AAV Vector-Mediated Gene Delivery to Substantia Nigra Dopamine Neurons: Implications for Gene Therapy and Disease Models
Abstract
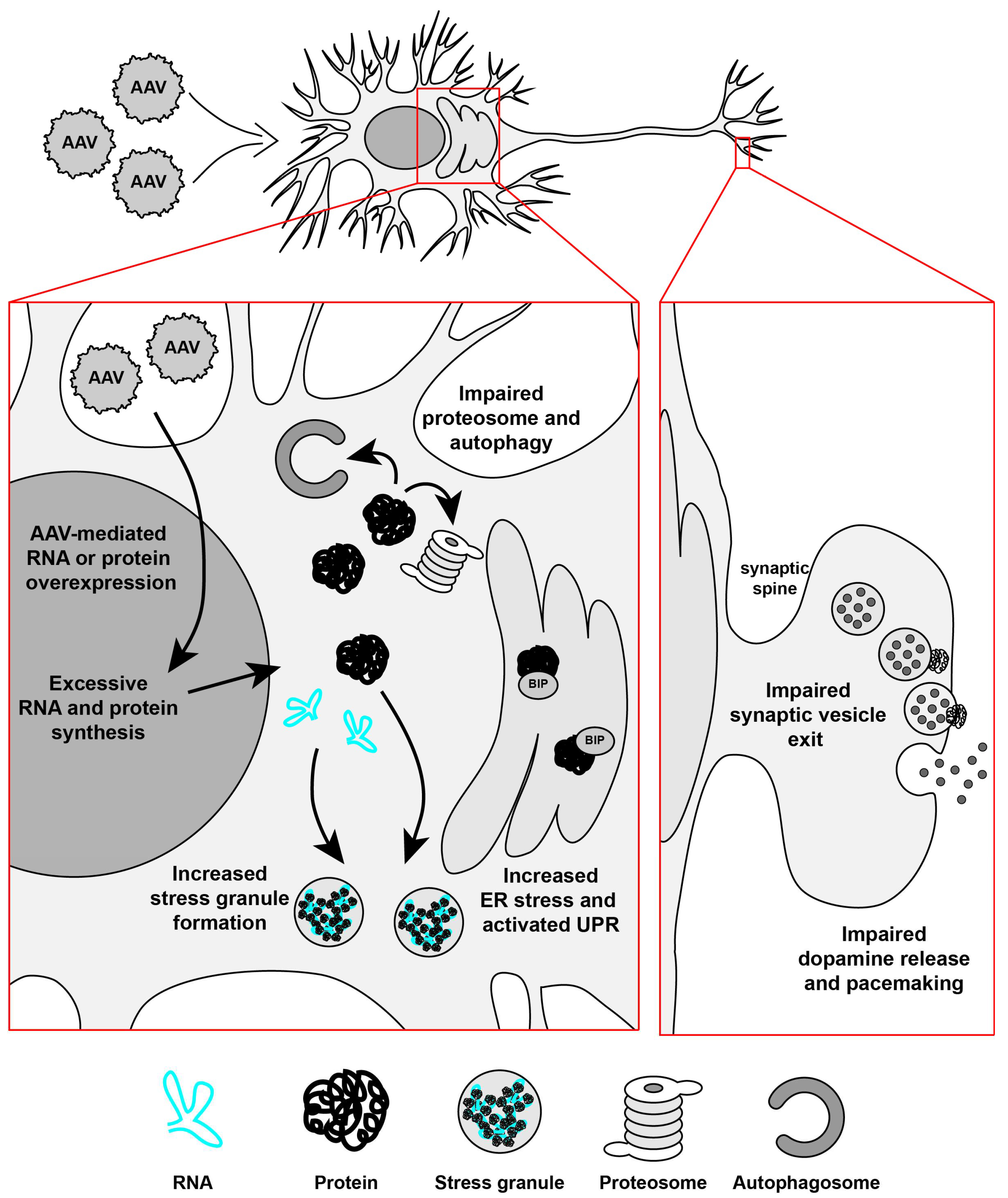
:1. Introduction
2. Delivering Neurotrophic Factor-Encoding Genes Carried by AAV to the Striatum
3. AAV to Target Substantia Nigra
4. AAV-α-syn Animal Models of Parkinson’s Disease
5. Challenges in Control AAV Vectors for Targeting Substantia Nigra Dopamine Neurons
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| α-syn | alpha-synuclein |
| AADC | aromatic l-amino decarboxylase |
| AAV | adeno-associated virus |
| BIP | binding immunoglobulin protein |
| bGH-polyA | bovine growth hormone polyadenylation sequence |
| CBA | chicken β-actin |
| cDNA | complementary deoxyribonucleic acid |
| CDNF | cerebral dopamine neurotrophic factor |
| CMV | cytomegalovirus |
| DIO | double-floxed inverse ORF |
| EF1a | elongation factor 1 alpha |
| GAD | glutamic acid decarboxylase |
| GFP | green fluorescent protein |
| GDNF | glial cell line-derived neurotrophic factor |
| HPLC | high performance liquid chromatography |
| IRES | internal ribosome entry site |
| iRFP | infrared fluorescent protein |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NRTN | neurturin |
| NSE | neuron-specific enolaseRNA: ribonucleic acid |
| rtTA | reverse tetracycline transactivator |
| SEM | standard error of the mean |
| SPECT/CT | single-photon emission computed tomography/computed tomography |
| TH | tyrosine hydroxylase |
| WPRE | woodchuck hepatitis virus posttranscriptional regulatory element |
| WT | wild-type |
References
- Kaplitt, M.G.; Leone, P.; Samulski, R.J.; Xiao, X.; Pfaff, D.W.; O′Malley, K.L.; During, M.J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Asokan, A.; Samulski, R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006, 14, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Airavaara, M.; Harvey, B.K. Viral vectors for neurotrophic factor delivery: A gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol. Res. 2010, 61, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Cearley, C.N.; Vandenberghe, L.H.; Parente, M.K.; Carnish, E.R.; Wilson, J.M.; Wolfe, J.H. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol. Ther. 2008, 16, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.; Gorbatyuk, O.S.; Velardo, M.J.; Peden, C.S.; Williams, P.; Zolotukhin, S.; Reier, P.J.; Mandel, R.J.; Muzyczka, N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 2004, 10, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, Z.; Malik, J.M.; Michel, U.; Bahr, M.; Kugler, S. Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Exp. Physiol. 2005, 90, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Schaffer, D.V. Designer gene delivery vectors: Molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm. Res. 2008, 25, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Kirik, D.; Cederfjall, E.; Halliday, G.; Petersen, A. Gene therapy for Parkinson’s disease: Disease modification by GDNF family of ligands. Neurobiol. Dis. 2016, 97, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.; Potschke, C.; Liss, B. Converging roles of ion channels, calcium, metabolic stress, and activity-pattern of substantia nigra dopaminergic neurons in health and Parkinson’s disease. J. Neurochem. 2016, 139, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Braak, H.; Ghebremedhin, E.; Rub, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Sherer, T.B.; Greenamyre, J.T. Animal models of Parkinson’s disease. Bioessays 2002, 24, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Foundation, N.P. Genetics and Parkinson’s Disease. Available online: http://www.parkinson.org/understanding-parkinsons/what-is-parkinsons/Genetics-and-Parkinsons-Disease (accessed on 7 November 2016).
