MNPs in various research areas cannot be overemphasized because of their intrinsic properties. Despite the vast works on MNPs and their applications, more research still needs to be conducted on the functionalization of MNPs with multiple functional groups for the removal of contaminants from aqueous solution.
Bidentate chelating ligand, 4-{[(E)-phenylmethylidene]amino}benzoic acid (Maph-COOH) consists of an imine functional group (-CH=N) and a terminal end -COOH group. In order to introduce imine functional groups onto the surface of MNPs, the terminal -COOH can be bonded to amine surface-modified MNPs (MNP-NH2). Maph-COOH possesses a high capability to form bonds with metal centers through one or more of its imine groups. Even though a lot of research has been done on MNPs for the uptake of contaminants from aqueous solutions, to the best of our knowledge, no study on the functionalization of MNPs-NH2 with Maph-COOH for the efficient removal of Zn(II) and As(III) from aqueous solutions has been reported.
Among the environmental problems today is the proliferation of heavy metals to the environment [
4]. Sources of these heavy metals into the environment are mainly through the industries (such as metal plating, paint manufacturing, electric device manufacturing, mining etc.). Zinc dominance in municipal and industrial water is essential in minute quantities for the growth and development of humans. In as much as it is essential to humans, it is also very harmful to human health, especially when consumed at higher concentrations. When ingested into the body, zinc causes anemia, stunted growth, and sometimes death [
5]. Similarly, arsenic is regarded as one of the most toxic heavy metals for aquatic bodies and the food chain, even at extremely low concentrations. Among the negative effects of exposure to arsenic include bladder cancer, kidney and lung issues, skin irritation, and death [
6,
7]. Different forms of arsenic exist, and the oxidation state of any heavy metal largely determines its toxicity, with As(III) being more toxic than any other forms of arsenic in aqueous solutions [
8].
Because of the enormous treat posed by these toxic metals to the environment and human beings, it is imperative to give great attention to water treatment. Far thus, various techniques such as ion exchange [
9,
10], coagulation [
11], membrane separation [
12,
13], reverse osmosis [
14], chemical precipitation [
15], chemical oxidation [
16], and adsorption [
17] have been employed for the removal of heavy metals from aqueous solutions, but due to the simplicity in its operation, cost effectiveness, efficiency, and potential to regenerate adsorbents, the adsorption technique has shown to be the most reliable technique for the removal of metal ions from aqueous solutions. Also, several adsorbents such as carbon nanotubes [
18], activated carbon [
19], bagasse [
20], lignocelluloses [
21], chitosan [
22,
23] etc. have been reported for the removal of toxic ions from municipal and industrial effluents, but noticeable drawbacks such as high cost, generation of secondary pollutants, difficulty of separation, and low adsorption capacity are characteristics of these materials. Functionalized magnetic nanoparticles ensure increased and enhanced adsorption capacity, aid easy separation of adsorbents from solution, and are very easy to prepare.
In this paper, we investigate the removal of Zn(II) and As(III) from aqueous solutions by adsorption onto imine functionalized magnetic nanoparticles (MNP-Maph) synthesized by the covalent bonding of 4-{[(E)-phenylmethylidene]amino}benzoic acid (Maph-COOH) with amine functionalized magnetic nanoparticles (MNP-NH2). Batch adsorption studies involving the effect of time, pH, temperature, and adsorbent dose on the adsorption of Zn(II) and As(III) from wastewater were also investigated. Also, a recyclability study was also conducted to ascertain whether Zn(II) and As(III) could be desorbed from the adsorbent so as to minimize the discharge of secondary pollutants into the environment. Furthermore, the potential of imine-functionalized magnetic nanoparticles for the removal of Zn(II) and As(III) from municipal wastewater samples obtained from the Eastern Cape Region of South Africa was evaluated at optimized conditions.
