De Novo Transcriptome Sequencing of Desert Herbaceous Achnatherum splendens (Achnatherum) Seedlings and Identification of Salt Tolerance Genes
Abstract
:1. Introduction
2. Materials and Methods
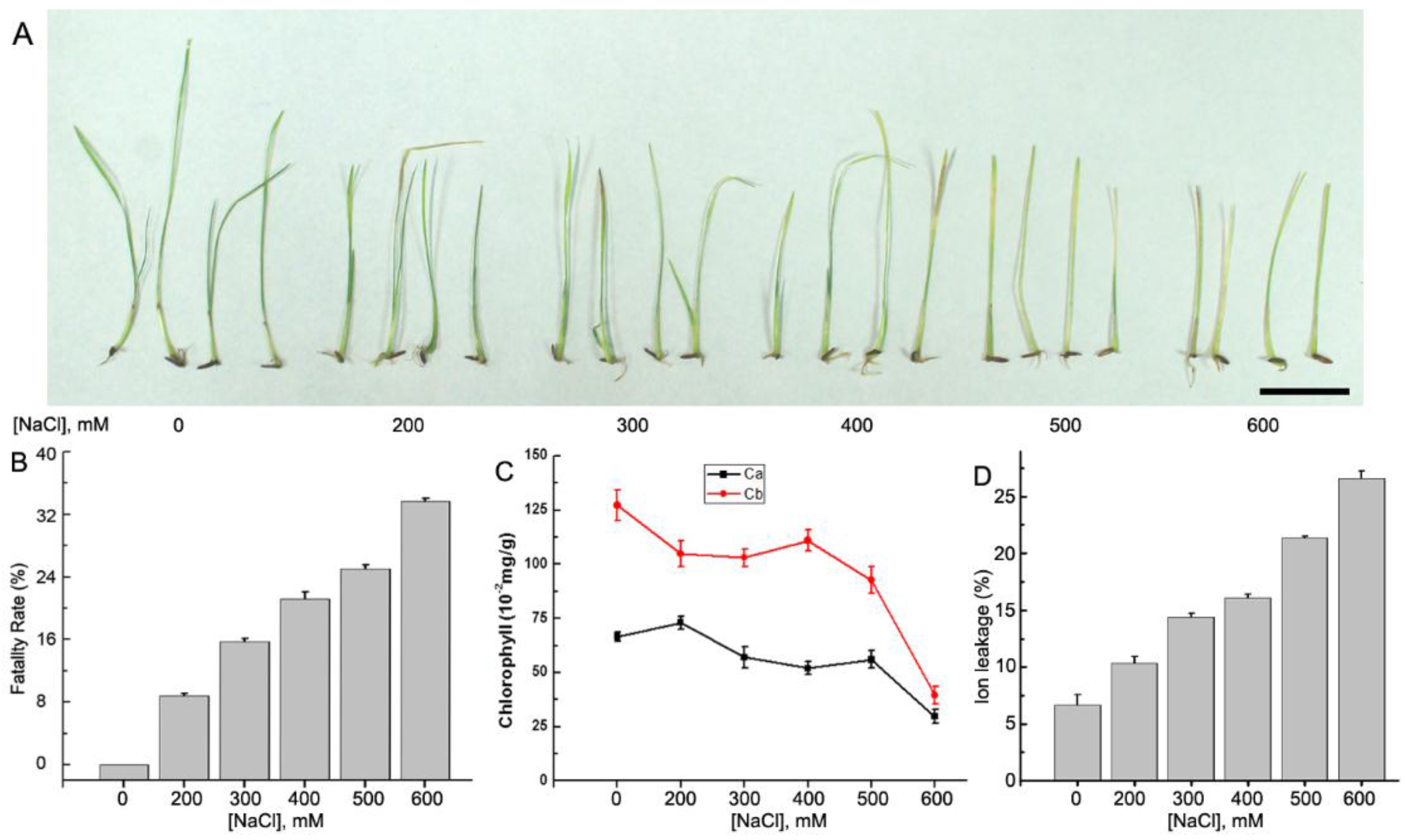
2.1. Plant Materials and Growth Conditions
2.2. Total RNA Extraction and Sequencing
2.3. Raw Sequence Processing and De Novo Assembly
2.4. Gene Expression Data
2.5. Identification and Functional Analysis of DEGs
2.6. Validation of Expression of Genes Related to Trichome Initiation by Quantitative Real-Time PCR (qRT-PCR)
3. Results and Discussion
3.1. RNA-Seq and De Novo Assembly of the A. Splendens Transcriptome
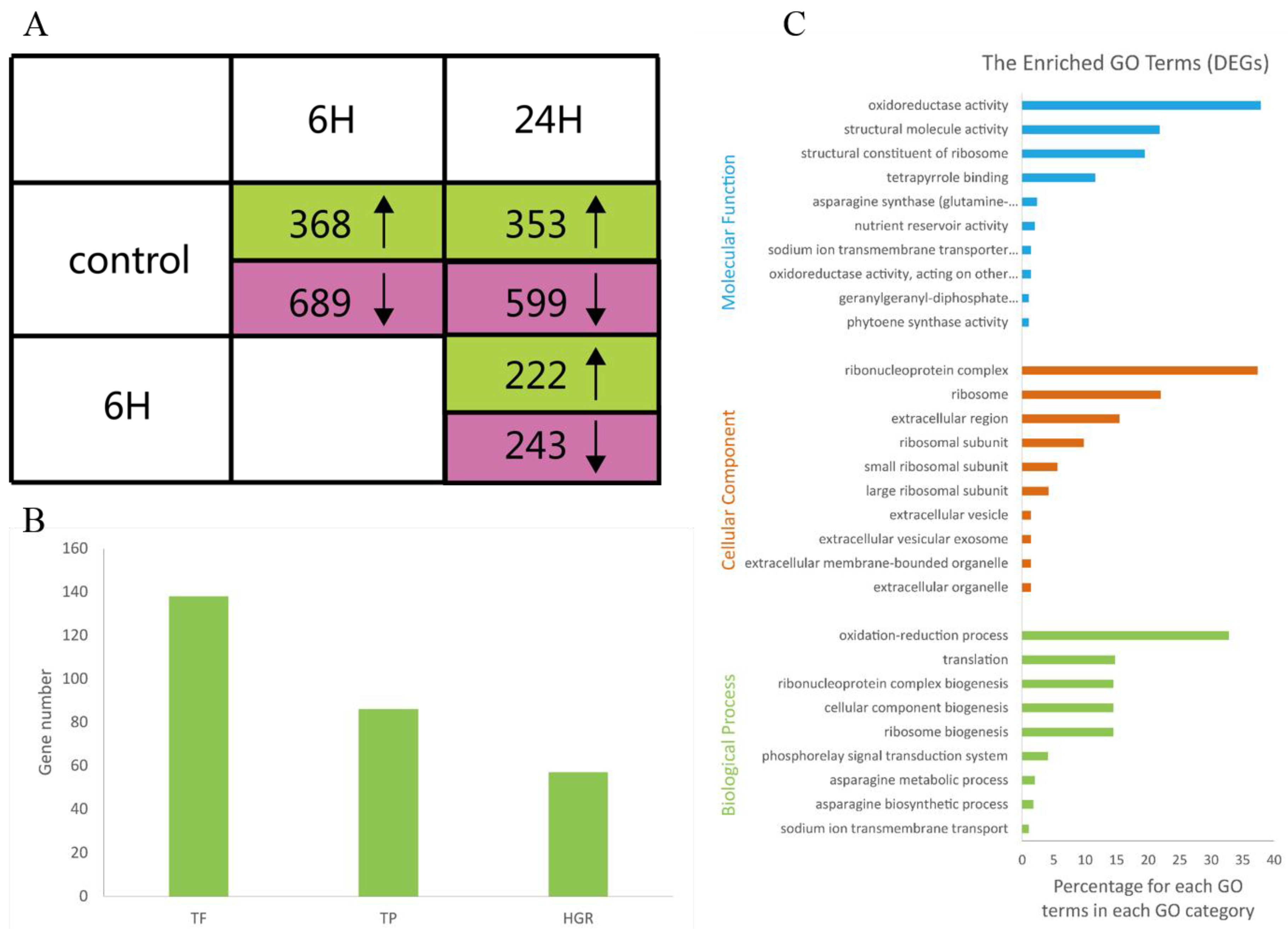
3.2. Differential Gene Expression and Functional Analysis
3.3. Functional Categories of DEGs Determined by Annotations to Arabidopsis Sequences
3.4. Identification of Salt Tolerance Genes Using Rice Homologs
3.5. Validation of DEGs by qRT-PCR
3.6. The Proposed Mechanism of the A. Splendens Response to Salt Stress
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A. Predicting the interaction between the effects of salinity and climate change on crop plants. Sci. Hortic. 1998, 78, 159–174. [Google Scholar] [CrossRef]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007, 428, 419–438. [Google Scholar] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Zhang, Z.; Wang, J.Y.; Oh, D.H.; Dassanayake, M.; Liu, B.; Huang, Q.; Sun, H.X.; Xia, R.; Wu, Y.; et al. Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc. Natl. Acad. Sci. USA 2012, 109, 12219–12224. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell. Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Waditee, R.; Hibino, T.; Nakamura, T.; Incharoensakdi, A.; Takabe, T. Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proc. Natl. Acad. Sci. USA 2002, 99, 4109–4114. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.S.; Tester, M. Salinity tolerance of Arabidopsis: A good model for cereals? Trends Plant Sci. 2007, 12, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell. 1995, 7, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Langridge, P.; Paltridge, N.; Fincher, G. Functional genomics of abiotic stress tolerance in cereals. Brief. Funct. Genom. Proteom. 