- Berman, S.B.; Hastings, T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: Implications for Parkinson’s disease. J. Neurochem. 1999, 73, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.N.; Sanchez-Padilla, J.; Wokosin, D.; Kondapalli, J.; Ilijic, E.; Schumacker, P.T.; Surmeier, D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 2010, 468, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 2003. [Google Scholar] [CrossRef] [PubMed]
- Chartier-Harlin, M.C.; Kachergus, J.; Roumier, C.; Mouroux, V.; Douay, X.; Lincoln, S.; Levecque, C.; Larvor, L.; Andrieux, J.; Hulihan, M.; et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004, 364, 1167–1169. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Cortico-basal ganglia-cortical circuitry in Parkinson’s disease reconsidered. Exp. Neurol. 2008, 212, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Domanskyi, A.; Saarma, M.; Airavaara, M. Prospects of neurotrophic factors for Parkinson’s disease: Comparison of protein and gene therapy. Hum. Gene Ther. 2015, 26, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Kirik, D.; Rosenblad, C.; Bjorklund, A.; Mandel, R.J. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: Intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 2000, 20, 4686–4700. [Google Scholar] [PubMed]
- Wang, L.; Muramatsu, S.; Lu, Y.; Ikeguchi, K.; Fujimoto, K.; Okada, T.; Mizukami, H.; Hanazono, Y.; Kume, A.; Urano, F.; et al. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson’s disease. Gene Ther. 2002, 9, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.; Kirik, D.; Rosenblad, C.; Georgievska, B.; Lundberg, C.; Mandel, R.J. Towards a neuroprotective gene therapy for Parkinson’s disease: Use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000, 886, 82–98. [Google Scholar] [CrossRef]
- Johnston, L.C.; Eberling, J.; Pivirotto, P.; Hadaczek, P.; Federoff, H.J.; Forsayeth, J.; Bankiewicz, K.S. Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum. Gene Ther. 2009, 20, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Lang, A.E. Disease-modifying strategies for Parkinson’s disease. Mov. Disord. 2015, 30, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- AAV2-GDNF for Advanced Parkinson’s Disease. Available online: https://foxtrialfinder.michaeljfox.org/trial/3121/ (accessed on 4 October 2016).
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Brown, L.; Wilson, A.; Kruegel, B.; Siffert, J.; Johnson, E.M., Jr.; Kordower, J.H.; Herzog, C.D. Properly scaled and targeted AAV2-NRTN (neurturin) to the substantia nigra is safe, effective and causes no weight loss: Support for nigral targeting in Parkinson’s disease. Neurobiol. Dis. 2011, 44, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.J., Jr.; Bartus, R.T.; Siffert, J.; Davis, C.S.; Lozano, A.; Boulis, N.; Vitek, J.; Stacy, M.; Turner, D.; Verhagen, L.; et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: A double-blind, randomised, controlled trial. Lancet Neurol. 2010, 9, 1164–1172. [Google Scholar] [CrossRef]
- Warren Olanow, C.; Bartus, R.T.; Baumann, T.L.; Factor, S.; Boulis, N.; Stacy, M.; Turner, D.A.; Marks, W.; Larson, P.; Starr, P.A.; et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann. Neurol. 2015, 78, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Johnson, E.M., Jr. Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 2: Where do we stand and where must we go next? Neurobiol. Dis. 2017, 97, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Runeberg-Roos, P.; Piccinini, E.; Penttinen, A.M.; Matlik, K.; Heikkinen, H.; Kuure, S.; Bespalov, M.M.; Peranen, J.; Garea-Rodriguez, E.; Fuchs, E.; et al. Developing therapeutically more efficient Neurturin variants for treatment of Parkinson’s disease. Neurobiol. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef]
- Christine, C.W.; Starr, P.A.; Larson, P.S.; Eberling, J.L.; Jagust, W.J.; Hawkins, R.A.; VanBrocklin, H.F.; Wright, J.F.; Bankiewicz, K.S.; Aminoff, M.J. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 2009, 73, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- AADC Gene Therapy for Parkinson’s Disease (AADC). Available online: https://clinicaltrials.gov/ct2/show/NCT01973543 (accessed on 7 November 2016).