2. Experimental
2.1. Chemicals and Materials
Nickel (II) chloride (NiCl2·6H2O) and iron (III) chloride (FeCl3·6H2O), absolute ethanol, 25% ammonia solution, sodium hydroxide pellets, and hydrochloric acid were purchased from Merck (Pty) Ltd., Gauteng, South Africa. Chemicals such as dicyclohexylcarbodiimide (DCC), 4-aminobenzoic acid, tetraethyl orthosilicate (TEOS) and 3-aminopropyltriethoxylsilane (APTES) were purchased from Sigma Aldrich, while 4-dimethylaminopyridine (DMAP) and benzaldehyde were purchased from SAAR chem (Mumbai, India) and dimethylformamide (DMF) was purchased from BDH Limited (Poole, UK). Pure zinc metal powder and diarsenic trioxide standard solution were purchased from Industrial Analytical (Pty) Ltd., Johannesburg, South Africa. All materials purchased were of analytical grade and used without further purification. Separation of magnetic adsorbents from aqueous solution was achieved by using a bar magnet.
2.2. Synthesis of Materials
2.2.1. Synthesis of 4-{[(E)-Phenylmethylidene]amino}Benzoic Acid (Maph-COOH)
Maph-COOH was synthesized using the method of Kumar et al. [
24] with slight modification. 4-aminobenzoic acid in (0.020 moles, 20 mL) of a mixture of ethanol and methanol (2:8
v/
v) was added to a solution of benzaldehyde (0.20 moles) previously dissolved in a mixture of ethanol and methanol (2:8) (20 mL). Two drops of glacial acetic acid was added to the reacting mixture and refluxed at 70 °C for 10 h, the product was allowed to cool at room temperature and left to stay overnight. The product was separated by filtration and the off white powder was recrystallized in ethanol (519.6 mg, 43%), m.pt. 190–192 °C; Infra Red (ATR) (Perkin Elmer, Midrand, South Africa), cm
−1) 2950, 1632, 1580, 1400, 1290, 830;
1H NMR (Nuclear Magnetic Resonance) (400 MHz, dimethyl sulfoxide, DMSO-d
6) δ: 10.0, 8.7, 8.0, 7.6, 7.3, 6.5, 2.5 and
13C NMR (400 MHz, DMSO-d
6) δ: 162.45, 155.20, 136.40, 132.20, 131.06, 130.05, 129.22, 121.86, 112.00, 40.22.
2.2.2. Synthesis of Magnetic Nanoparticles (MNPs)
Magnetic nanoparticles (NiFe
2O
4) were prepared by the co-precipitation method [
25]. Solutions of FeCl
3·6H
2O and NiCl
2·6H
2O in molar ratio 2:1 were prepared and 30 mL of each solution were mixed and stirred under inert atmosphere for 30 min. Sodium hydroxide (10 M) was slowly added to the reacting mixture to take the pH of the mixture to 11. The temperature of the reaction mixture was taken up to 80 °C and this mixture was stirred for 3 h more under an inert condition. The precipitate generated was collected magnetically, washed two times with ethanol and severally with deionized water until the pH was neutral and dried overnight at 80 °C. Finally, the dried powder was calcined at 450 °C for 2 h.
2.2.3. Synthesis of Silica Coated Magnetic Nanoparticles (MNP-Si)
MNPs surface was coated with silica as follows: MNP (300 mg) was placed in a 250 mL round bottom flask and dispersed in ethanol (40 mL) by using a water bath ultrasonicator for 30 min. Furthermore, tetraethoxysilane (TEOS) (3 mL) was added to the mixture and aqueous ammonia solution was used to take the pH of the reaction to 10. This mixture was continuously stirred under nitrogen for 9 h and refluxed at 65 °C overnight. The precipitate obtained was collected by magnetically after severally washing with water and dried for 12 h at 80 °C followed by calcinating at a temperature of 450 °C within 2 h.
2.2.4. Synthesis of Amine Functionalized Magnetic Nanoparticles (MNP-NH2)
In brief, silica-coated MNPs (200 mg) dissolved in absolute ethanol (40 mL) were ultrasonicated for 20 min in order to obtain a homogeneous mixture. Slowly added to this mixture was APTES (2 mL) and the mixture was allowed to reflux under an inert condition at 70 °C for 10 h. The dark brown crude obtained was separated, washed severally with distilled water and dried in an oven for 2 days at 100 °C [
26].