2006, 4, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Xia, L.Q.; Chen, M.; Cheng, X.G.; Zhang, R.Y.; Li, L.C.; Zhao, Y.X.; Lu, Y.; Ni, Z.Y.; Liu, L.; et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol. Biol. 2007, 65, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Dai, X.; Xu, Y.; Guo, J.; Liu, Y.; Chen, N.; Xiao, J.; Zhang, D.; Xu, Z.; Zhang, X.; et al. Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and Ectopic expression of stress genes. Plant Physiol. 2009, 150, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Kurihara, Y.; Seki, M.; Shinozaki, K. ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr. Opin. Plant Biol. 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell. Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Meiri, D.; Breiman, A. Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 2009, 59, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Narusaka, M.; Abe, H.; Kasuga, M.; Yamaguchi-Shinozaki, K.; Carninci, P.; Hayashizaki, Y.; Shinozaki, K. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 2001, 13, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Z.; Osborne, C.P. Leaf cold acclimation and freezing injury in C3 and C4 grasses of the Mongolian Plateau. J. Exp. Bot. 2008, 59, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, Y.R. Germination testing methods on Achnatherum splendens seeds. Pratacult. Sci. 1998, 15, 29–32. [Google Scholar]
- Zhou, G.Y.; Chen, G.C.; Wei, G.L.; Han, Y.J.; Zhu, C.G. Distribution patterns of major populations in Achnatherum splendens communities of Qinghai lake area. Acta Bot. Boreal. Occident. Sin. 2006, 26, 579–584. [Google Scholar]
- Wei, S.Z. Characteristics, utilization and cultivation of A. Splendens. China Herbiv. Sci. 2012, 2, 38–39. [Google Scholar]
