PM1 Chemical Characterization during the ACU15 Campaign, South of Mexico City
Abstract
:1. Introduction
2. Experiments
2.1. ACU15 Campaign
2.2. Aerosol Chemical Speciation Monitor (ACSM)
2.3. Other Co-Located Measurements
3. Results and Discussion
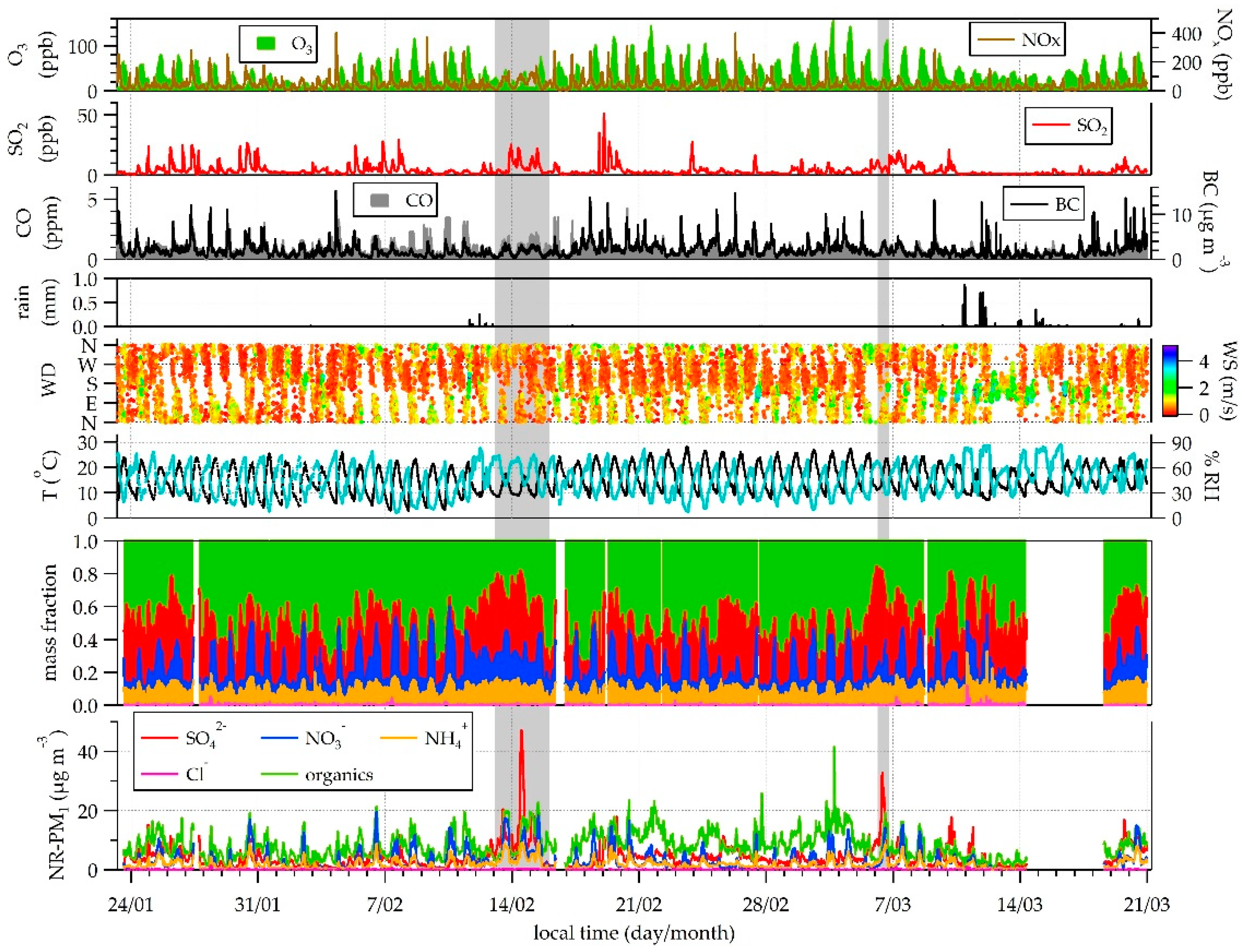
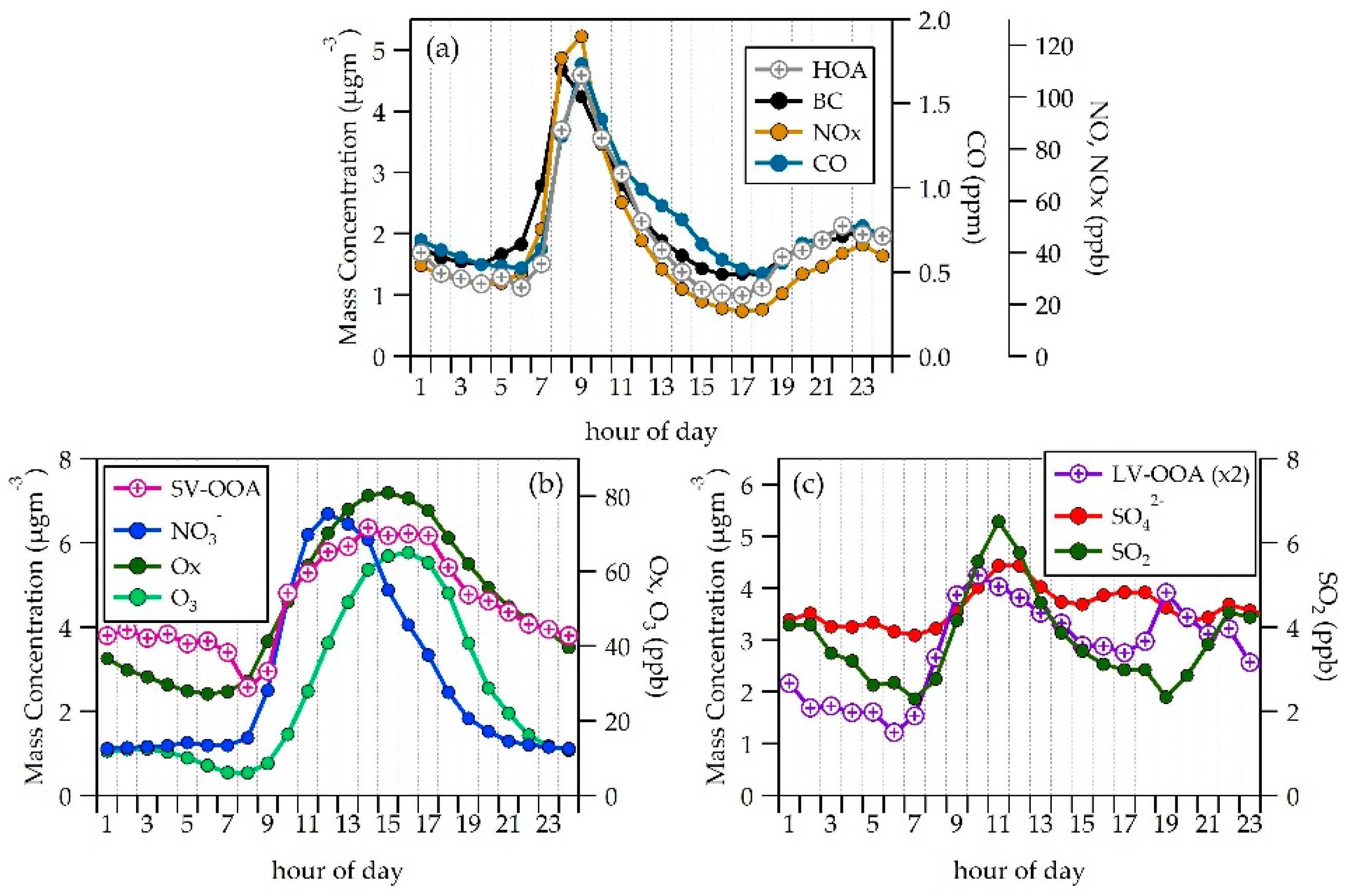
3.1. PM Concentrations
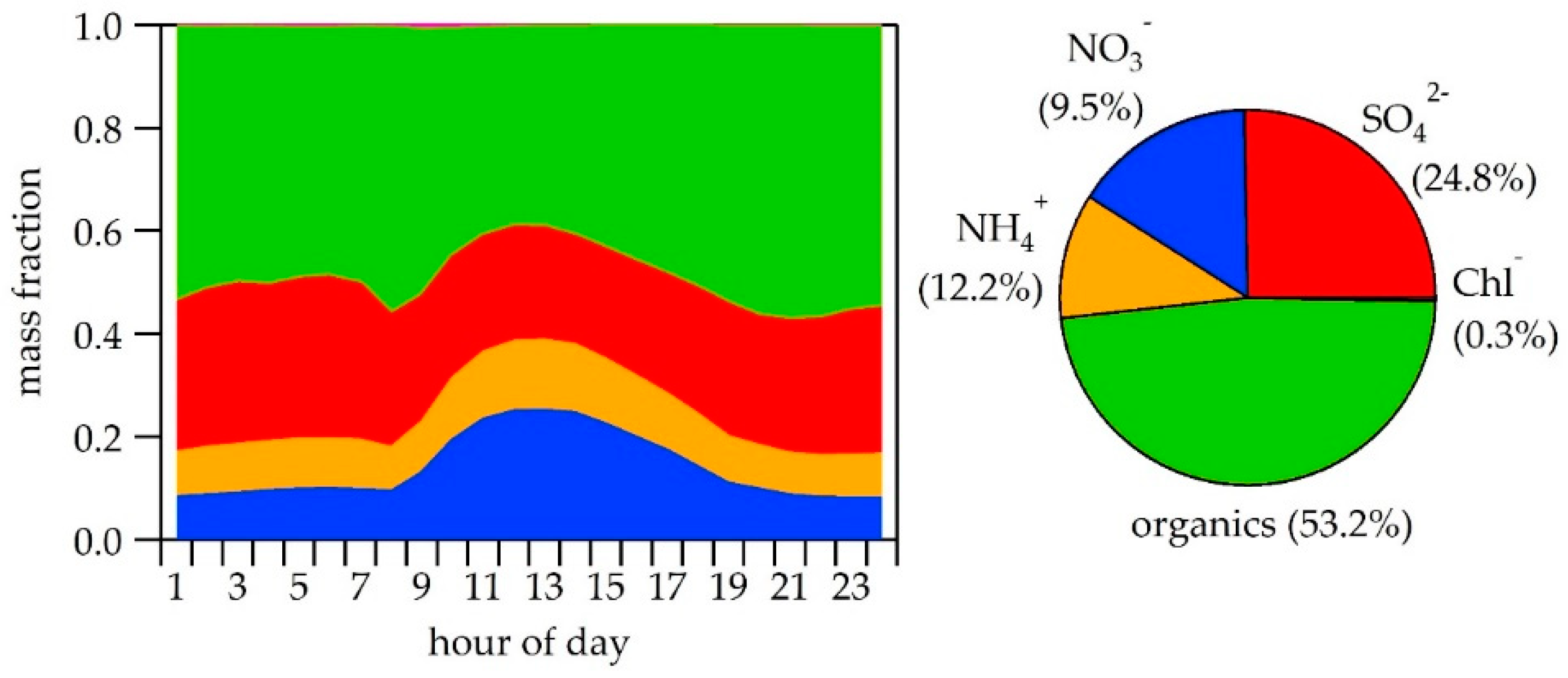
3.2. NR-PM1 Chemical Composition