2.2.5. Synthesis of Imine Functionalized MNPs (MNP-Maph)
Functionalization of MNP-NH
2 with 4-{[(E)-phenylmethylidene]amino}benzoic acid (Maph-COOH) to afford imine functionalized MNPs (MNP-Maph), a novel nanomaterial was achieved by covalent bonding of the carboxylic acid group of Maph-COOH with the amino group of MNP-NH
2 [
27,
28]. Maph-COOH (100 mg) previously dissolved in anhydrous DMF was stirred with DCC (100 mg) for 24 h at 20 °C. Solution of MNP-NH
2 (200 mg) in anhydrous DMF and DMAP (70 mg) was added dropwise into the mixture. The suspension was further stirred for 48 h and the product obtained was collected via magnetic means in absolute ethanol to remove DMF, and washed three times with deionized water to remove DCC residues and then oven dried for two days at 100 °C.
2.3. Adsorbate Preparation
Pure zinc metal powder (1.0 g) dissolved in nitric acid (25 mL, 5 M) and made up to 1000 mL with deionized water and diarsenic trioxide standard solution purchased from the supplier were used as stock solutions. Working solutions were prepared from the different stock solutions by correctly diluting an aliquot of the latter to obtain the desired concentrations.
2.4. Adsorption Experiment
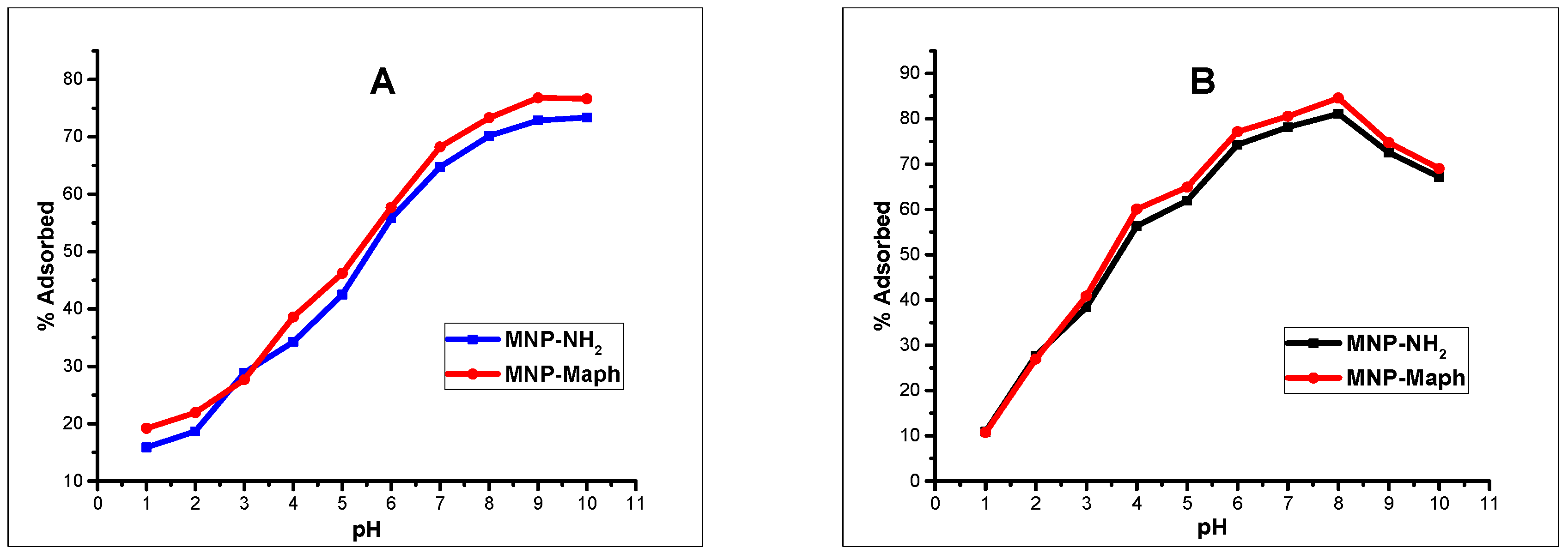
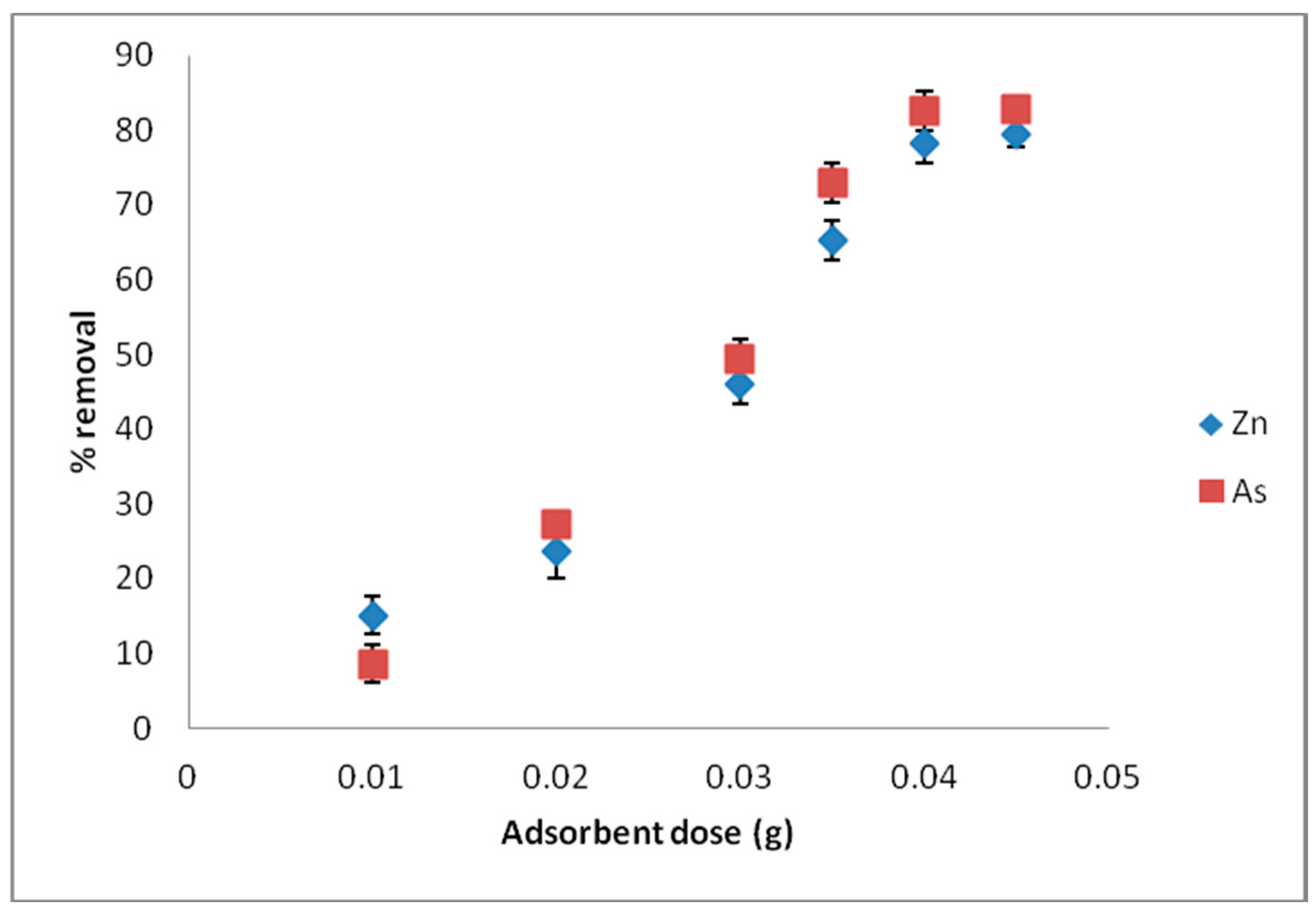
The effect of pH, contact time, adsorbent dose, adsorbate concentration, and temperature was assessed through some batch adsorption experiments involving the sorption of Zn(II) and As(III) onto MNP-Maph. In order to determine the optimum conditions for the removal of Zn(II) and As(III) from aqueous solutions, the various experimental conditions were carefully studied.
