Optical Properties of Fine Particulate Matter in Upper Silesia, Poland
Abstract
:1. Introduction
2. Methods
2.1. Sampling Sites and PM Collecting
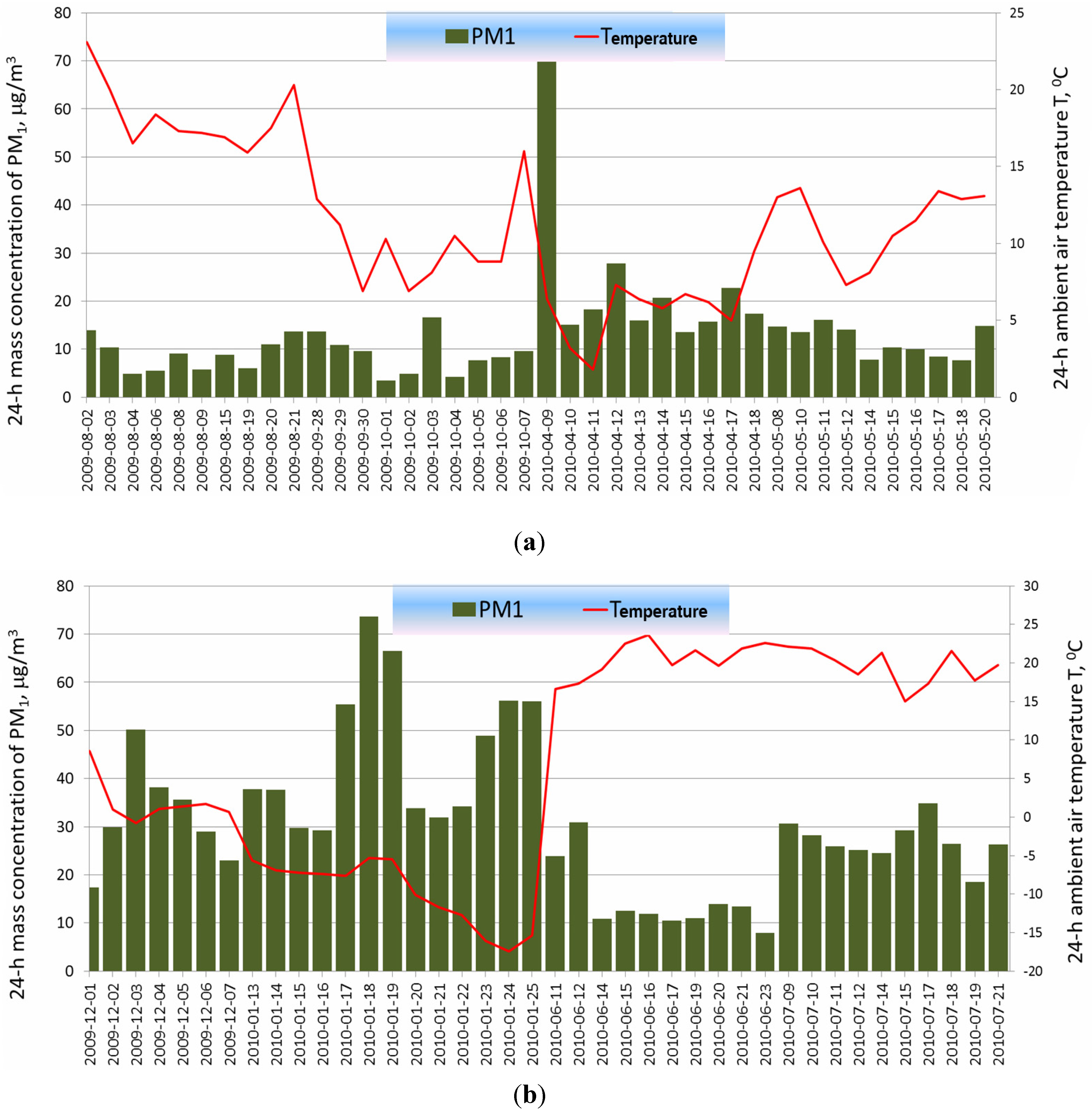
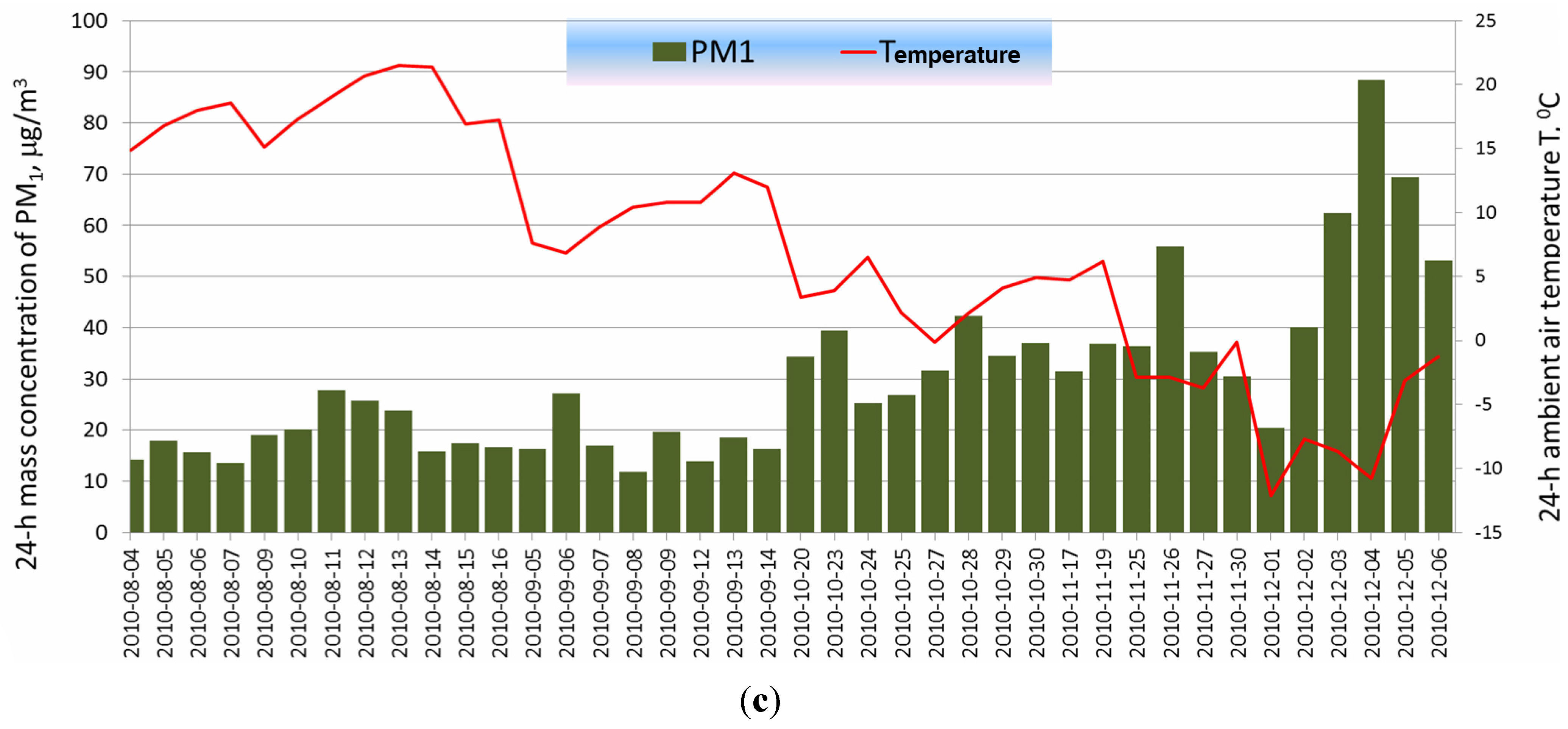
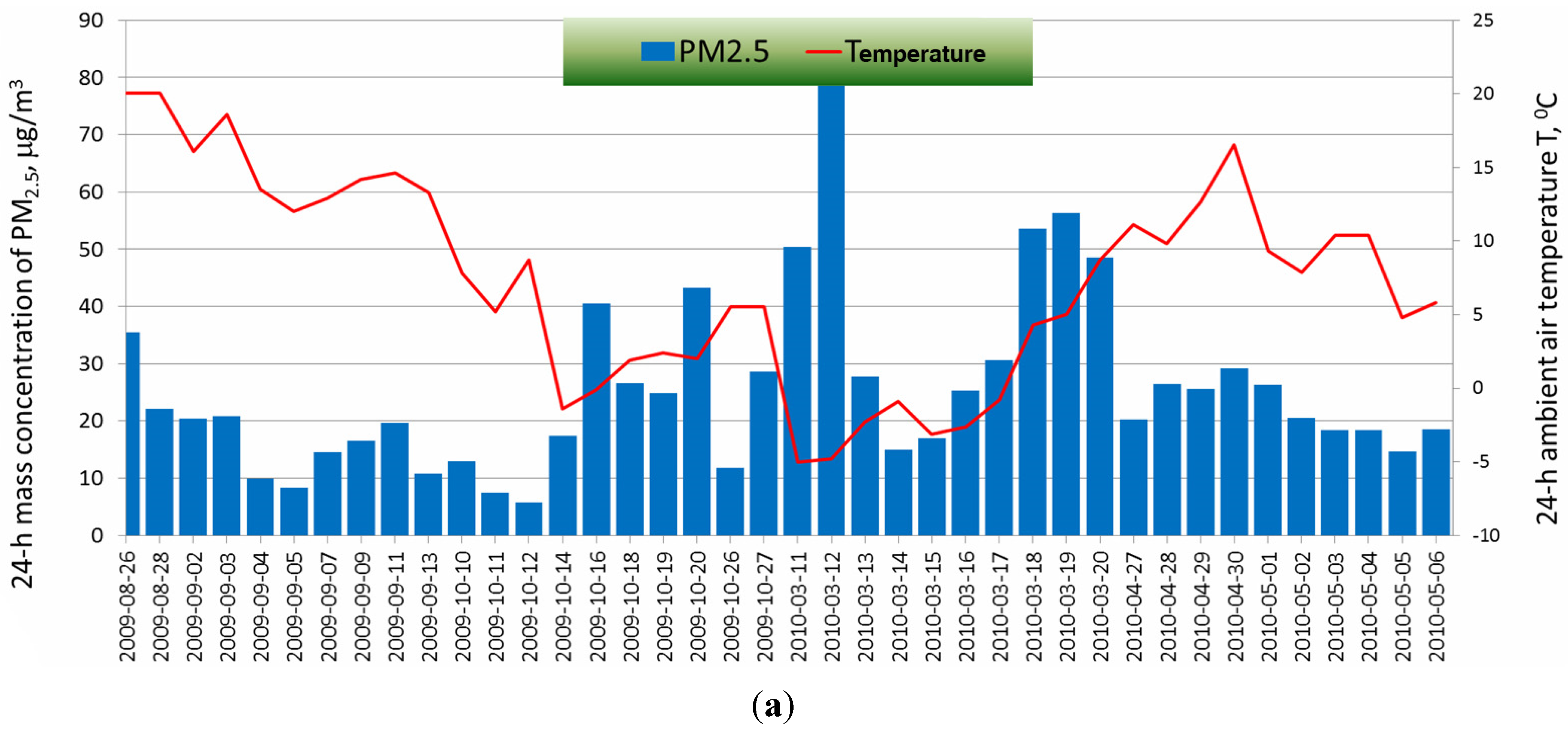
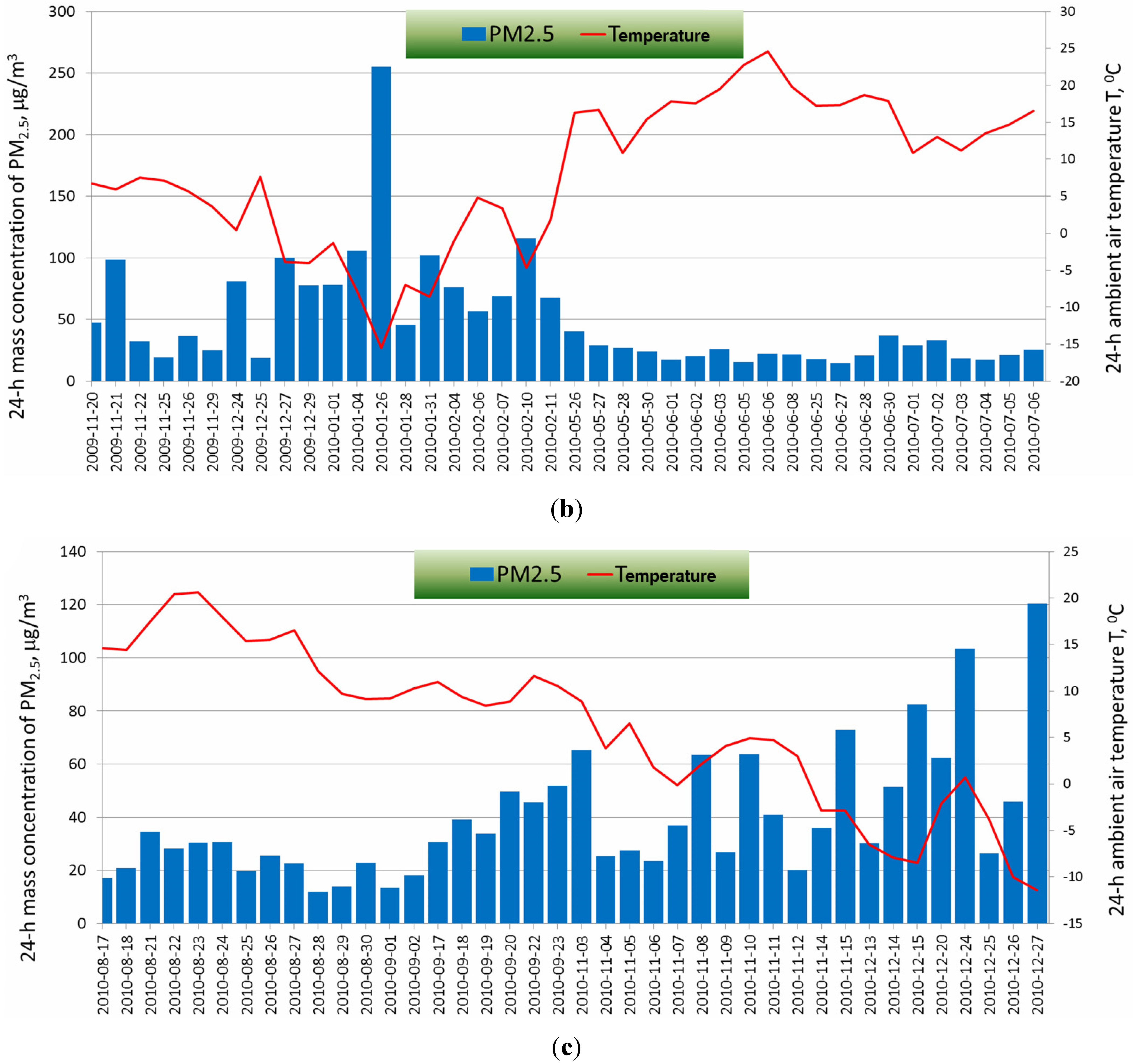
2.2. Determination of the Optical Parameters of PM
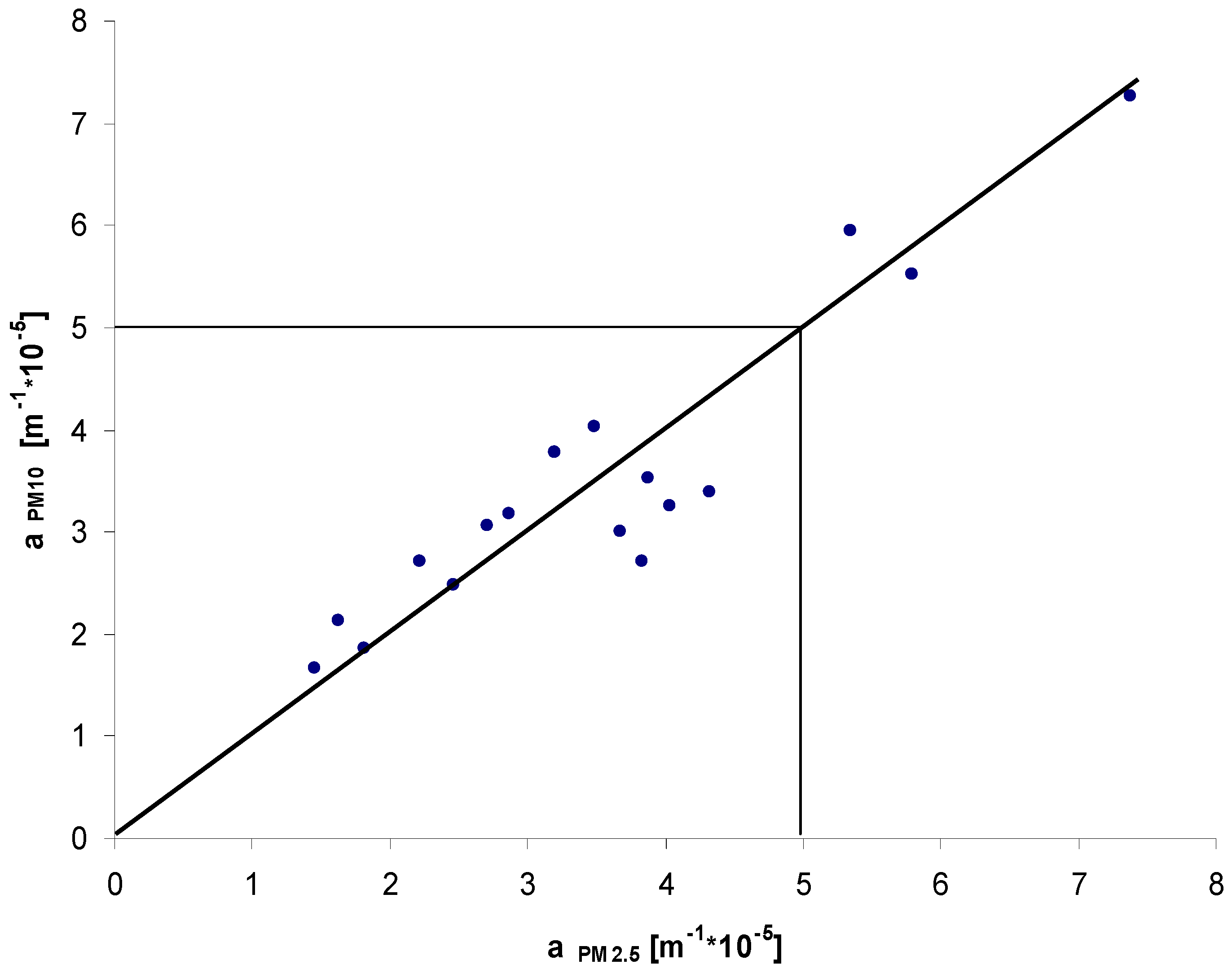
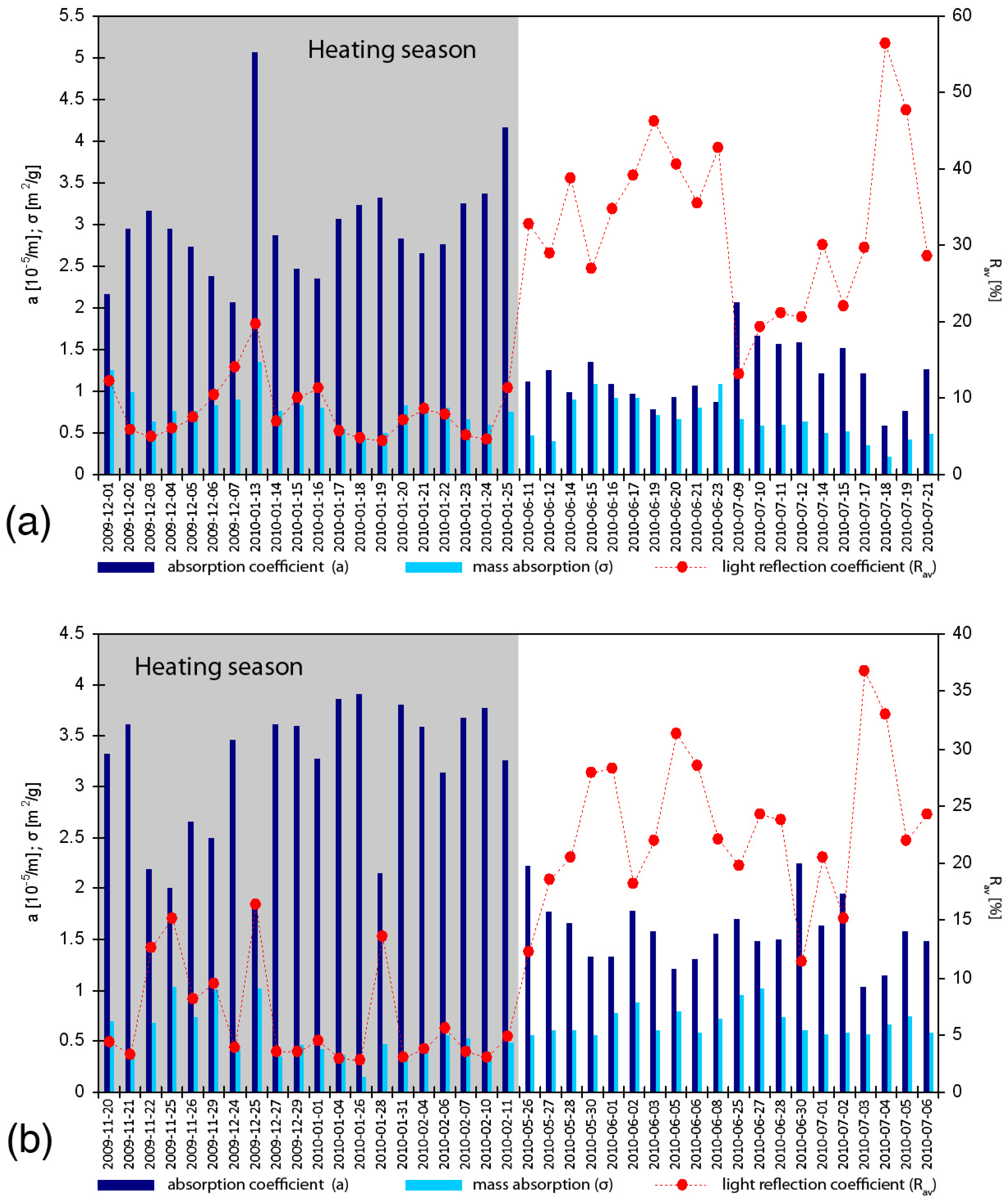
3. Results and Discussion


| Research Period (N = 40) | Heating Season (N = 20) | Non-Heating Season (N = 20) | ||||
|---|---|---|---|---|---|---|
| Parameter | Min/Max | Arithmetic Mean ± St.dev. | Min/Max | Arithmetic Mean ± St.dev. | Min/Max | Arithmetic Mean ± St.dev |
| PM1 (µg/m3) | 3.49/71.41 | 13.34 ± 10.80 | 3.49/71.41 | 16.37 ± 14.42 | 4.82/16.06 | 10.32 ± 3.51 |
| Rav (%) | 26.00/66.30 | 46.15 ± 11.32 | 26.00/63.10 | 39.26 ± 10.73 | 40.10/66.30 | 53.03 ± 6.94 |
| a (10−5/m) | 0.44/2.20 | 0.87 ± 0.34 | 0.47/2.20 | 1.06 ± 0.38 | 0.44/0.97 | 0.67 ± 0.14 |
| σ (m2/g) | 0.31/1.58 | 0.78 ± 0.33 | 0.21/1.58 | 0.87 ± 0.41 | 0.44/1.12 | 0.70 ± 0.20 |
| PM2.5 (µg/m3) | 5.80/78.74 | 25.48 ± 15.28 | 5.80/78.74 | 31.12 ± 19.18 | 8.28/35.44 | 19.83 ± 6.65 |
| Rav (%) | 9.80/58.20 | 33.44 ± 14.33 | 9.80/56.90 | 25.20 ± 13.97 | 27.20/58.20 | 41.69 ± 9.13 |
