Telomere Transcripts Target Telomerase in Human Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
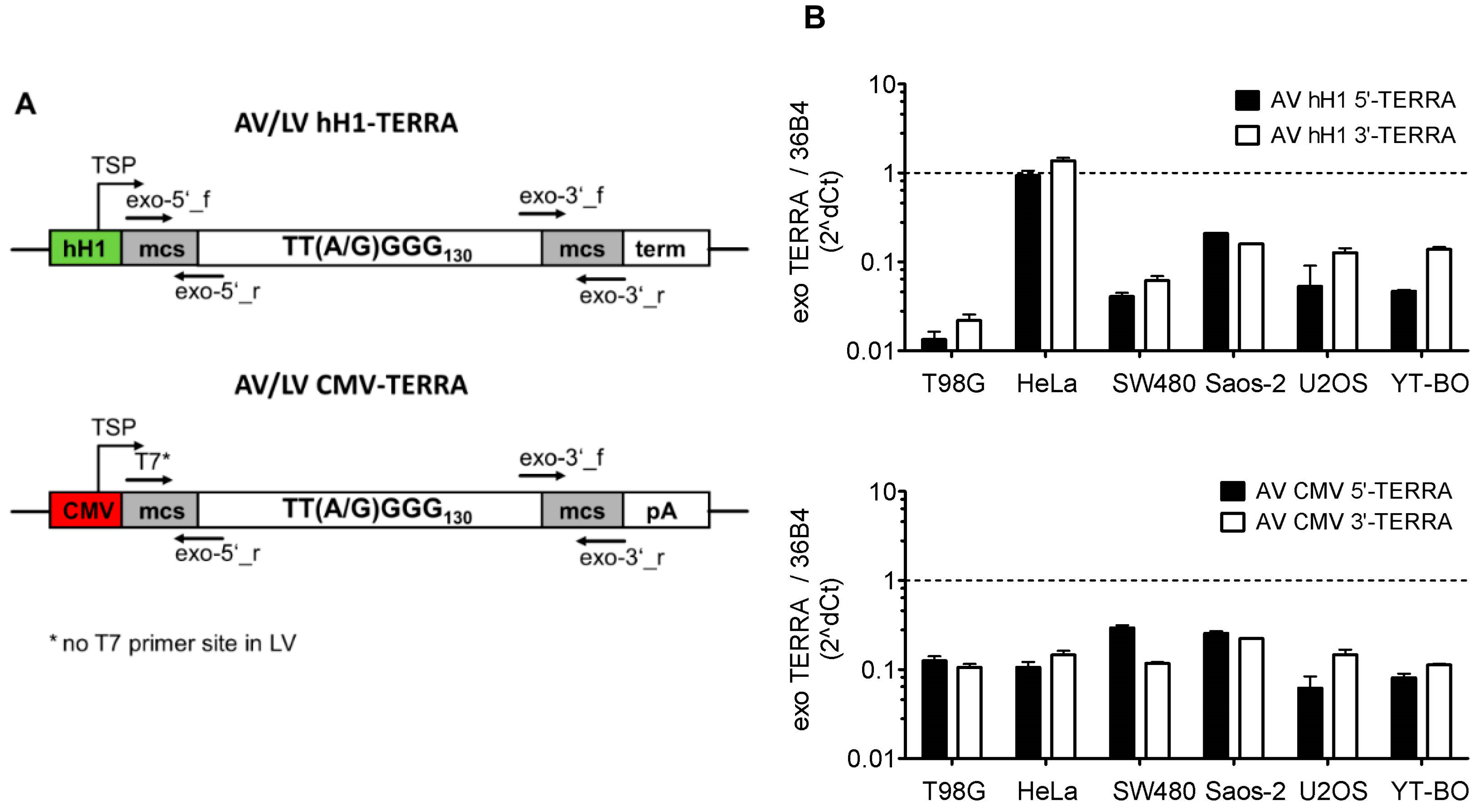
2.2. Adeno- and Lentivirus Constructs
2.3. Relative Telomere Length and Expression
2.4. Telomerase Activity
2.5. Cell Growth and Viability
2.6. Clonogenicity Assay
2.7. Senescence-Associated β-Galactosidase Activity
2.8. Statistical Analysis
3. Results
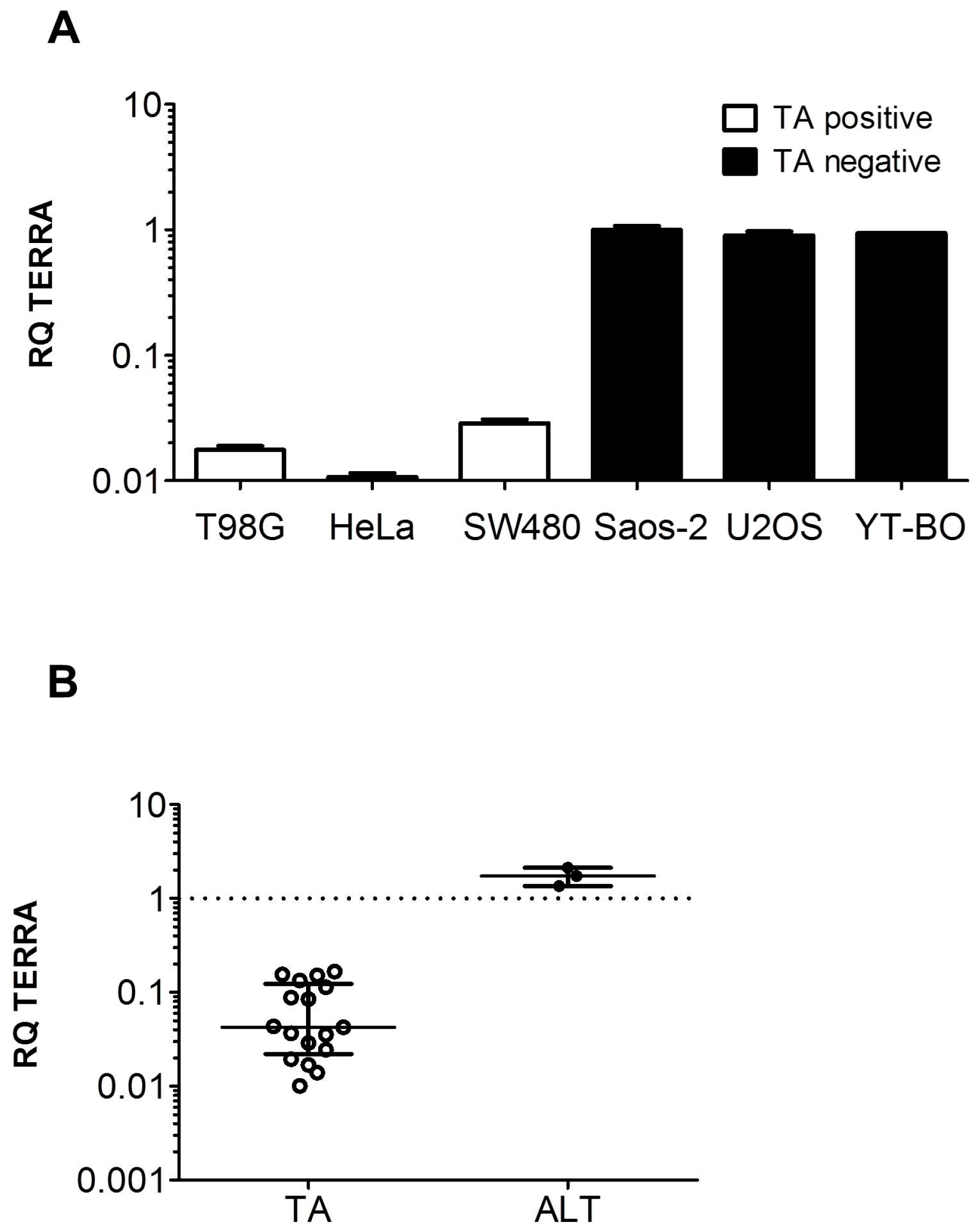
3.1. TERRA Expression in Human Cell Models
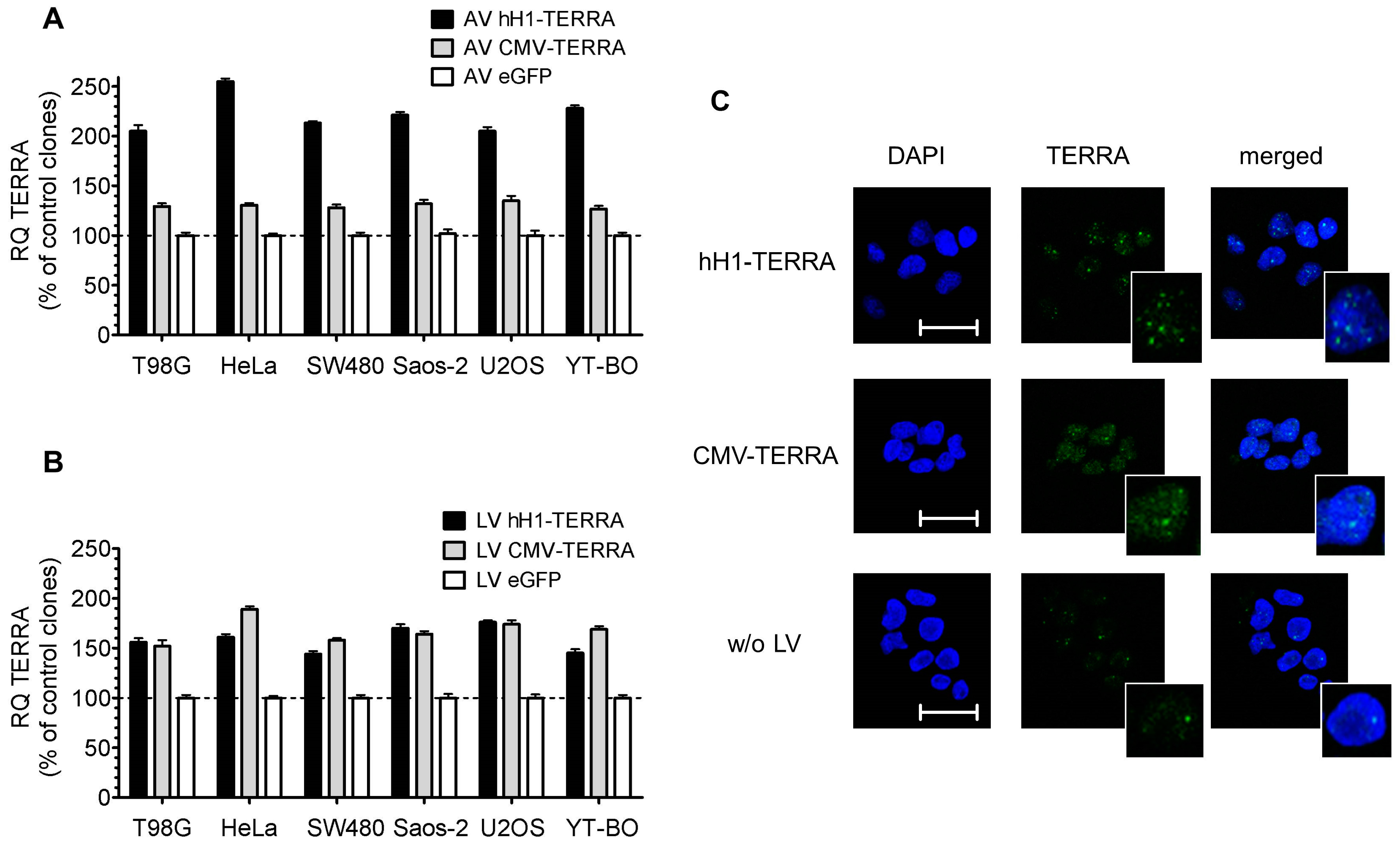
3.2. Transient and Stable Expression of Recombinant TERRA in Human Cell Lines
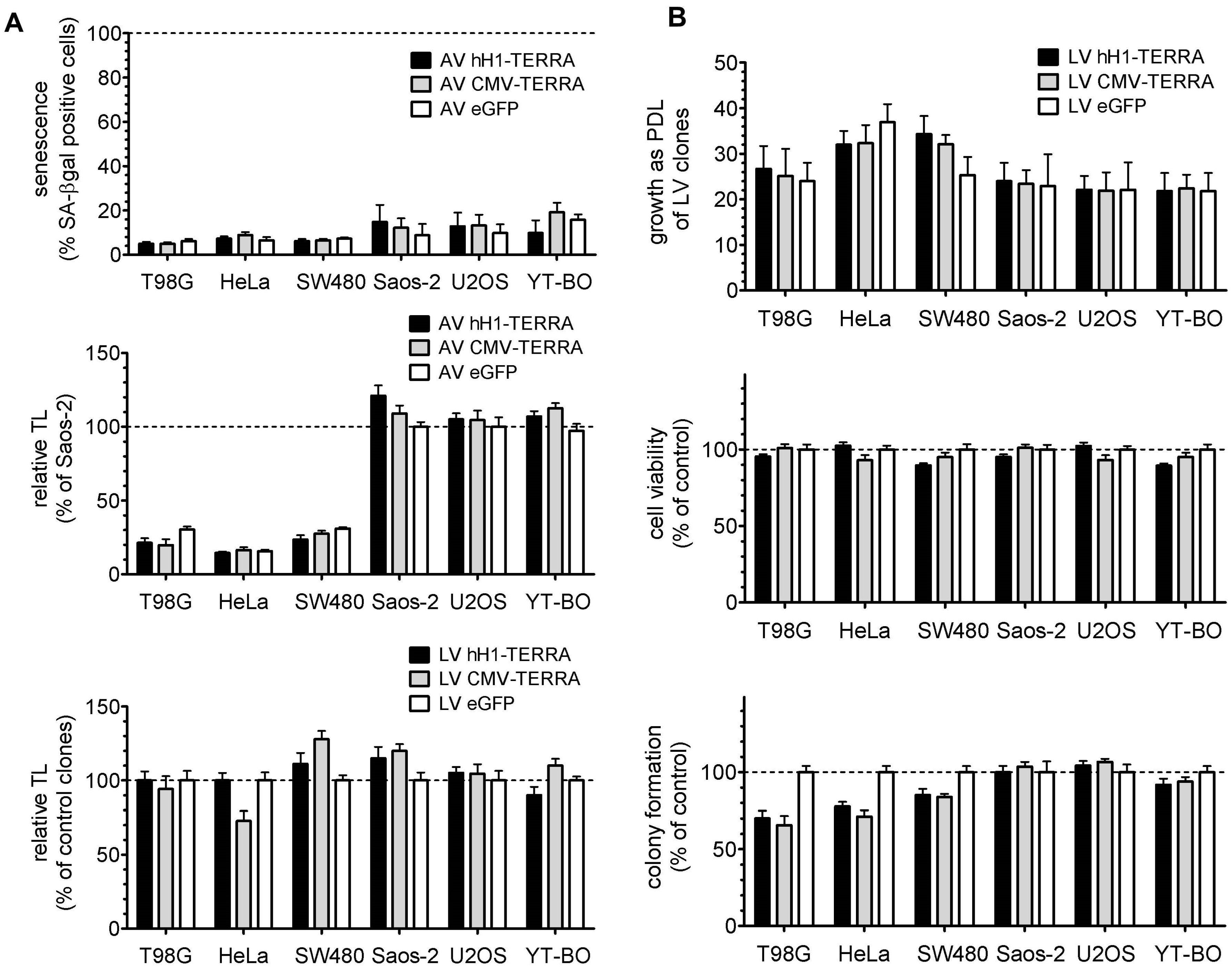
3.3. Telomere Length and Cell Growth Behavior
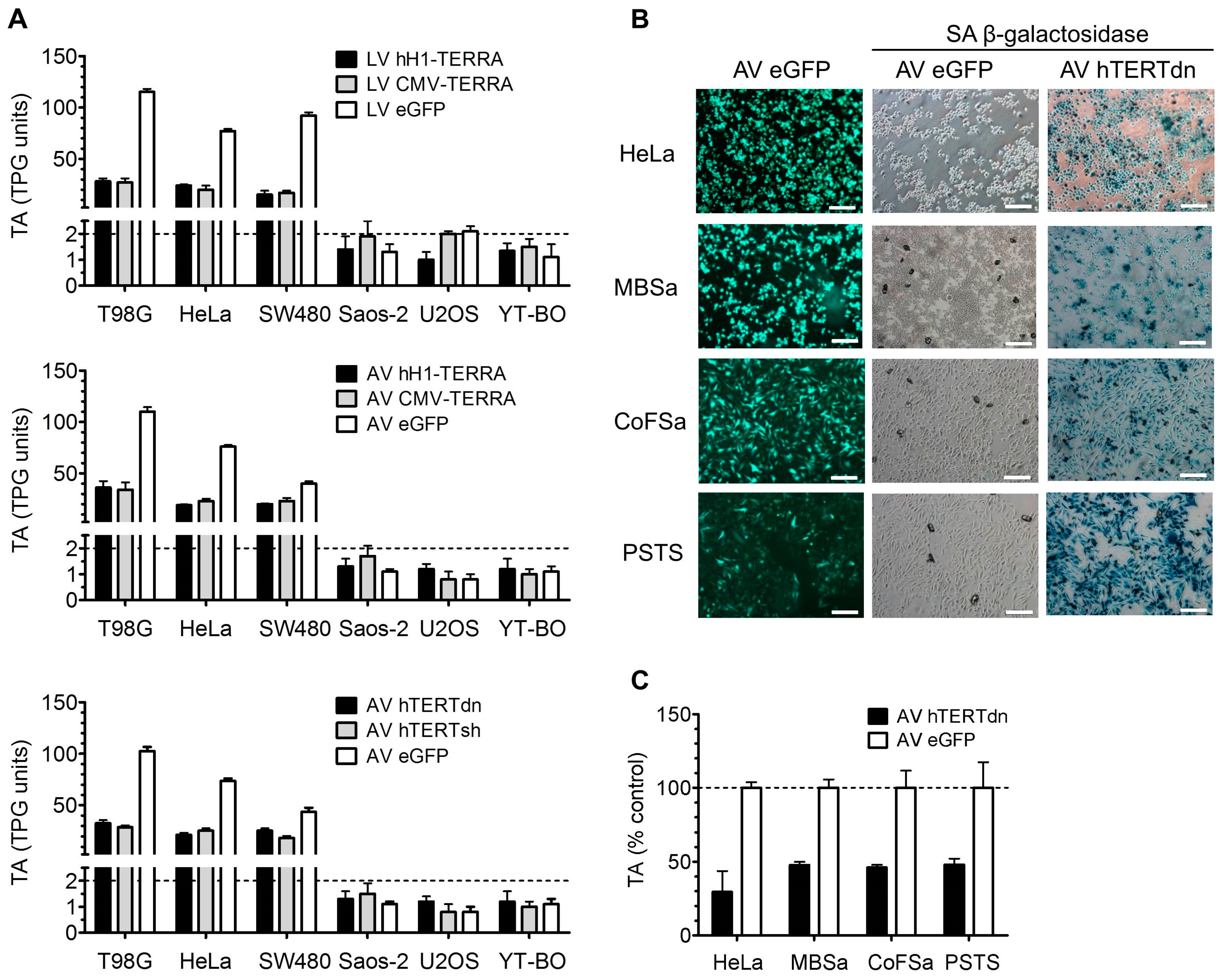
3.4. Telomerase Activity in Cell Models with Recombinant TERRA
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.J.; Rube, H.T.; Xavier-Magalhães, A.; Costa, B.M.; Mancini, A.; Song, J.S.; Costello, J.F. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol. Cancer Res. 2016, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNAand RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Nergadze, S.G.; Farnung, B.O.; Wischnewski, H.; Khoriauli, L.; Vitelli, V.; Chawla, R.; Giulotto, E.; Azzalin, C.M. CpG-island promoters drive transcription of human telomeres. RNA 2009, 15, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Redon, S.; Reichenbach, P.; Lingner, J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010, 38, 5797–5806. [Google Scholar] [CrossRef] [PubMed]