Adsorption studies between adsorbent and Zn(II) and As(III) were investigated by agitating 100 mL polypropylene plastic vials containing working solutions of Zn(II) and As(III) (20 mL, 100 mg L
−1) freshly prepared daily from their stock solutions using a thermostated shaker (200 rpm) at 20 °C and an adsorbent dose (40 mg) for 24 h. Appropriate amounts of NaOH or HCl (0.1 M) were used to adjust the solutions so as to attain the desired solution pH. The adsorbent was magnetically separated from the solution after the required time interval and the concentration of metal ions in solution determined by using Thermo Fischer iCE 3500 atomic adsorption spectrophotometer (AAS) (Thermo Fischer, Berlin, Germany). The % adsorbed (adsorption efficiency) and
qe (adsorption capacity) of Zn(II) and As(III) were calculated by using Equations (1) and (2) respectively.
Ci and Ceq is the initial and equilibrium Zn2+ and As3+ concentration in mg L−1, qe is the adsorption capacity in mg g−1, m in mg is the mass of the adsorbent and V is the volume of the adsorbate solution used in L.
Kinetics, Isotherm and Thermodynamics Studies
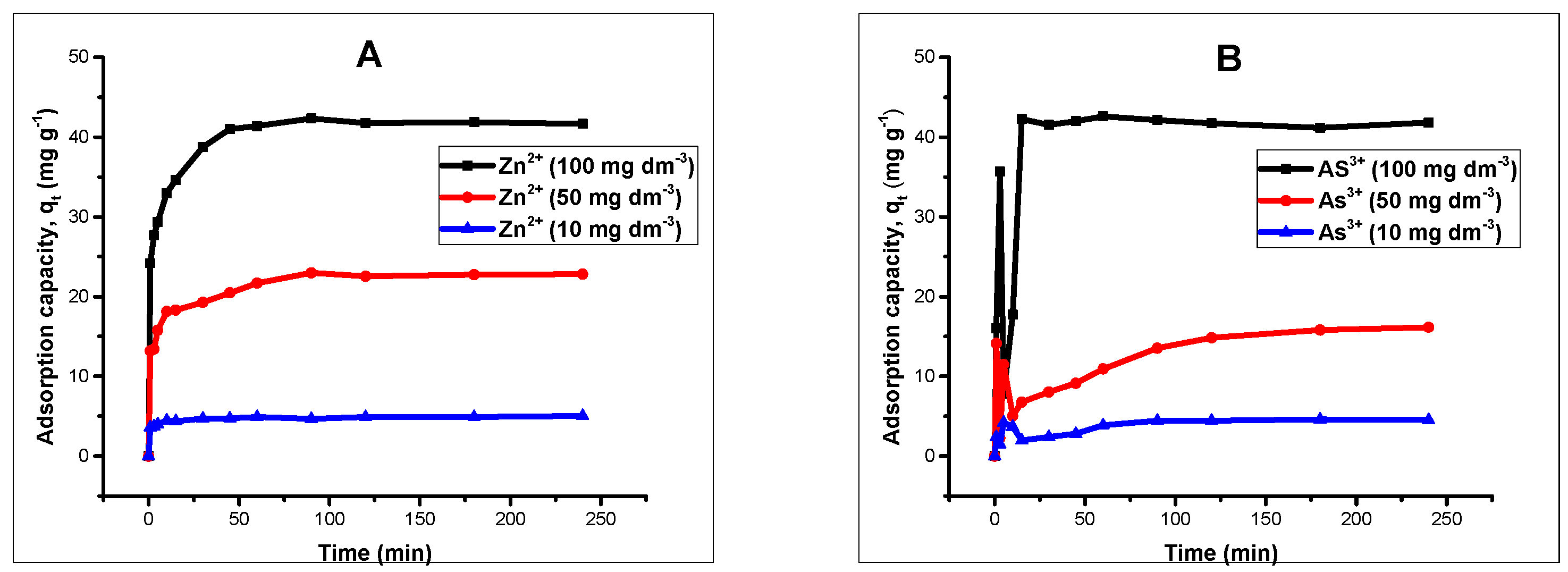
Kinetic studies were conducted by agitating 20 mL 100 mg L of Zn(II) and As(III) solution with an adsorbent amount of 40 mg on a thermostated shaker at 20 °C for different time intervals ranging from 1 to 240 min. The solutions were adjusted to pH 5 and 6 for Zn(II) and As(III) respectively by using 0.1 molar HCl/NaOH. After attaining the set time, the samples were separated magnetically and the concentrations of Zn(II) and As(III) were determined by AAS. Kinetic models such as pseudo-first order, pseudo-second order, intraparticle diffusion and Elovich kinetic models [
19,
29,
30] were used to determine the process which the experimental data obeyed.