| a (10−5/m) | 0.57/2.49 | 1.27 ± 0.51 | 0.59/2.49 | 1.56 ± 0.55 | 0.57/1.40 | 0.97 ± 0.22 |
| σ (m2/g) | 0.14/1.12 | 0.58 ± 0.20 | 0.14/1.12 | 0.62 ± 0.23 | 0.34/0.95 | 0.54 ± 0.14 |
| Research Period (N = 40) | Heating Season (N = 20) | Non-Heating Season (N = 20) | ||||
|---|---|---|---|---|---|---|
| Parameter | Min/Max | Arithmetic Mean ± St.dev. | Min/Max | Arithmetic Mean ± St.dev. | Min/Max | Arithmetic Mean ± St.dev. |
| PM1 (µg/m3) | 7.99/73.59 | 30.77 ± 15.61 | 17.33/73.59 | 40.70 ± 14.82 | 7.99/34.86 | 20.83 ± 8.50 |
| Rav (%) | 4.40/56.30 | 20.56 ± 14.72 | 4.40/19.70 | 8.40 ± 3.89 | 13.10/56.30 | 32.73 ± 10.88 |
| a (10−5/m) | 0.59/5.07 | 2.09 ± 1.06 | 2.06/5.07 | 2.99 ± 0.69 | 0.59/2.06 | 1.19 ± 0.36 |
| σ (m2/g) | 0.22/1.34 | 0.72 ± 0.24 | 0.44/1.34 | 0.79 ± 0.22 | 0.22/1.09 | 0.65 ± 0.24 |
| PM2.5 (µg/m3) | 14.56/255.08 | 49.60 ± 44.94 | 18.50/255.08 | 75.39 ± 51.91 | 14.56/40.17 | 23.80 ± 7.04 |
| Rav (%) | 2.80/36.70 | 14.70 ± 10.09 | 2.80/16.40 | 6.40 ± 4.52 | 11.40/36.70 | 23.01 ± 6.58 |
| a (10−5/m) | 1.04/3.92 | 2.37 ± 0.96 | 1.89/3.92 | 3.17 ± 0.67 | 1.04/2.25 | 1.58 ± 0.32 |
| σ (m2/g) | 0.15/1.03 | 0.62 ± 0.21 | 0.15/1.03 | 0.55 ± 0.24 | 0.55/1.02 | 0.69 ± 0.14 |
| Research Period (N = 40) | Heating Season (N = 20) | Non-Heating Season (N = 20) | ||||
|---|---|---|---|---|---|---|
| Parameter | Min/Max | Arithmetic Mean ± St.dev. | Min/Max | Arithmetic Mean ± St.dev. | Min/Max | Arithmetic Mean ± St.dev. |