- Sampl, S.; Pramhas, S.; Stern, C.; Preusser, M.; Marosi, C.; Holzmann, K. Expression of telomeres in astrocytoma WHO grade 2 to 4: TERRA level correlates with telomere length, telomerase activity, and advanced clinical grade. Transl. Oncol. 2012, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.J.; Cropley, J.E.; Pickett, H.A.; Reddel, R.R.; Suter, C.M. Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res. 2009, 37, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Caslini, C. Transcriptional regulation of telomeric non-coding RNA: Implications on telomere biology, replicative senescence and cancer. RNA Biol. 2010, 7, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Feuerhahn, S.; Iglesias, N.; Panza, A.; Porro, A.; Lingner, J. TERRA biogenesis, turnover and implications for function. FEBS Lett. 2010, 584, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Maicher, A.; Kastner, L.; Luke, B. Telomeres and disease: Enter TERRA. RNA Biol. 2012, 9, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Maicher, A.; Lockhart, A.; Luke, B. Breaking new ground: Digging into TERRA function. Biochim. Biophys. Acta 2014, 1839, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Rippe, K.; Luke, B. TERRA and the state of the telomere. Nat. Struct. Mol. Biol. 2015, 22, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Blasco, M.A. Chromatin regulation and non-coding RNAs at mammalian telomeres. Semin. Cell Dev. Biol. 2010, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Nasir, L.; Devlin, P.; McKevitt, T.; Rutteman, G.; Argyle, D.J. Telomere lengths and telomerase activity in dog tissues: A potential model system to study human telomere and telomerase biology. Neoplasia 