Table 1 shows the equation for each model.
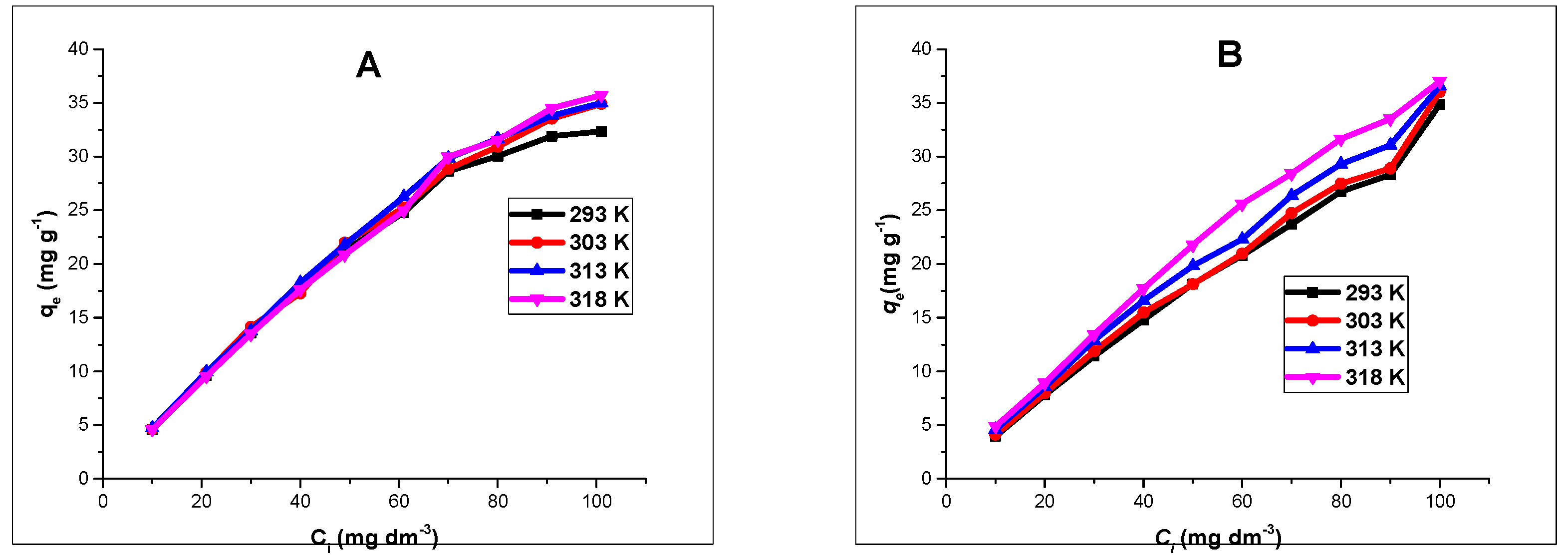
Similarly, isotherm experiments were studied by using different Zn(II) and As(III) concentrations ranging from 10–100 mg L
−1, at a fixed pH of 5 and 6 respectively. Adsorbents dose of 40 mg were agitated with a 20 mL aliquot of Zn(II) and As(III) on a thermostated oven shaker at temperature ranges of 293, 303, 313 and 318 K for 4 h. The solutions were separated magnetically and the concentration of Zn(II) and As(III) in the filtrate determined by using AAS. The experimental adsorption data were fitted into two isotherm models; Langmuir and Freundlich models [
30,
31,
32].