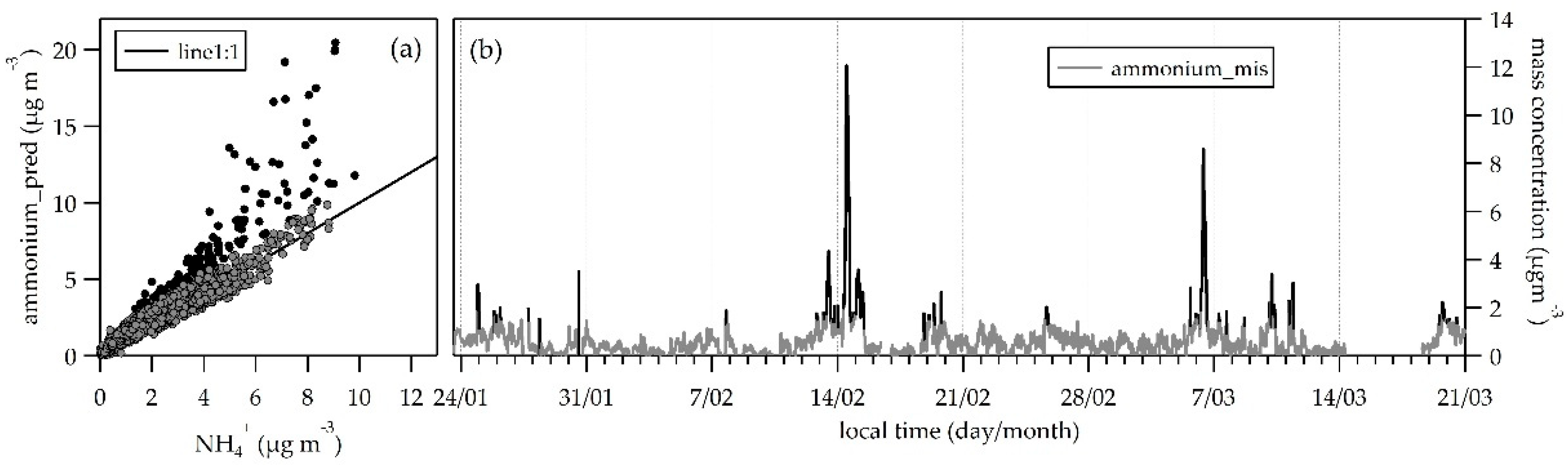
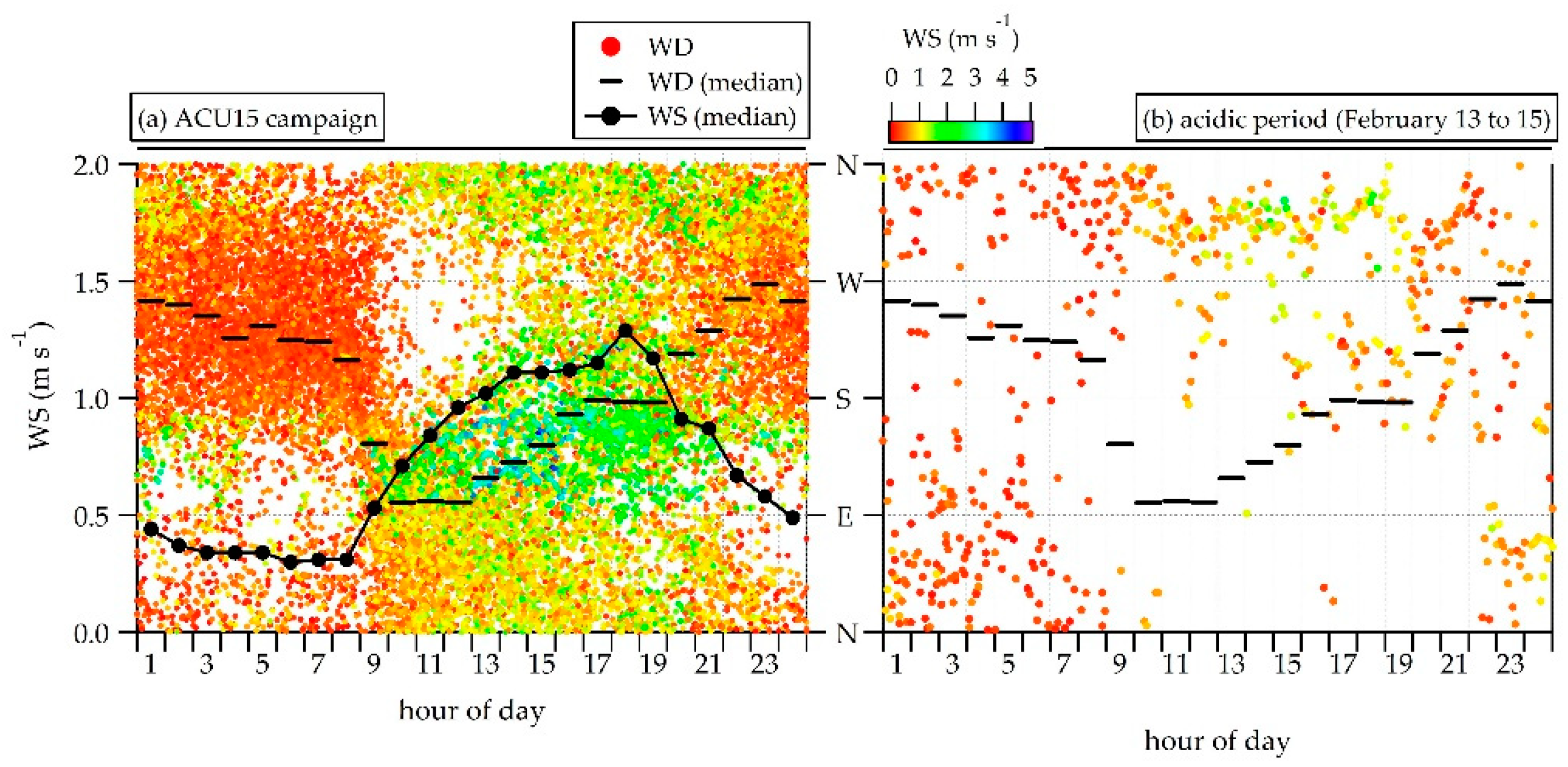
3.3. NR-PM1 Acidity
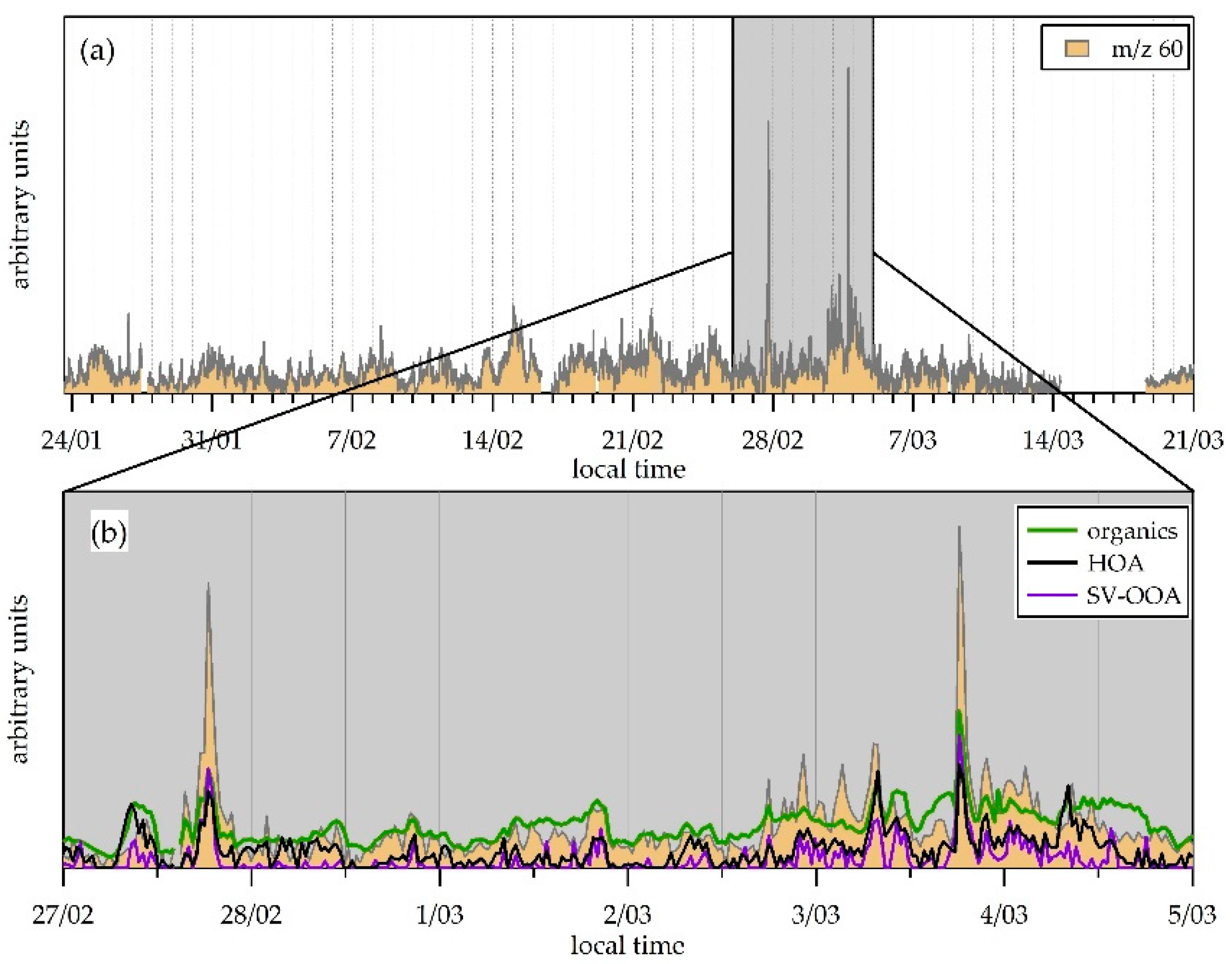
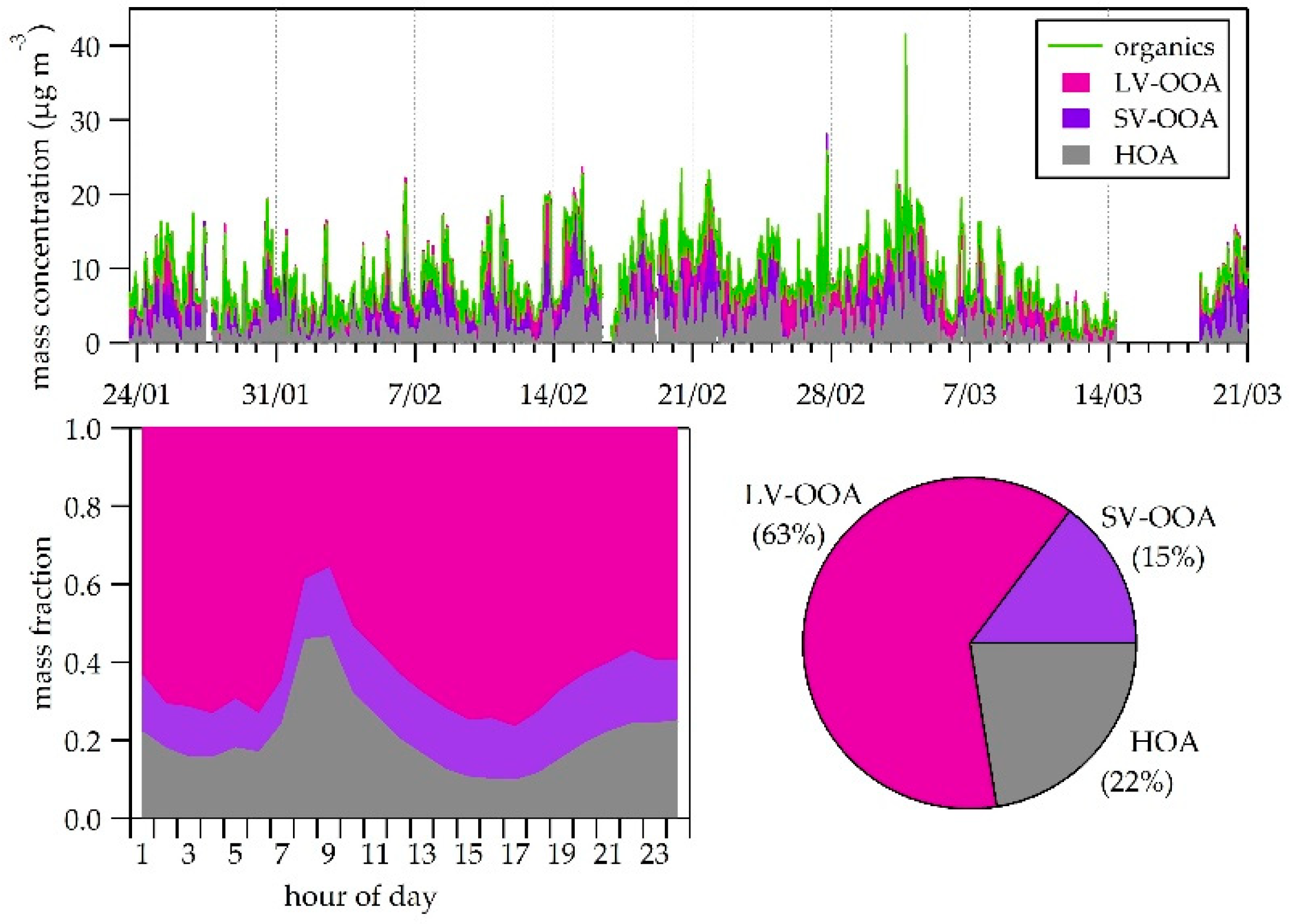
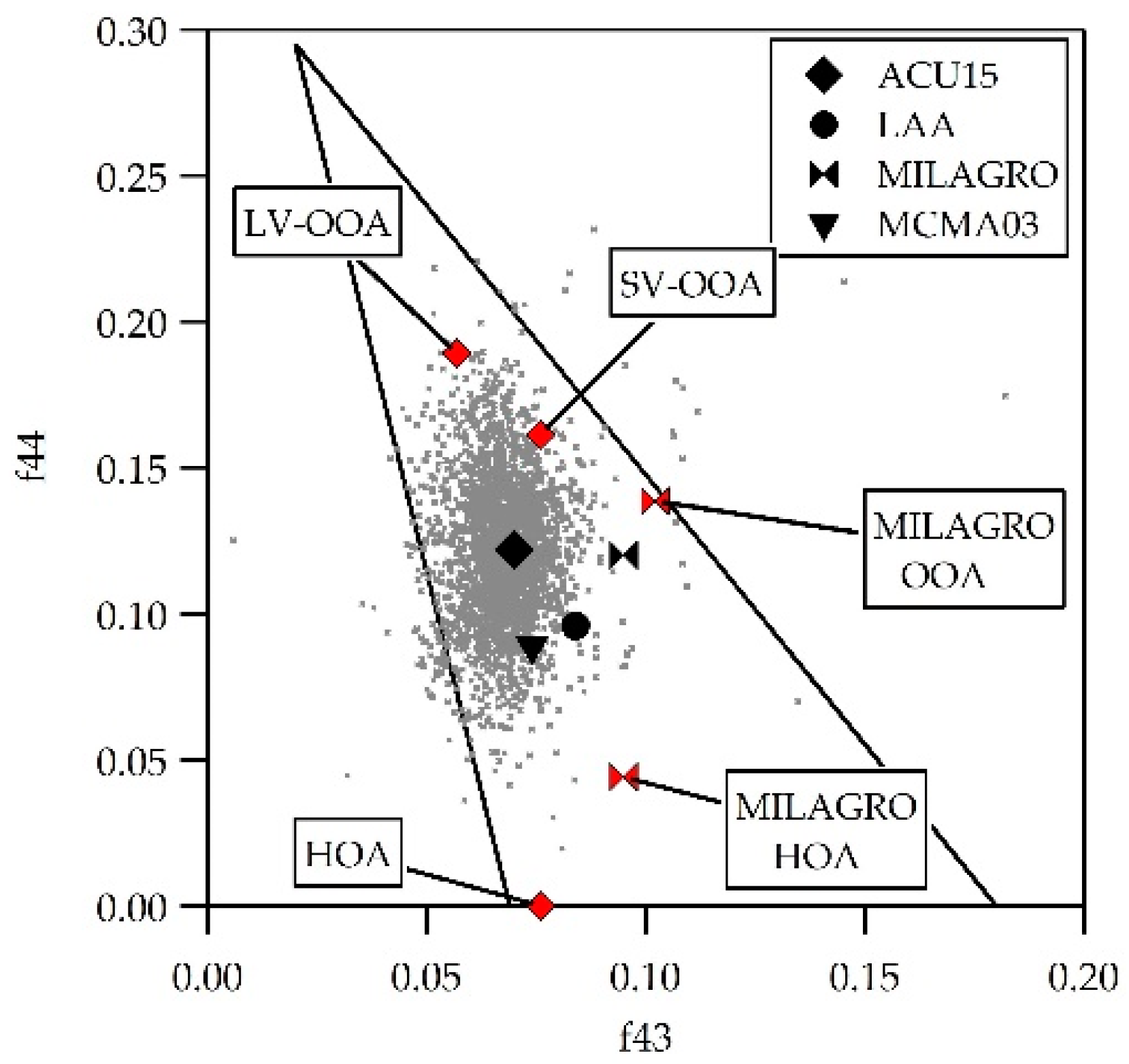
3.4. Organic Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Secretaría del Medio Ambiente de la Ciudad de México (SEDEMA). Calidad del Aire en la Ciudad de México Informe 2014; Secretaría del Medio Ambiente de la Ciudad de México: Ciudad de México, Mexico, 2015.