2001, 3, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Jurek, D.; Wrba, F.; Kaserer, K.; Wurzer, G.; Karner-Hanusch, J.; Marian, B. Cells obtained from colorectal microadenomas mirror early premalignant growth patterns in vitro. Eur. J. Cancer 2002, 38, 1937–1945. [Google Scholar] [CrossRef]
- Willson, J.K.; Bittner, G.N.; Oberley, T.D.; Meisner, L.F.; Weese, J.L. Cell culture of human colon adenomas and carcinomas. Cancer Res. 1987, 47, 2704–2713. [Google Scholar] [PubMed]
- Schweiger, N.; Hauck, M.; Steinhoff, H.; Sampl, S.; Reifinger, M.; Walter, I.; Kreilmeier, T.; Marian, B.; Grusch, M.; Berger, W.; et al. Canine and human sarcomas exhibit predominant FGFR1 expression and impaired viability after inhibition of signaling. Mol. Carcinog. 2015, 54, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Kiyozuka, Y. Correlation of telomerase activity and telomere length to chemosensitivity. Methods Mol. Med. 2005, 111, 97–108. [Google Scholar] [PubMed]
- Griffith, J.; Bianchi, A.; de Lange, T. TRF1 promotes parallel pairing of telomeric tracts in vitro. J. Mol. Biol. 1998, 278, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, K.; Gerner, C.; Poltl, A.; Schafer, R.; Obrist, P.; Ensinger, C.; Grimm, R.; Sauermann, G. A human common nuclear matrix protein homologous to eukaryotic translation initiation factor 4A. Biochem. Biophys. Res. Commun. 2000, 267, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ferraiuolo, M.A.; Lee, C.S.; Ler, L.W.; Hsu, J.L.; Costa-Mattioli, M.; Luo, M.J.; Reed, R.; Sonenberg, N. A nuclear translation-like factor eIF4AIII is recruited to the mRNA during splicing and functions in nonsense-mediated decay. Proc. Natl. Acad. Sci. USA 2004, 101, 4118–4123. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Yu, E.Y.; Khurts, S.; Ben-Porath, I.; Currier, J.L.; Metz, G.B.; Brooks, M.W.; Kaneko, S.; Murakami, S.; DeCaprio, J.A.; et al. Telomerase maintains telomere structure in normal human cells. Cell 2003, 114, 241–253. [Google Scholar] [CrossRef]
- Guiducci, C.; Anglana, M.; Wang, A.; Bacchetti, S. Transient expression of wild-type or biologically inactive telomerase allows the formation of artificial telomeres in mortal human cells. Exp. Cell Res. 2001, 265, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Losert, A.; Mauritz, I.; Erlach, N.; Herbacek, I.; Schulte-Hermann, R.; Holzmann, K.; Grusch, M. Monitoring viral decontamination procedures with green fluorescent protein-expressing adenovirus. Anal. Biochem. 2006, 355, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Aschacher, T.; Wolf, B.; Enzmann, F.; Kienzl, P.; Messner, B.; Sampl, S.; Svoboda, M.; Mechtcheriakova, D.; Holzmann, K.; Bergmann, M. LINE-1 induces hTERT and ensures telomere maintenance in tumour cell lines. Oncogene 2016, 35, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Aschacher, T.; Sampl, S.; Kaser, L.; Bernhard, D.; Spittler, A.; Holzmann, K.; Bergmann, M. The combined use of known antiviral reverse transcriptase inhibitors AZT and DDI induce anticancer effects at low concentrations. Neoplasia 2012, 14, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wege, H.; Chui, M.S.; Le, H.T.; Tran, J.M.; Zern, M.A. SYBR Green real-time telomeric repeat amplification protocol for the rapid quantification of telomerase activity. Nucleic Acids Res. 2003, 31, E3–E3. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Heinzle, C.; Gsur, A.; Hunjadi, M.; Erdem, Z.; Gauglhofer, C.; Stattner, S.; Karner, J.; Klimpfinger, M.; Wrba, F.; Reti, A.; et al. Differential effects of polymorphic alleles of FGF receptor 4 on colon cancer growth and metastasis. Cancer Res. 2012, 72, 5767–5777. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Gatza, C.; Donehower, L.A.; Medrano, E.E. Analysis of Cellular Senescence in Culture in Vivo: The Senescence-Associated β-Galactosidase Assay. Curr. Protoc. Cell Biol. 2005. [Google Scholar] [CrossRef]
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Low, K.C.; Tergaonkar, V. Telomerase: Central regulator of all of the hallmarks of cancer. Trends Biochem. Sci. 2013, 38, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Kolevska, T.; Spigel, D.R.; Hager, S.; Rarick, M.; Gadgeel, S.; Blais, N.; Von Pawel, J.; Hart, L.; Reck, M.; et al. A randomized phase IIstudy of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann. Oncol. 2015, 26, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, G.M.; Oppliger Leibundgut, E.; Ottmann, O.G.; Spitzer, G.; Odenike, O.; McDevitt, M.A.; Roth, A.; Daskalakis, M.; Burington, B.; Stuart, M.; et al. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. New Engl. J. Med. 2015, 373, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Begna, K.H.; Patnaik, M.M.; Zblewski, D.L.; Finke, C.M.; Laborde, R.R.; Wassie, E.; Schimek, L.; Hanson, C.A.; et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. New Engl. J. Med. 2015, 373, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Mender, I.; Gryaznov, S.; Dikmen, Z.G.; Wright, W.E.; Shay, J.W. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2′-deoxyguanosine. Cancer Discov. 2015, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.; Feuerhahn, S.; Reichenbach, P.; Lingner, J. Molecular dissection of telomeric repeat-containing RNA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol. Cell. Biol. 2010, 30, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Lee, Y.; Wischnewski, H.; Brun, C.M.; Schwarz, T.; Azzalin, C.M. RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat. Commun. 2014, 5, 5220. [Google Scholar] [CrossRef] [PubMed]
- Episkopou, H.; Draskovic, I.; Van Beneden, A.; Tilman, G.; Mattiussi, M.; Gobin, M.; Arnoult, N.; Londono-Vallejo, A.; Decottignies, A. Alternative lengthening of telomeres is characterized by reduced compaction of telomeric chromatin. Nucleic Acids Res. 2014, 42, 4391–4405. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, C.A.; Li, W.; Reisenweber, S.; Thongthip, S.; Bruno, J.; de Lange, T.; De, S.; Petrini, J.H.; Sung, P.A.; Jasin, M.; et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012, 8, e1002772. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Kao, Y.W.; Lin, J.J. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc. Natl. Acad. Sci. USA 2014, 111, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Nasir, L.; Gault, E.; Campbell, S.; Veeramalai, M.; Gilbert, D.; McFarlane, R.; Munro, A.; Argyle, D.J. Isolation and expression of the