| PM1 (µg/m3) | 11.83/88.34 | 29.98 ± 16.76 | 20.50/88.34 | 41.55 ± 16.56 | 11.83/27.72 | 18.40 ± 4.48 |
| Rav (%) | 3.70/22.00 | 8.67 ± 4.78 | 3.70/22.00 | 7.18 ± 4.23 | 4.70/19.80 | 10.17 ± 4.94 |
| a (10−5/m) | 1.62/3.43 | 2.66 ± 0.52 | 1.62/3.43 | 2.87 ± 0.45 | 1.67/3.13 | 2.45 ± 0.50 |
| σ (m2/g) | 0.39/1.64 | 1.05 ± 0.35 | 0.39/1.05 | 0.75 ± 0.16 | 1.09/1.64 | 1.35 ± 0.18 |
| PM2.5 (µg/m3) | 11.80/120.46 | 39.59 ± 24.20 | 20.02/120.46 | 51.22 ± 27.91 | 11.80/51.78 | 27.97 ± 11.78 |
| Rav (%) | 3.10/28.60 | 7.79 ± 5.41 | 3.10/15.20 | 6.06 ± 2.96 | 3.40/28.60 | 9.52 ± 6.71 |
| a (10−5/m) | 1.32/3.80 | 2.91 ± 0.57 | 1.99/3.80 | 3.07 ± 0.46 | 1.32/3.57 | 2.75 ± 0.64 |
| σ (m2/g) | 0.31/2.07 | 0.91 ± 0.36 | 0.31/1.27 | 0.73 ± 0.29 | 0.67/2.07 | 1.09 ± 0.35 |
| a | PM2.5 | Rav |
|---|---|---|
| Złoty Potok RB | ||
| heating season PM1 | 0.817 | −0.867 |
| non-heating season PM1 | 0.749 | −0.984 |
| heating season PM2.5 | 0.557 | −0.871 |
| non-heating season PM2.5 | 0.735 | −0.991 |
| Katowice UB | ||
| heating season PM1 | 0.530 | 0.201 |
| non-heating season PM1 | 0.555 | −0.977 |
| heating season PM2.5 | 0.720 | −0.978 |
| non-heating season PM2.5 | 0.813 | −0.983 |
| Katowice UT | ||
| heating season PM1 | 0.745 | −0.962 |
| non-heating season PM1 | 0.720 | −0.954 |