Table 2 shows the equations of the models. Also, thermodynamic parameters such as change in Gibbs energy (∆
G°), change in enthalpy (∆
H°), and change in entropy (∆
S°) were calculated from the data obtained from the effect of temperature.
2.5. Recyclability Studies
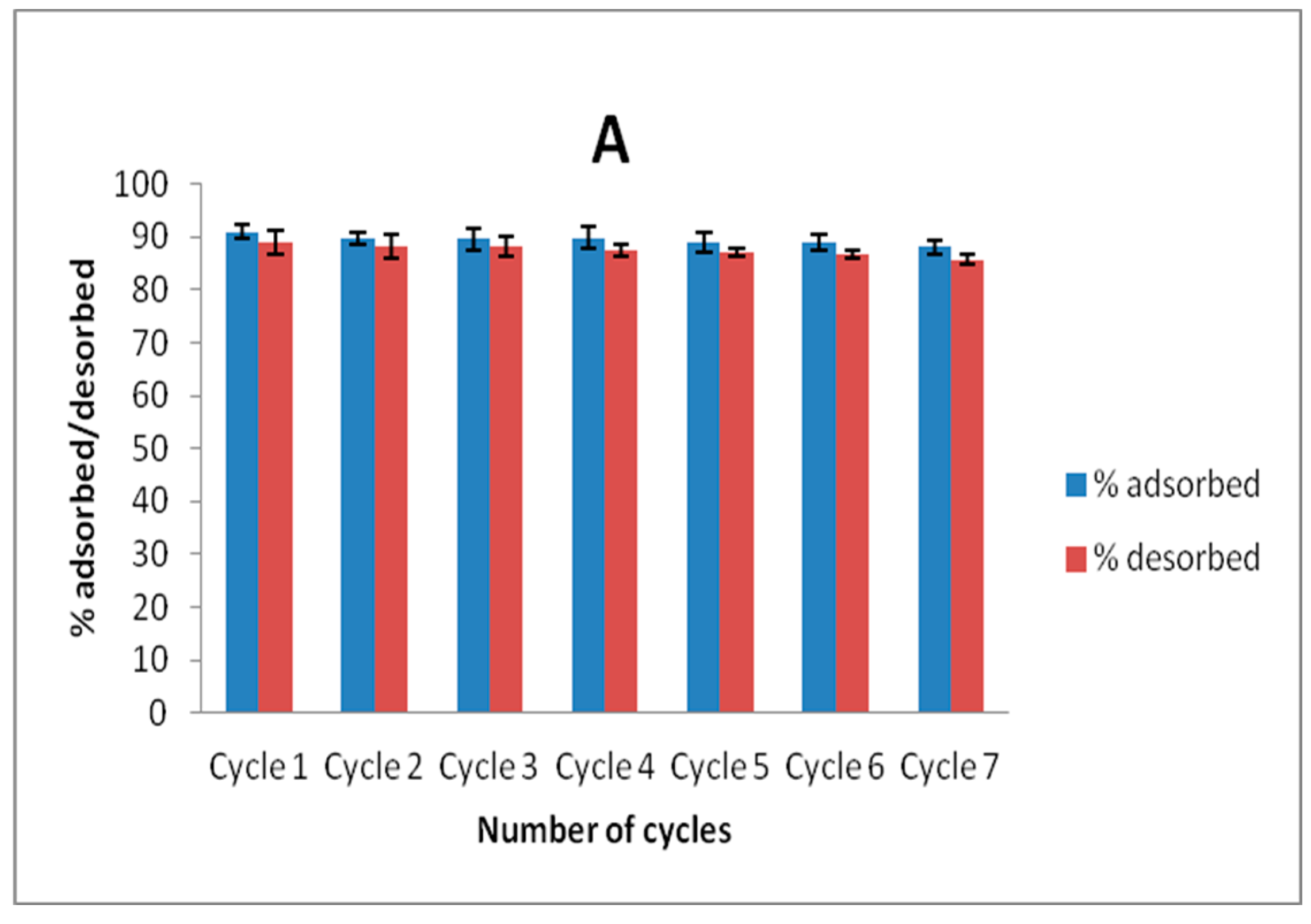
Recyclability studies were conducted by agitating 20 mL volume of 100 mg L−1 solution of Zn(II) and As(III) and adsorbent dose of 40 mg for 6 h. The solutions were magnetically separated and Zn(II) and As(III) concentrations in solution after separation were determined. The spent adsorbents obtained after magnetic separation were dried in an oven at 100 °C for 12 h, and 30 mg of this adsorbent was agitated with 10 mL solution of a 50:50 mixture of 0.1 mol L−1 HNO3 and HCl for 1 h on a shaker at room temperature and the concentrations of Zn(II) and As(III) removed from the adsorbent were then determined by using AAS. This process was allowed to undergo seven cycles to ascertain the stability of MNP-Maph towards Zn(II) and As(III) removal from aqueous solutions.