- Fan, D.S.; Ogawa, M.; Fujimoto, K.I.; Ikeguchi, K.; Ogasawara, Y.; Urabe, M.; Nishizawa, M.; Nakano, I.; Yoshida, M.; Nagatsu, I.; et al. Behavioral recovery in 6-hydroxydopamine-lesioned rats by cotransduction of striatum with tyrosine hydroxylase and aromatic l-amino acid decarboxylase genes using two separate adeno-associated virus vectors. Hum. Gene Ther. 1998, 9, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Cederfjall, E.; Sahin, G.; Kirik, D.; Bjorklund, T. Design of a single AAV vector for coexpression of TH and GCH1 to establish continuous DOPA synthesis in a rat model of Parkinson’s disease. Mol. Ther. 2012, 20, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Kordower, J.H.; Johnson, E.M., Jr.; Brown, L.; Kruegel, B.R.; Chu, Y.; Baumann, T.L.; Lang, A.E.; Olanow, C.W.; Herzog, C.D. Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with alpha-synucleinopathies. Neurobiol. Dis. 2015, 78, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Green, F.; Samaranch, L.; Zhang, H.S.; Manning-Bog, A.; Meyer, K.; Forsayeth, J.; Bankiewicz, K.S. Axonal transport of AAV9 in nonhuman primate brain. Gene Ther. 2016, 23, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.P.; Forsayeth, J.; Bankiewicz, K.S. Glial-derived neurotrophic factor gene transfer for Parkinson’s disease: Anterograde distribution of AAV2 vectors in the primate brain. Neurobiol. Dis. 2012, 48, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Chinea, P.; Cruz-Muros, I.; Afonso-Oramas, D.; Castro-Hernandez, J.; Salas-Hernandez, J.; Chtarto, A.; Luis-Ravelo, D.; Humbert-Claude, M.; Tenenbaum, L.; Gonzalez-Hernandez, T. Long-term controlled GDNF over-expression reduces dopamine transporter activity without affecting tyrosine hydroxylase expression in the rat mesostriatal system. Neurobiol. Dis. 2016, 88, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Georgievska, B.; Kirik, D.; Bjorklund, A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp. Neurol. 2002, 177, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, J.; Maddalena, A.; Bahr, M.; Kugler, S. Pharmacologically controlled, discontinuous GDNF gene therapy restores motor function in a rat model of Parkinson’s disease. Neurobiol. Dis. 2014, 65, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Kells, A.P.; Eberling, J.; Su, X.; Pivirotto, P.; Bringas, J.; Hadaczek, P.; Narrow, W.C.; Bowers, W.J.; Federoff, H.J.; Forsayeth, J.; et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J. Neurosci. 2010, 30, 9567–9577. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Harvey, B.K.; Hoffman, A.F.; Wang, Y.; Chiang, Y.H.; Lupica, C.R. MPTP-induced deficits in striatal synaptic plasticity are prevented by glial cell line-derived neurotrophic factor expressed via an adeno-associated viral vector. FASEB J. 2008, 22, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, M.; Herzog, C.D.; Brandon, E.P.; Cunningham, J.J.; Ramirez, G.A.; Ketchum, E.T.; Bartus, R.T. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Mol. Ther. 2007, 15, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.D.; Dass, B.; Holden, J.E.; Stansell, J., 3rd; Gasmi, M.; Tuszynski, M.H.; Bartus, R.T.; Kordower, J.H. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov. Disord. 2007, 22, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.D.; Dass, B.; Gasmi, M.; Bakay, R.; Stansell, J.E.; Tuszynski, M.; Bankiewicz, K.; Chen, E.Y.; Chu, Y.; Bishop, K.; et al. Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol. Ther. 2008, 16, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Herzog, C.D.; Dass, B.; Bakay, R.A.; Stansell, J., 3rd; Gasmi, M.; Bartus, R.T. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann. Neurol. 2006, 60, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Peranen, J.; Galli, E.; Pulkkila, P.; Lonka-Nevalaita, L.; Tamminen, T.; Voutilainen, M.H.; Raasmaja, A.; Saarma, M.; Mannisto, P.T.; et al. Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson’s disease. Brain Behav. 2013, 3, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, T.; Gong, X.; Hu, G.; Ding, W.; Wang, X. AAV2-mediated striatum delivery of human CDNF prevents the deterioration of midbrain dopamine neurons in a 6-hydroxydopamine induced parkinsonian rat model. Exp. Neurol. 