- Molina, L.; Molina, M. Air Quality in the Mexico Megacity. An Integrated Assessment; Springer: Dordrecht, The Netherlands, 2002; Volume 2. [Google Scholar]
- Secretaría del Medio Ambiente de la Ciudad de México (SEDEMA). Calidad del Aire en la Ciudad de México Informe 2015; Secretaría del Medio Ambiente de la Ciudad de México: Ciudad de México, Mexico, 2016.
- Secretaría del Medio Ambiente de la Ciudad de México (SEDEMA). Calidad del Aire en la Ciudad de México Informe 2016; Secretaría del Medio Ambiente de la Ciudad de México: Ciudad de México, Mexico, 2017.
- Secretaría del Medio Ambiente del Gobierno del Distrito Federal (SMADF). Calidad del Aire en la Ciudad de México Informe 2011; Secretaría del Medio Ambiente del Gobierno del Distrito Federal: Mexico City, Mexico, 2012.
- Secretaría del Medio Ambiente del Gobierno del Distrito Federal (SMADF). Calidad del Aire en la Ciudad de México Informe 2013; Secretaría del Medio Ambiente del Gobierno del Distrito Federal: Mexico City, Mexico, 2014.
- Instituto Nacional de Estadística y Geografía (INEGI). Encuesta Origen Destino en Hogares de la Zona Metropolitana del Valle de México 2017; Instituto Nacional de Estadística y Geografía: Aguascalientes, Mexico, 2018.