reverse transcriptase component of the Canis familiaris telomerase ribonucleoprotein (dogTERT). Gene 2004, 336, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Argyle, D.J.; Nasir, L. Telomerase: A potential diagnostic and therapeutic tool in canine oncology. Vet. Pathol. 2003, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.Y.; Argyle, D. Cancer stem cells and telomerase as potential biomarkers in veterinary oncology. Vet. J. 2010, 185, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cusanelli, E.; Romero, C.A.; Chartrand, P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol. Cell 2013, 51, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Smekalova, E.; Baumann, P. TERRA-A calling card for telomerase. Mol. Cell 2013, 51, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Lv, N.; Wysham, W.Z.; Roque, D.R.; Zhang, T.; Jiao, S.; Song, D.; Chen, J.; Bae-Jump, V.L.; Zhou, C. Knockdown of hTERT and treatment with BIBR1532 inhibit cell proliferation and invasion in endometrial cancer cells. J. Cancer 2015, 6, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Rao, Y.; Zhu, W.; Guo, F. Inhibition of cell growth and telomerase activity in osteosarcoma cells by DN-hTERT. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mar, V.; Zhou, W.; Harrington, L.; Robinson, M.O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999, 13, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Bojovic, B.; Crowe, D.L. Resistance to telomerase inhibition by human squamous cell carcinoma cell lines. Int. J. Oncol. 2011, 38, 1175–1181. [Google Scholar] [PubMed]