| heating season PM2.5 | 0.811 | −0.983 |
| non-heating season PM2.5 | 0.693 | −0.799 |
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, S.; Lee, M.; Lee, G.; Kim, S.; Yoon, S.; Kang, K. Ionic and carbonaceous compositions of PM10, PM2.5 and PM1.0 at Gosan ABC Superstation and their ratios as source signature. Atmos. Chem. Phys. 2012, 12, 2007–2024. [Google Scholar] [CrossRef]
- Wang, Y.; Khalizov, A.; Levy, M.; Zhang, R. New directions: Light absorbing aerosols and their atmospheric impacts. Atmos. Environ. 2013, 81, 713–715. [Google Scholar] [CrossRef]
- Jacobson, M.C.; Hansson, H.-C.; Noone, K.J.; Charlson, R.J. Organic atmospheric aerosols: Review and state of science. Rev. Greophys. 2000, 38, 267–294. [Google Scholar] [CrossRef]
- Sztyler, A. Solar radiation and its attenuation in the central part of the Upper Silesia industrial region (in Polish). Prz. Geofiz. 1987, 3, 263–276. [Google Scholar]
- Highwood, E.J.; Kinnersley, R.P. When smoke gets in our eyes: The multiple impacts of atmospheric black carbon on climate, air quality and health. Environ. Int. 2006, 32, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Quinn, P.K.; Stohl, A.; Arneth, A.; Berntsen, T.; Burkhart, J.F.; Christensen, J.; Flanner, M.; Kupiainen, K.; Lihavainen, H.; Shepherd, M.; et al. The Impact of Black Carbon on Arctic Climate; Arctic Monitoring and Assessment Programme: Oslo, Norway, 2011. [Google Scholar]