2013, 248, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Hamby, M.E.; Gong, Y.; Hirko, A.C.; Wang, S.; Hughes, J.A.; King, M.A.; Meyer, E.M. Dose and Promoter Effects of Adeno-Associated Viral Vector for Green Fluorescent Protein Expression in the Rat Brain. Exp. Neurol. 2002, 176, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Taymans, J.M.; Vandenberghe, L.H.; Haute, C.V.; Thiry, I.; Deroose, C.M.; Mortelmans, L.; Wilson, J.M.; Debyser, Z.; Baekelandt, V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum. Gene Ther. 2007, 18, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Low, K.; Aebischer, P.; Schneider, B.L. Direct and retrograde transduction of nigral neurons with AAV6, 8, and 9 and intraneuronal persistence of viral particles. Hum. Gene Ther. 2013, 24, 613–629. [Google Scholar] [CrossRef] [PubMed]
- McFarland, N.R.; Lee, J.S.; Hyman, B.T.; McLean, P.J. Comparison of transduction efficiency of recombinant AAV serotypes 1, 2, 5, and 8 in the rat nigrostriatal system. J. Neurochem. 2009, 109, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Masamizu, Y.; Okada, T.; Ishibashi, H.; Takeda, S.; Yuasa, S.; Nakahara, K. Efficient gene transfer into neurons in monkey brain by adeno-associated virus 8. Neuroreport 2010, 21, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Markakis, E.A.; Vives, K.P.; Bober, J.; Leichtle, S.; Leranth, C.; Beecham, J.; Elsworth, J.D.; Roth, R.H.; Samulski, R.J.; Redmond, D.E., Jr. Comparative transduction efficiency of AAV vector serotypes 1–6 in the substantia nigra and striatum of the primate brain. Mol. Ther. 2010, 18, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.D.; Brown, L.; Kruegel, B.R.; Wilson, A.; Tansey, M.G.; Gage, F.H.; Johnson, E.M., Jr.; Bartus, R.T. Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120). Neurobiol. Dis. 2013, 58, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Landeck, N.; Buck, K.; Kirik, D. Toxic effects of human and rodent variants of alpha-synuclein in vivo. Eur. J. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Voutilainen, M.H.; Domanskyi, A.; Ahola, S.; Piepponen, T.P.; Tuominen, R.K.; Harvey, B.K.; Airavaara, M.; University of Helsinki, Helsinki, Finland. AAV mediated overexpression of alpha-synuclein protein and foreign RNA in the substantia nigra: Dissociation between dopamine damage and motor impairment. Unpublished work. 2017. [Google Scholar]
- Quintino, L.; Manfre, G.; Wettergren, E.E.; Namislo, A.; Isaksson, C.; Lundberg, C. Functional neuroprotection and efficient regulation of GDNF using destabilizing domains in a rodent model of Parkinson′s disease. Mol. Ther. 2013, 21, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Chtarto, A.; Humbert-Claude, M.; Bockstael, O.; Das, A.T.; Boutry, S.; Breger, L.S.; Klaver, B.; Melas, C.; Barroso-Chinea, P.; Gonzalez-Hernandez, T.; et al. A regulatable AAV vector mediating GDNF biological effects at clinically-approved sub-antimicrobial doxycycline doses. Mol. Ther. Methods Clin. Dev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, A.; Tereshchenko, J.; Bahr, M.; Kugler, S. Adeno-associated virus-mediated, mifepristone-regulated transgene expression in the brain. Mol. Ther. Nucleic Acids 2013. [Google Scholar] [CrossRef] [PubMed]
- Samaranch, L.; San Sebastian, W.; Kells, A.P.; Salegio, E.A.; Heller, G.; Bringas, J.R.; Pivirotto, P.; DeArmond, S.; Forsayeth, J.; Bankiewicz, K.S. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol. Ther. 2014, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, H.S.; Lelos, M.J.; Dunnett, S.B. Do alpha-synuclein vector injections provide a better model of Parkinson’s disease than the classic 6-hydroxydopamine model? Exp. Neurol. 2012, 237, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Mattsson, B.; Bjorklund, A. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson’s disease. Exp. Neurol. 2012, 235, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Salva, M.; Van der Perren, A.; Casadei, N.; Stroobants, S.; Nuber, S.; D′Hooge, R.; Van den Haute, C.; Baekelandt, V. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 2013. [Google Scholar] [CrossRef] [PubMed]