- Zhou, Y.; Wang, Y.; Li, M.; Gao, Z.; Hu, Q.; Gao, S. Anatomical and biochemical characteristics of Achnatherum splendens (Trin.) Nevski seedlings stressed on Pb2+. Appl. Biochem. Biotechnol. 2014, 172, 3176–3193. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.B.; Lei, Y.; Mo, L.; Zheng, Y.K.; Cheng, B.; Liu, L.Y. Studies on improving microclimate of desert by A. Splendens. J. Shihezi Univ. Nat. Sci. 2006, 3, 213–216. [Google Scholar]
- Koski, V.M. Chlorophyll formation in seedlings of Zea mays L. Arch. Biochem. Biophys. 1950, 29, 339–343. [Google Scholar]
- Ishitani, M.; Xiong, L.; Lee, H.J.; Stevenson, B.; Zhu, J.K. HOS1, a genetic locus involved in cold-Responsive Gene expression in Arabidopsis. Plant Cell. 1998, 10, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Cock, P.; Fields, C.; Goto, N.; Heuer, M.; Rice, P. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucl. Acids Res. 2009, 38, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, S.; Tang, Q.; Chen, P.; Yu, Y.; Tang, S. De novo assembly and characterization of transcriptome using Illumina paired-end sequencing and identification of CesA gene in ramie (Boehmeria nivea L. Gaud). BMC Genom. 2013. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; Tsai, J.; Quackenbush, J. TIGR gene indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.CRAN.Rproject.org (accessed on 8 January 2016).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Ma, T.; Hu, Q.; Liu, B.; Wu, Y.; Zhou, H.; Wang, Q.; Wang, J.; Liu, J. Genome-scale transcriptome analysis of the desert poplar, Populus euphratica. Tree Physiol. 2011, 31, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, J.; Zhou, G.; Yue, Z.; Hu, Q.; Chen, Y.; Liu, B.; Qiu, Q.; Wang, Z.; Zhang, J.; Wang, K. Genomic insights into salt adaptation in a desert poplar. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, J.; Lu, J.; Yang, Y.; Zhang, X.; Wan, D.; Liu, J. Transcriptome differences between two sister desert poplar species under salt stress. BMC Genom. 2014. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Barkla, B.J.; Vera-Estrella, R.; Pantoja, O.; Lee, S.Y.; Bohnert, H.J.; Dassanayake, M. Cell type-specific responses to salinity—The epidermal bladder cell transcriptome of Mesembryanthemum crystallinum. New Phytol. 2015, 207, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y. Salt stress or salt shock: Which genes are we studying? J. Exp. Bot. 2013, 64, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, S.; Dai, S.; Wang, R.; Li, N.; Shen, X.; Zhou, X.; Lu, C.; Zheng, X.; Hu, Z.; et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009, 149, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.Y.; Zhang, H.C.; Chen, J.H.; Xia, X.L.; Yin, W.L. Molecular characterization of putative vacuolar NHX-type Na(+)/H(+) exchanger genes from the salt-resistant tree Populus euphratica. Physiol. Plant 2009, 137, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, D.; Liu, B.; Luo, W.; Lu, J.; Ma, T.; Wan, D. Transcriptome dynamics of a desert poplar (Populus pruinosa) in response to continuous salinity stress. Plant Cell. Rep. 2014, 33, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, Q.; Li, S.; Tang, L.; Wen, J. De novo transcriptome sequencing of Acer palmatum and comprehensive analysis of differentially expressed genes under salt stress in two contrasting genotypes. Mol. Genet. Genom. 2015. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2001, 14, S165–S183. [Google Scholar]
- Barragán, V.; Leidi, E.O.; Andre’s, Z.; Rubio, L.; De Luca, A.; Ferna’ndez, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]




| Transcription Factor Family | Number of Genes |
|---|---|
| bHLH | 15 |
| bZIP | 11 |
| C2C2-Dof | 3 |
| DBB | 5 |
| GARP-G2-like | 14 |
| HB | 3 |
| HSF | 4 |
| MYB | 4 |
| MYB-like | 7 |
| NAC | 8 |
| NF-YC | 6 |
| Nin-like | 4 |
| OFP | 3 |
| Pseudo-ARR-B | 10 |
| TRAF | 3 |
| Trihelix | 3 |
| WRKY | 5 |
| Gene ID | Control | 6 h | 24 h | FDR | Rice Homolog | Function |
|---|---|---|---|---|---|---|
| m.155459 | 10.5 | 7.9 | 21.26 | 8.07 × 10−8 | OsHsfC1b | heat shock transcription factor |
| m.184302 | 9.53 | 12.6 | 5.12 | 7.74 × 10−8 | OsNHX1 | vacuolar Na+/H+ antiporter |