- Yang, S.; Xu, B.; Cao, J.; Zender, C.S.; Wang, M. Climate effects of black carbon aerosol in a Tibetan Plateau Glacier. Atmos. Environ. 2015, 111, 71–78. [Google Scholar] [CrossRef]
- Krishnan, R.; Ramanathan, V. Evidence of surface cooling from absorbing aerosols. J. Geophys. Res. 2002, 29, 1340–1340. [Google Scholar] [CrossRef]
- Abel, S.J.; Haywood, J.M.; Highwood, E.J.; Li, J.; Buseck, P.R. Evolution of biomass burning aerosol properties from an agricultural fire in Southern Africa. Geophys. Res. Lett. 2003, 30, 1783. [Google Scholar] [CrossRef]
- Adler, G.; Flores, J.M.; Riziq, A.; Borrmann, S.; Rudich, Y. Chemical, physical, and optical evolution of biomass burning aerosols: A case study. Atmos. Chem. Phys. 2012, 11, 1491–1503. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Klejnowski, K.; Rogula-Kopiec, P.; Ośródka, L.; Krajny, E.; Błaszczak, B.; Mathews, B. Spatial and seasonal variability of the mass concentration and chemical composition of PM2.5 in Poland. Air Qual. Atmos. Heath 2014, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Klejnowski, K.; Rogula-Kopiec, P.; Mathews, B.; Szopa, S. A study on the seasonal mass closure of ambient fine and coarse dusts in Zabrze, Poland. Bull. Environ. Contam. Toxico. 