- Carreón-Sierra, S.; Salcido, A.; Castro, T.; Celada-Murillo, A.-T. Cluster analysis of the wind events and seasonal wind circulation patterns in the mexico city region. Atmosphere 2015, 6, 1006–1031. [Google Scholar] [CrossRef]
- De Foy, B.; Krotkov, N.A.; Bei, N.; Herndon, S.C.; Huey, L.G.; Martínez, A.P.; Ruiz-Suárez, L.G.; Wood, E.C.; Zavala, M.; Molina, L.T. Hit from both sides: Tracking industrial and volcanic plumes in Mexico city with surface measurements and OMI SO2 retrievals during the milagro field campaign. Atmos. Chem. Phys. 2009, 9, 9599–9617. [Google Scholar] [CrossRef]
- Molina, L.T.; Kolb, C.E.; de Foy, B.; Lamb, B.K.; Brune, W.H.; Jimenez, J.L.; Ramos-Villegas, R.; Sarmiento, J.; Paramo-Figueroa, V.H.; Cardenas, B.; et al. Air quality in north america’s most populous city—Overview of the MCMA-2003 campaign. Atmos. Chem. Phys. 2007, 7, 2447–2473. [Google Scholar] [CrossRef]
- Molina, L.T.; Madronich, S.; Gaffney, J.S.; Apel, E.; de Foy, B.; Fast, J.; Ferrare, R.; Herndon, S.; Jimenez, J.L.; Lamb, B.; et al. An overview of the milagro 2006 campaign: Mexico city emissions and their transport and transformation. Atmos. Chem. Phys. 2010, 10, 8697–8760. [Google Scholar] [CrossRef] [Green Version]
- Aiken, A.C.; de Foy, B.; Wiedinmyer, C.; DeCarlo, P.F.; Ulbrich, I.M.; Wehrli, M.N.; Szidat, S.; Prevot, A.S.H.; Noda, J.; Wacker, L.; et al. Mexico city aerosol analysis during milagro using high resolution aerosol mass spectrometry at the urban supersite (T0)—Part 2: Analysis of the biomass burning contribution and the non-fossil carbon fraction. Atmos. Chem. Phys. 2010, 10, 5315–5341. [Google Scholar] [CrossRef] [Green Version]
- Aiken, A.C.; Salcedo, D.; Cubison, M.J.; Huffman, J.A.; DeCarlo, P.F.; Ulbrich, I.M.; Docherty, K.S.; Sueper, D.; Kimmel, J.R.; Worsnop, D.R.; et al. Mexico city aerosol analysis during milagro using high resolution aerosol mass spectrometry at the urban supersite (T0)—Part 1: Fine particle composition and organic source apportionment. Atmos. Chem. Phys. 2009, 9, 6633–6653. [Google Scholar] [CrossRef]
- Baumgardner, D.; Grutter, M.; Allan, J.; Ochoa, C.; Rappenglueck, B.; Russell, L.M.; Arnott, P. Physical and chemical properties of the regional mixed layer of mexico’s megapolis. Atmos. Chem. Phys. 2009, 9, 5711–5727. [Google Scholar] [CrossRef]
- DeCarlo, P.; Dunlea, E.; Kimmel, J.; Aiken, A.; Sueper, D.; Crounse, J.; Wennberg, P.; Emmons, L.; Shinozuka, Y.; Clarke, A. Fast airborne aerosol size and chemistry measurements above Mexico city and central Mexico during the milagro campaign. Atmos. Chem. Phys. 2008, 8, 4027–4048. [Google Scholar] [CrossRef]
- Dzepina, K.; Arey, J.; Marr, L.C.; Worsnop, D.R.; Salcedo, D.; Zhang, Q.; Onasch, T.B.; Molina, L.T.; Molina, M.J.; Jimenez, J.L. Detection of particle-phase polycyclic aromatic hydrocarbons in Mexico city using an aerosol mass spectrometer. Int. J. Mass Spectrom. 2007, 263, 152–170. [Google Scholar] [CrossRef]
- Salcedo, D.; Onasch, T.B.; Aiken, A.C.; Williams, L.R.; de Foy, B.; Cubison, M.J.; Worsnop, D.R.; Molina, L.T.; Jimenez, J.L. Determination of particulate lead using aerosol mass spectrometry: Milagro/MCMA-2006 observations. Atmos. Chem. Phys. 2010, 10, 5371–5389. [Google Scholar] [CrossRef] [Green Version]
- Salcedo, D.; Onasch, T.B.; Dzepina, K.; Canagaratna, M.R.; Zhang, Q.; Huffman, J.A.; DeCarlo, P.F.; Jayne, J.T.; Mortimer, P.; Worsnop, D.R.; et al. Characterization of ambient aerosols in Mexico city during the MCMA-2003 campaign with aerosol mass spectrometry: Results from the cenica supersite. Atmos. Chem. Phys. 2006, 6, 925–946. [Google Scholar] [CrossRef]
- Guerrero, F.; Álvarez-Ospina, H.; Retama, A.; López-Medina, A.; Castro, T.; Salcedo, D. Seasonal changes in the PM1 chemical composition north of Mexico city. Atmósfera 2017, 30, 243–273. [Google Scholar] [CrossRef]
- Ng, N.L.; Herndon, S.C.; Trimborn, A.; Canagaratna, M.R.; Croteau, P.L.; Onasch, T.B.; Sueper, D.; Worsnop, D.R.; Zhang, Q.; Sun, Y.L.; et al. An aerosol chemical speciation monitor (ACSM) for routine monitoring of the composition and mass concentrations of ambient aerosol. Aerosol Sci. Technol. 2011, 45, 780–794. [Google Scholar] [CrossRef]
- Retama, A.; Baumgardner, D.; Raga, G.B.; McMeeking, G.R.; Walker, J.W. Seasonal and diurnal trends in black carbon properties and co-pollutants in Mexico city. Atmos. Chem. Phys. 2015, 15, 9693–9709. [Google Scholar] [CrossRef]
- Von der Weiden, S.L.; Drewnick, F.; Borrmann, S. Particle loss calculator—A new software tool for the assessment of the performance of aerosol inlet systems. Atmos. Meas. Tech. 2009, 2, 479–494. [Google Scholar] [CrossRef]
- Canagaratna, M.R.; Jayne, J.T.; Jimenez, J.L.; Allan, J.D.; Alfarra, M.R.; Zhang, Q.; Onasch, T.B.; Drewnick, F.; Coe, H.; Middlebrook, A.; et al. Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer. Mass Spectrom. Rev. 2007, 26, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Ulbrich, I.M.; Canagaratna, M.R.; Zhang, Q.; Worsnop, D.R.; Jimenez, J.L. Interpretation of organic components from positive matrix factorization of aerosol mass spectrometric data. Atmos. Chem. Phys. 2009, 9, 2891–2918. [Google Scholar] [CrossRef]
- Wavemetrics. Igor Pro v6.36 User's Guide; Wavemetrics Inc.: Lake Oswego, OR, USA, 2014. [Google Scholar]
- Patrick Arnott, W.; Moosmüller, H.; Fred Rogers, C.; Jin, T.; Bruch, R. Photoacoustic spectrometer for measuring light absorption by aerosol: Instrument description. Atmos. Environ. 1999, 33, 2845–2852. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, R.J.; Cao, J.; Han, Y.; Wang, G.; Li, G.; Wang, Y.; Dai, W.; Zhang, R.; Zhou, Y. Mixing state of black carbon aerosol in a heavily polluted urban area of China: Implications for light absorption enhancement. Aerosol Sci. Technol. 2014, 48, 689–697. [Google Scholar] [CrossRef]
- Bond, T.C.; Habib, G.; Bergstrom, R.W. Limitations in the enhancement of visible light absorption due to mixing state. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Liou, K.N.; Takano, Y.; Zhang, R.; Levy Zamora, M.; Yang, P.; Li, Q.; Leung, L.R. Variation of the radiative properties during black carbon aging: Theoretical and experimental intercomparison. Atmos. Chem. Phys. 2015, 15, 11967–11980. [Google Scholar] [CrossRef]
- Pavia-Hernández, R. Eficiencia de Absorción de Masa de Carbono Elemental y Propiedades Ópticas de Partículas Atmosféricas PM2.5; Universidad Nacional Autónoma de México (UNAM): Mexico City, Mexico, 2016. [Google Scholar]
- SIMAT. Available online: http://www.aire.cdmx.gob.mx (accessed on 24 April 2018).