2.6. Real Sample Analysis
At two different wastewater treatment plants in the Eastern Cape Province, five water samples were collected. Physicochemical parameters of the sampled wastewater were recorded on site and on arrival into our laboratory, the samples were processed for analysis of their initial Zn(II) and As(III) concentrations. An aliquot of 20 mL of the water samples adjusted to pH 6.7 and 6.0 for Zn(II) and As(III) respectively was agitated with 40 mg of adsorbent in polypropylene bottles at 20 °C for 60 min. After shaking by means of a thermostated orbital shaker, the solutions were magnetically separated and the final concentrations of Zn(II) and As(III) in the filtrate were analyzed. To compare the performance of the adsorbent for Zn(II) and As(III) removal in real wastewater and simulated water, a wastewater deliberately contaminated with the metal ions were also prepared and subjected to the same experimental conditions as that of the real wastewater. After adsorption, the percentage removal and adsorption capacity of Zn(II) and As(III) calculated by using Equations (1) and (2), respectively for real wastewater were compared to that of the simulated water sample.
2.7. Statistical Analysis
Linear least square regression routine in the origin software statistical computing systems was used to fit the experimental data into the kinetic and isotherm models [
31]. The origin statistical software considers the adjustment of the regression coefficient square,
R2 and the minimization of the residual sum of squares (
RSS). The model with the highest
R2 and the least
RSS values was chosen to be adequate after a comparison of all
R2 and
RSS values was done.
4. Conclusions
Functionalized magnetic nanoparticles obtained via the covalent coupling of an imine ligand, 4-{[(E)-phenylmethylidene]amino}benzoic acid (Maph-COOH) with amine modified magnetic nanoparticles (MNP-NH2) was applied for the uptake of Zn(II) and As(III) from aqueous solutions through some batch adsorption experiments.
Characterization techniques including Raman spectroscopy showed the introduction of imine groups to the surface of magnetic nanoparticles, confirming the successful functionalization of magnetic nanoparticles with imine.
Batch adsorption experiments revealed that the processes were influenced by the pH of the solutions, contact time, and adsorbent dose. Changes in solution pH showed that higher uptake was favored at alkaline pH, and the effect of time indicated that in the first few minutes of contact between adsorbent and adsorbate solutions, there existed a sharp increase in the percentage adsorption in both divalent and trivalent ion solutions, until equilibrium was attained and the processes obeyed a pseudo-second order rate kinetic model. The Langmuir adsorption isotherm model was observed to be the most suitable isotherm model for the spontaneous processes.
Although, the uptake of As(III) was observed to be higher than that of Zn(II) in this study, the adsorbent still proved efficient for the removal of both metal ions from aqueous solutions, going by their considerably high adsorption capacity compared to that observed from previously reported studies on these contaminants. Application of this adsorbent to real wastewater samples for the uptake of Zn(II) and As(III) showed a remarkable sorption of the metal ions from aqueous solution, indicating the capability of this material to selectively remove metal ions from a complex matrix. We therefore infer that imine-functionalized magnetic nanoparticles (MNP-Maph) are a good contender as a potential adsorbent for the uptake of Zn(II) and As(III) from aqueous solutions without separation difficulty.