2012, 88, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W. Size-segregated urban particulate matter: Chemical composition, primary and secondary matter content and mass closure. Air Qual. Atmos. Health 2015, 2015, 1873–9318. [Google Scholar] [CrossRef]
- Pastuszka, J.S. Studies on the relationship between visibility and mass size distribution of aerosol in Katowice. In Aerosols: Science, Industry, Health and Environment; Pergamon Press: Oxford, UK, 1990; pp. 290–293. [Google Scholar]
- Tsai, Y.I. Atmospheric visibility trends in urban area in Taiwan 1961–2003. Atmos. Environ. 2005, 39, 5555–5567. [Google Scholar] [CrossRef]
- Majewski, G.; Rogula-Kozłowska, W.; Czechowski, P.O.; Badyda, A.; Brandyk, A. The impact of selected parameters on visibility: First results from a long-term campaign in Poland. Atmosphere 2015, 6, 1154–1174. [Google Scholar] [CrossRef]
- Colbeck, I.; Atkinson, B.; Johar, Y. The morphology and optical properties of soot produced by different fuels. J. Aerosol Sci. 1997, 28, 715–723. [Google Scholar] [CrossRef]
- Naone, H.; Hasegawa, S.; Heintzenberg, J.; Okada, K.; Uchiyama, A.; Zaizen, Y.; Kobayashi, E.; Yamazaki, A. State of mixture of atmospheric submicrometer black carbon particles and its effect on particulate light absorption. Atmos. Environ. 2009, 43, 1296–1301. [Google Scholar] [CrossRef]