- Querol, X.; Pey, J.; Minguillón, M.C.; Pérez, N.; Alastuey, A.; Viana, M.; Moreno, T.; Bernabé, R.M.; Blanco, S.; Cárdenas, B.; et al. Pm speciation and sources in Mexico during the milagro-2006 campaign. Atmos. Chem. Phys. 2008, 8, 111–128. [Google Scholar] [CrossRef]
- Secretaría del Medio Ambiente de la Ciudad de México (SEDEMA). Inventario de Emisiones de la CDMX 2014. Contaminates Criterio, Tóxicos y de Efecto Invernadero; Secretaría del Medio Ambiente de la Ciudad de México: Ciudad de México, Mexico, 2016.
- De Foy, B.; Caetano, E.; Magaña, V.; Zitácuaro, A.; Cárdenas, B.; Retama, A.; Ramos, R.; Molina, L.T.; Molina, M.J. Mexico city basin wind circulation during the MCMA-2003 field campaign. Atmos. Chem. Phys. 2005, 5, 2267–2288. [Google Scholar] [CrossRef]
- De Foy, B.; Fast, J.D.; Paech, S.J.; Phillips, D.; Walters, J.T.; Coulter, R.L.; Martin, T.J.; Pekour, M.S.; Shaw, W.J.; Kastendeuch, P.P.; et al. Basin-scale wind transport during the milagro field campaign and comparison to climatology using cluster analysis. Atmos. Chem. Phys. 2008, 8, 1209–1224. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rollins, A.W.; Fry, J.L.; Hunter, J.F.; Kroll, J.H.; Worsnop, D.R.; Singaram, S.W.; Cohen, R.C. Elemental analysis of aerosol organic nitrates with electron ionization high-resolution mass spectrometry. Atmos. Meas. Tech. 2010, 3, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jimenez, J.L.; Canagaratna, M.R.; Ulbrich, I.M.; Ng, N.L.; Worsnop, D.R.; Sun, Y. Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: A review. Anal. Bioanal. Chem. 2011, 401, 3045–3067. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.L.; Canagaratna, M.R.; Zhang, Q.; Jimenez, J.L.; Tian, J.; Ulbrich, I.M.; Kroll, J.H.; Docherty, K.S.; Chhabra, P.S.; Bahreini, R.; et al. Organic aerosol components observed in northern hemispheric datasets from aerosol mass spectrometry. Atmos. Chem. Phys. 2010, 10, 4625–4641. [Google Scholar] [CrossRef]