- Samy, M.; Gattolliat, C.H.; Pendino, F.; Hillion, J.; Nguyen, E.; Bombard, S.; Douc-Rasy, S.; Benard, J.; Segal-Bendirdjian, E. Loss of the malignant phenotype of human neuroblastoma cells by a catalytically inactive dominant-negative hTERT mutant. Mol. Cancer Ther. 2012, 11, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomeres: Structure and synthesis. J. Biol. Chem. 1990, 265, 5919–5921. [Google Scholar] [PubMed]
- Lipps, H.J.; Rhodes, D. G-quadruplex structures: In vivo evidence and function. Trends Cell Biol. 2009, 19, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, K.; Seimiya, H. Telomeric repeat-containing RNA/G-quadruplex-forming sequences cause genome-wide alteration of gene expression in human cancer cells in vivo. Nucleic Acids Res. 2015, 43, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Magdinier, F.; Stadler, G.; Wagner, K.R.; Shay, J.W.; Wright, W.E. Telomere position effect: Regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 2014, 28, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreilmeier, T.; Mejri, D.; Hauck, M.; Kleiter, M.; Holzmann, K. Telomere Transcripts Target Telomerase in Human Cancer Cells. Genes 2016, 7, 46. https://doi.org/10.3390/genes7080046
Kreilmeier T, Mejri D, Hauck M, Kleiter M, Holzmann K. Telomere Transcripts Target Telomerase in Human Cancer Cells. Genes. 2016; 7(8):46. https://doi.org/10.3390/genes7080046
Chicago/Turabian StyleKreilmeier, Theresa, Doris Mejri, Marlene Hauck, Miriam Kleiter, and Klaus Holzmann. 2016. "Telomere Transcripts Target Telomerase in Human Cancer Cells" Genes 7, no. 8: 46. https://doi.org/10.3390/genes7080046