- St Martin, J.L.; Klucken, J.; Outeiro, T.F.; Nguyen, P.; Keller-McGandy, C.; Cantuti-Castelvetri, I.; Grammatopoulos, T.N.; Standaert, D.G.; Hyman, B.T.; McLean, P.J. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 2007, 100, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Svarcbahs, R.; Julku, U.H.; Myohanen, T.T. Inhibition of prolyl oligopeptidase restores spontaneous motor behavior in the alpha-synuclein virus vector-based Parkinson’s disease mouse model by decreasing alpha-synuclein oligomeric species in mouse brain. J. Neurosci. 2016, 36, 12485–12497. [Google Scholar] [CrossRef] [PubMed]
- Kirik, D.; Rosenblad, C.; Burger, C.; Lundberg, C.; Johansen, T.E.; Muzyczka, N.; Mandel, R.J.; Bjorklund, A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J. Neurosci. 2002, 22, 2780–2791. [Google Scholar] [PubMed]
- Decressac, M.; Mattsson, B.; Lundblad, M.; Weikop, P.; Bjorklund, A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol. Dis. 2012, 45, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Gombash, S.E.; Manfredsson, F.P.; Kemp, C.J.; Kuhn, N.C.; Fleming, S.M.; Egan, A.E.; Grant, L.M.; Ciucci, M.R.; MacKeigan, J.P.; Sortwell, C.E. Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS ONE 2013, 8, e81426. [Google Scholar] [CrossRef] [PubMed]
- Shahaduzzaman, M.; Nash, K.; Hudson, C.; Sharif, M.; Grimmig, B.; Lin, X.; Bai, G.; Liu, H.; Ugen, K.E.; Cao, C.; et al. Anti-human alpha-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-alpha-synuclein rat model of Parkinson’s disease. PLoS ONE 2015, 10, e0116841. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Iwatsubo, T.; Mizuno, Y.; Mochizuki, H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: Resemblance to pathogenetic changes in Parkinson’s disease. J. Neurochem. 2004, 91, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Febbraro, F.; Sahin, G.; Farran, A.; Soares, S.; Jensen, P.H.; Kirik, D.; Romero-Ramos, M. Ser129D mutant alpha-synuclein induces earlier motor dysfunction while S129A results in distinctive pathology in a rat model of Parkinson’s disease. Neurobiol. Dis. 2013, 56, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Koprich, J.B.; Johnston, T.H.; Huot, P.; Reyes, M.G.; Espinosa, M.; Brotchie, J.M. Progressive neurodegeneration or endogenous compensation in an animal model of Parkinson’s disease produced by decreasing doses of alpha-synuclein. PLoS ONE 2011, 6, e17698. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, M.N.; Genc, O.; Bobela, W.; Mohanna, S.; Ardah, M.T.; El-Agnaf, O.M.; Cantoni, M.; Bensadoun, J.C.; Schneggenburger, R.; Knott, G.W.; et al. Nigrostriatal overabundance of alpha-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 2012, 123, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, M.; Dovero, S.; Engeln, M.; Bido, S.; Bastide, M.F.; Dutheil, N.; Vollenweider, I.; Baud, L.; Piron, C.; Grouthier, V.; et al. Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by alpha-synuclein overexpression. Acta Neuropathol. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Van der Perren, A.; Toelen, J.; Casteels, C.; Macchi, F.; Van Rompuy, A.S.; Sarre, S.; Casadei, N.; Nuber, S.; Himmelreich, U.; Osorio Garcia, M.I.; et al. Longitudinal follow-up and characterization of a robust rat model for Parkinson’s disease based on overexpression of alpha-synuclein with adeno-associated viral vectors. Neurobiol. Aging 2015, 36, 1543–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gully, J.C.; Sergeyev, V.G.; Bhootada, Y.; Mendez-Gomez, H.; Meyers, C.A.; Zolotukhin, S.; Gorbatyuk, M.S.; Gorbatyuk, O.S. Up-regulation of activating transcription factor 4 induces severe loss of dopamine nigral neurons in a rat model of Parkinson’s disease. Neurosci. Lett. 2016, 627, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.D.; Qu, G.; Sferra, T.J.; Clark, R.; Chen, R.; Johnson, P.R. Adeno-associated virus-mediated gene transfer to the brain: Duration and modulation of expression. Hum. Gene Ther. 1999, 10, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Hensley, S.E.; Amalfitano, A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol. Ther. 2007, 15, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Dayton, R.D.; Leidenheimer, N.J.; Jansen, K.; Golde, T.E.; Zweig, R.M. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol. Ther. 2006, 13, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Update Regarding MJFF & UNC-Generated AAV Viral Vectors. Available online: https://www.med.unc.edu/genetherapy/vectorcore/files/updateregardingmjffviralvectors-final (accessed on 4 November 2016).