- Lu, K.; Zhang, Y.; Su, H.; Brauers, T.; Chou Charles, C.; Hofzumahaus, A.; Liu Shaw, C.; Kita, K.; Kondo, Y.; Shao, M.; et al. Oxidant (O3 + NO2) production processes and formation regimes in beijing. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
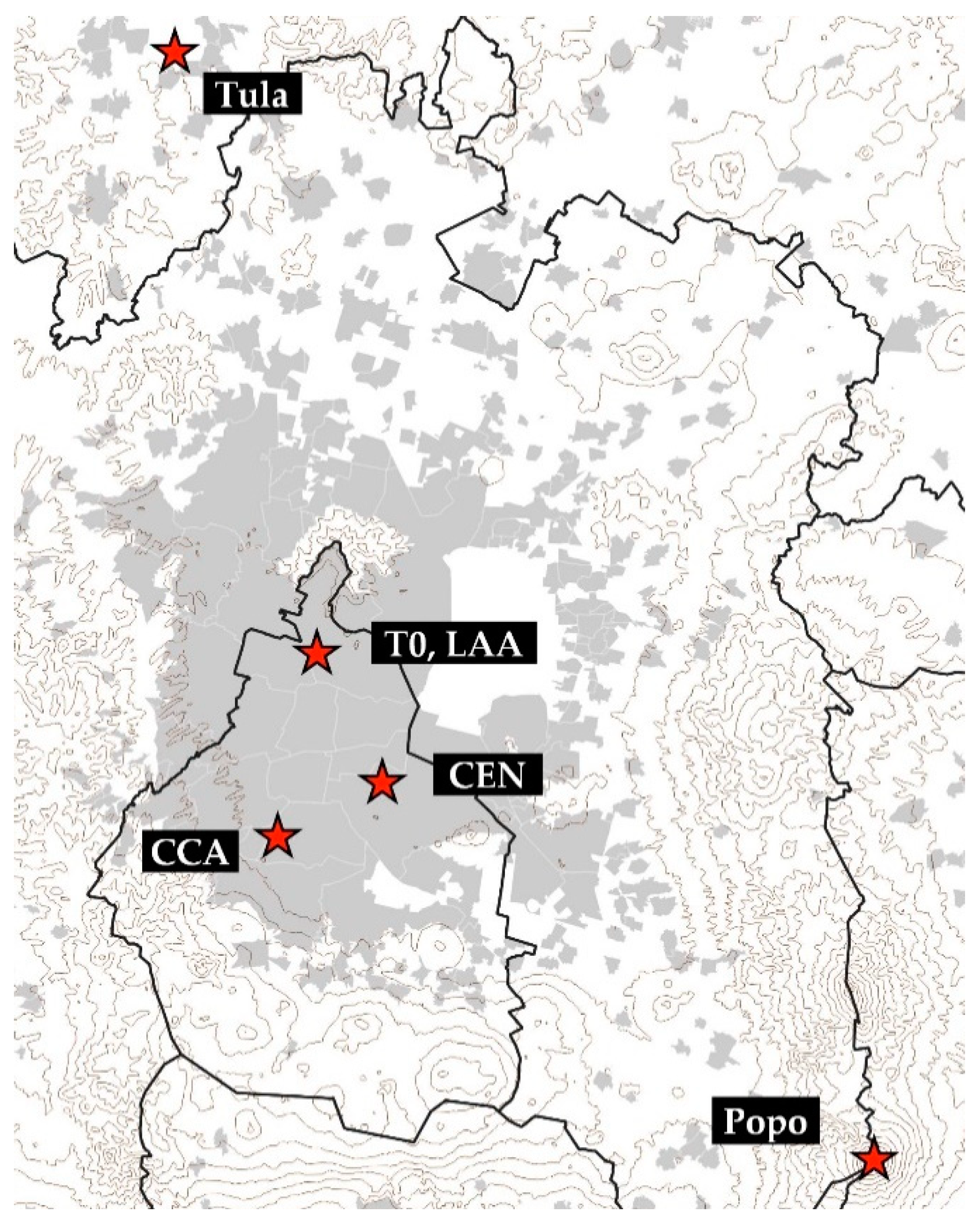
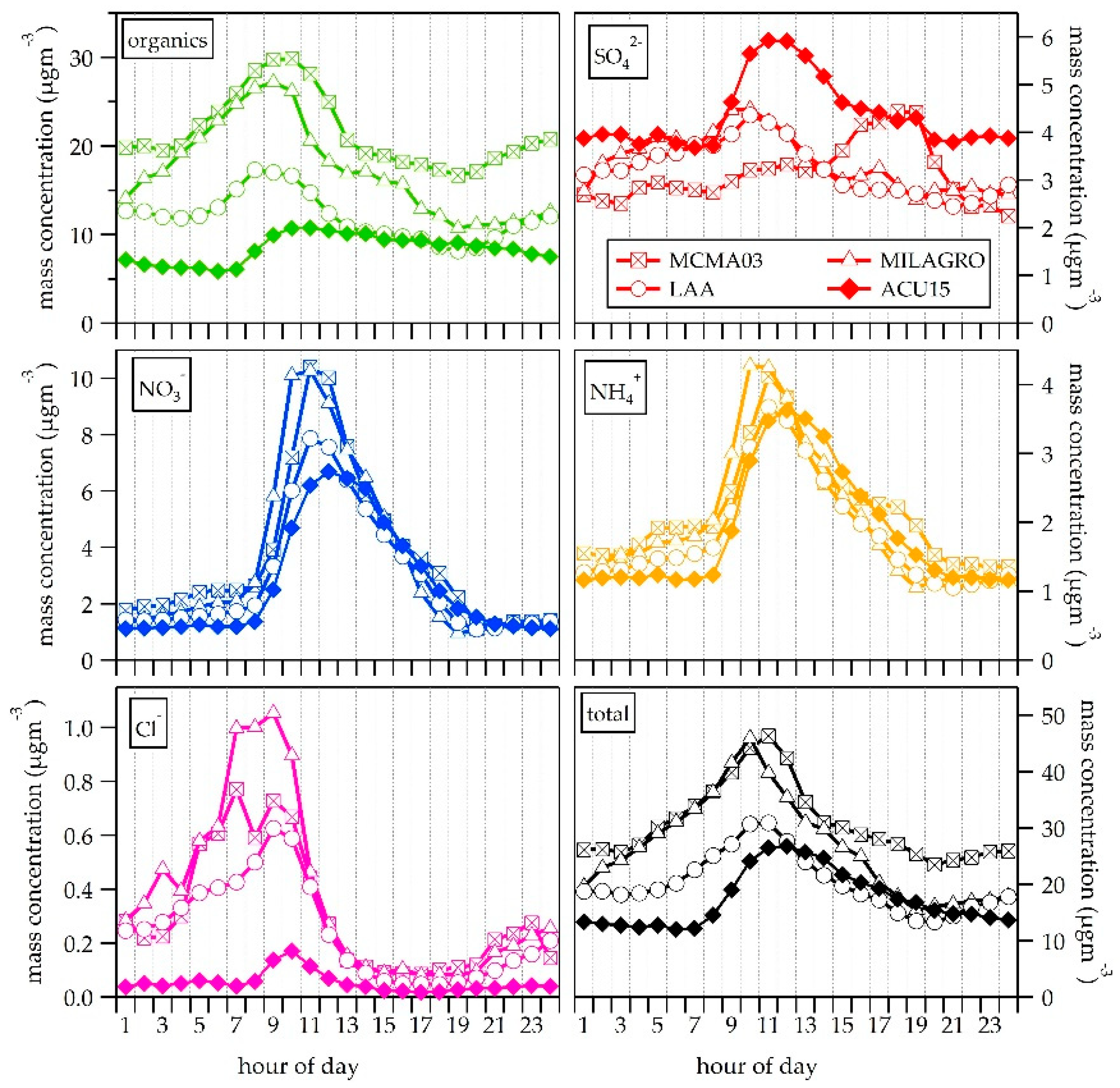

| ACU15 (CCA) | LAA [19] | MILAGRO (T0) [13] | MCMA03 (CEN) [18] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 21 January–23 March 2015 | 13 November 2013–30 April 2014 | 1 March–4 April 2006 | 31 March–4 May 2003 | ||||||
| (µg m−3) | % | (µg m−3) | % | (µg m−3) | % | (µg m−3) | % | ||
| NR-PM1 | organics | 8.1 | 53.2 | 12.0 | 59.3 | 17.3 | 64.6 | 21.6 | 69.9 |
| sulfate | 4.3 | 24.8 | 3.2 | 16.1 | 3.6 | 13.4 | 3.1 | 10.1 | |
| nitrate | 2.7 | 12.2 | 2.9 | 14.4 | 3.5 | 13.1 | 3.7 | 11.9 | |
| ammonium | 1.8 | 9.5 | 1.8 | 9.0 | 2.0 | 7.7 | 2.2 | 7.0 | |
| chloride | 0.05 | 0.3 | 0.2 | 1.2 | 0.4 | 1.5 | 0.3 | 1.0 | |
| ACSM total | 16.9 | 20.2 | 26.8 | 30.9 | |||||
| BC (PM2.5) | 2.1a | 3.03 a | 4.2 *,c | 3.4 *,c | |||||
| soil (PM2.5) | 1.7 § | 2.1 | |||||||
| metals (PM2.5) | 1.0 | ||||||||
| PM2.5 | 17.5 b, 16.1 c | 37.0 b | 40.0 d [33] | 35.7 b, 40.0 e | |||||
| PM1 | 27.8 b | 33.0 d [33] | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salcedo, D.; Alvarez-Ospina, H.; Peralta, O.; Castro, T. PM1 Chemical Characterization during the ACU15 Campaign, South of Mexico City. Atmosphere 2018, 9, 232. https://doi.org/10.3390/atmos9060232
Salcedo D, Alvarez-Ospina H, Peralta O, Castro T. PM1 Chemical Characterization during the ACU15 Campaign, South of Mexico City. Atmosphere. 2018; 9(6):232. https://doi.org/10.3390/atmos9060232
Chicago/Turabian StyleSalcedo, Dara, Harry Alvarez-Ospina, Oscar Peralta, and Telma Castro. 2018. "PM1 Chemical Characterization during the ACU15 Campaign, South of Mexico City" Atmosphere 9, no. 6: 232. https://doi.org/10.3390/atmos9060232