- Shen, G.; Xue, M.; Yuan, S.; Zhang, J.; Zhang, Q.; Li, B.; Wu, H.; Ding, A. Chemical composition and reconstructed light extinction coefficients of particulate matter in a mega-city in the western Yangtze River Delta, China. Atmos. Environ. 2014, 83, 14–20. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Gao, J.; Wang, H.; Chai, F.; Wang, S. Aerosol chemical composition and light scattering during a winter season in Beijing. Atmos. Environ. 2015, 110, 36–44. [Google Scholar] [CrossRef]
- Pastuszka, J.S. Study of PM10 and PM2.5 concentrations in Southern Poland. J. Aerosol Sci. 1997, 28, 227–228. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Kozielska, B.; Błaszczak, B.; Klejnowski, K. The Mass Distribution of Particle Bound PAH among Aerosol Fractions: A Case-Study of An Urban Area in Poland; InTech Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Rogula-Kozłowska, W.; Majewski, G.; Czechowski, P.O. The size distribution and origin of elements bound to ambient particles: A case study of a Polish urban area. Environ. Monit. Assess. 2015, 187, 240. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W. Traffic-generated changes in the chemical characteristics of size-segregated urban aerosols. Bull. Environ. Contam. Toxico. 2014, 93, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; 2005/0183/COD; Council of the European Union: Brussels, Belgium, 2008.