- Bartels, T.; Choi, J.G.; Selkoe, D.J. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Jappelli, R.; Maji, S.K.; Desplats, P.A.; Boyer, L.; Aigner, S.; Hetzer, C.; Loher, T.; Vilar, M.; Campioni, S.; et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA 2011, 108, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Animal | Insert | Serotype/Promoter | Duration | STR TH Fibre Density | SN TH+ Cells | STR Dopamine | Behaviour | Ref. |
|---|---|---|---|---|---|---|---|---|
| C57BL/6 mice | WTA53T | AAV7/CMV (w/WPRE) | 8 weeks | N/A | ↓ | N/A | WT: motor deficits | [72] |
| C57BL/6 mice | WT | rAAV/CMV (w/WPRE and IRES) | 24 weeks | N/A | ↓ | N/A | N/A | [73] |
| Sprague Dawley rats | WT | rAAV/CBA (w/CMV elements) | 27 weeks | ↓ | ↓ | ↓ | No motor deficits * | [75] |
| Sprague Dawley rats | WT | AAV6/CBA (w/and w/out WPRE) | 16 weeks | ↓ | ↓ | ↓ | Motor deficits | [76] |
| Sprague Dawley rats | WT | AAV5/CBA/CMV | 8 weeks | N/A | ↓ | N/A | Some motor and non-motor deficits | [77] |
| Fisher 344 rats | WT | AAV9/CBA | 12 weeks | N/A | ↓ | N/A | Motor deficits | [78] |
| Sprague Dawley rats | WT | rAAV/CMV | 13 weeks | N/A | ↓ | ↔ | No motor deficits | [79] |
| Sprague Dawley rats | WTS129AS129D | AAV5/CMV/CBA | 15 weeks | ↓ | ↓ | N/A | Some motor deficit | [80] |
| Sprague Dawley rats | A53T | AAV1/2/CBA/CMV (w/WPRE and bGH-polyA) | 6 weeks | ↓ | ↓ | ↓ | Some motor deficit | [81] |
| Sprague Dawley rats | WTA30P | AAV6/CMV (w/WPRE) | 16 weeks | ↓• | ↓• | ↓• | Some motor deficit | [82] |
| Wistar rats | WTA53T | AAV7/CMV/synapsin | 29 days | N/A | A53T: ↓ | A53T: ↓ | A53T: Some motor deficit | [84] |
| Sprague Dawley rats | WT | AAV5/human U6 | 8 weeks | N/A | ↓ | ↓ | No motor deficit | [85] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albert, K.; Voutilainen, M.H.; Domanskyi, A.; Airavaara, M. AAV Vector-Mediated Gene Delivery to Substantia Nigra Dopamine Neurons: Implications for Gene Therapy and Disease Models. Genes 2017, 8, 63. https://doi.org/10.3390/genes8020063
Albert K, Voutilainen MH, Domanskyi A, Airavaara M. AAV Vector-Mediated Gene Delivery to Substantia Nigra Dopamine Neurons: Implications for Gene Therapy and Disease Models. Genes. 2017; 8(2):63. https://doi.org/10.3390/genes8020063
Chicago/Turabian StyleAlbert, Katrina, Merja H. Voutilainen, Andrii Domanskyi, and Mikko Airavaara. 2017. "AAV Vector-Mediated Gene Delivery to Substantia Nigra Dopamine Neurons: Implications for Gene Therapy and Disease Models" Genes 8, no. 2: 63. https://doi.org/10.3390/genes8020063