- Horvath, H. Atmospheric light absorption: A review. Atmos. Environ. 1993, 27, 293–317. [Google Scholar] [CrossRef]
- Hogan, A.; Ahmed, N.; Black, J.; Barnard, S. Some physical properties of a black aerosol. J. Aerosol Sci. 1985, 5, 391–397. [Google Scholar] [CrossRef]
- Wolff, G.T.; Groblicki, P.J.; Cadle, S.H.; Countness, R.J. Particulate carbon at various locations in the United States. In Particulate Carbon: Atmospheric Life Cycle; Plenum Press: London, UK, 1982. [Google Scholar]
- Ambient Air-Determination of A Black Smoke Index; ISO 9835; International Organization for Standardization: Geneva, Switzerland, 1993.
- Eeftens, M.; Phuleria, H.C.; Meier, R.; Aguilera, I.; Corradi, E.; Davey, M.; Ducret-Stich, R.; Fierz, M.; Gehring, R.; Ineichen, A.; et al. Spatial and temporal variability of ultrafine particles, NO2, PM2.5, PM2.5 absorbance, PM10 and PMcoarse in Swiss study areas. Atmos. Environ. 2015, 111, 60–70. [Google Scholar] [CrossRef]
- Pastuszka, J.S.; Wawroś, A.; Talik, E.; Paw, U.K.T. Optical and chemical characteristics of the atmospheric aerosol in four towns in southern Poland. Sci. Total Environ. 2003, 309, 237–251. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W. Chemical composition and mass closure of ambient particulate matter at a crossroads and a highway in Katowice, Poland. Environ. Prot. Eng. 2015, 41, 15–29. [Google Scholar]
- Deshmukh, D.K.; Deb, M.K.; Tsai, Y.I.; Mkoma, S.L. Atmospheric ionic species in PM2.5 and PM1 aerosols in the ambient air of eastern central India. J. Atmos. Chem. 2010, 66, 81–100. [Google Scholar] [CrossRef]
- Đordević, D.; Mihajlidi-Zelić, A.; Relić, D.; Ignjatović, L.; Huremović, J.; Stortini, A.M.; Gambaro, A. Size-segregated mass concentration and water soluble inorganic ions in an urban aerosol of the Central Balkans (Belgrade). Atmos. Environ. 2012, 46, 309–317. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Viana, M.; Moreno, T.; Reche, C.; Minguillón, M.C.; Ripoll, A.; Pandolfi, M.; Amato, F.; Karanasiou, A.; et al. Variability of carbonaceous aerosols in remote, rural, urban and industrial environments in Spain: Implications for air quality policy. Atmos. Chem. Phys. 2013, 13, 6185–6206. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Pastuszka, J.S.; Talik, E. Influence of vehicular traffic on concentration and particle surface composition of PM10 and PM2.5 in Zabrze, Poland. Pol. J. Environ. Stud. 2008, 4, 539–548. [Google Scholar]
- Eeftens, M.; Tsai, M.-Y.; Ampe, C.; Anwander, B.; Beelen, R.; Bellander, T.; Cesaroni, G.; Cirach, M.; Cyrys, J.; de Hoogh, K.; et al. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PM coarse concentrations between and within 20 European study areas and the relationship with NO2: Results of the ESCAPE project. Atmos. Environ. 2012, 62, 303–317. [Google Scholar]
- Pastuszka, J.S.; Kolarczyk, J.; Sztyler, A. Preliminary studies of elemental carbon mass size distribution in Katowice. J. Aerosol Sci. 1989, 20, 1265–1268. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastuszka, J.S.; Rogula-Kozłowska, W.; Klejnowski, K.; Rogula-Kopiec, P. Optical Properties of Fine Particulate Matter in Upper Silesia, Poland. Atmosphere 2015, 6, 1521-1538. https://doi.org/10.3390/atmos6101521
Pastuszka JS, Rogula-Kozłowska W, Klejnowski K, Rogula-Kopiec P. Optical Properties of Fine Particulate Matter in Upper Silesia, Poland. Atmosphere. 2015; 6(10):1521-1538. https://doi.org/10.3390/atmos6101521
Chicago/Turabian StylePastuszka, Jozef S., Wioletta Rogula-Kozłowska, Krzysztof Klejnowski, and Patrycja Rogula-Kopiec. 2015. "Optical Properties of Fine Particulate Matter in Upper Silesia, Poland" Atmosphere 6, no. 10: 1521-1538. https://doi.org/10.3390/atmos6101521