| m.37867 | 0.11 | 2.34 | 0.25 | 8.81 × 10−5 | OsLEA3-2 | late embryogenesis abundant protein |
| m.39376 | 1.29 | 2.34 | 0.94 | 1.37 × 10−5 | OsNHX1 | vacuolar Na+/H+ antiporter |
| m.40521 | 6.24 | 18.4 | 4.11 | 1.54 × 10−9 | Oshox22 | homeobox-leucine zipper protein HOX22 |
| m.43934 | 0.16 | 4.2 | 0.92 | 0.00086 | OsLEA3-2 | late embryogenesis abundant protein |
| m.44033 | 5.49 | 2.87 | 11.06 | 5.52 × 10−14 | DCA1 | Transcriptional co-activator of DST; CHY zinc finger protein |
| m.83348 | 6.35 | 9.8 | 4.25 | 4.10 × 10−8 | OsNHX1 | vacuolar Na+/H+ antiporter |
| m.135953 | 17.7 | 9.44 | 8.2 | 4.98 × 10−6 | OsiSAP8 | O. sativa subspecies indica stress-associated protein |
| m.47323 | 6.26 | 4.5 | 2.71 | 0.00051 | OsMKK1 | mitogen-activated protein kinase kinase |
| m.54442 | 7.06 | 2.26 | 1.95 | 5.50 × 10−14 | OsHAK1 | potassium transporter |
| m.54443 | 6.08 | 2.32 | 2.18 | 3.53 × 10−11 | OsHAK1 | potassium transporter |
| m.68120 | 1.64 | 0.1 | 0.01 | 2.64 × 10−13 | OsCYP2 | peptidyl-prolyl cis/trans isomerase; cyclophilin 2 |
| m.76341 | 92.1 | 52.3 | 37.69 | 4.99 × 10−12 | OsiSAP8 | O. sativa subspecies indica stress-associated protein |
| m.159577 | 21.6 | 54.4 | 29.45 | 4.17 × 10−5 | Oshox22 | Homeobox-leucine zipper protein HOX22 |
| m.6654 | 0.27 | 2.23 | 0.23 | 0.00027 | OsLEA3-2 | late embryogenesis abundant protein |
| m.68132 | 2.73 | 0.21 | 1.2 | 0.00011 | OsKAT1 | potassium channel |
| m.83942 | 9.49 | 22.9 | 13.8 | 1.66 × 10−17 | OsGMST1 | Golgi-localized monosaccharide transporter |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhou, Y.; Luo, C.; Xiang, Y.; An, L. De Novo Transcriptome Sequencing of Desert Herbaceous Achnatherum splendens (Achnatherum) Seedlings and Identification of Salt Tolerance Genes. Genes 2016, 7, 12. https://doi.org/10.3390/genes7040012
Liu J, Zhou Y, Luo C, Xiang Y, An L. De Novo Transcriptome Sequencing of Desert Herbaceous Achnatherum splendens (Achnatherum) Seedlings and Identification of Salt Tolerance Genes. Genes. 2016; 7(4):12. https://doi.org/10.3390/genes7040012
Chicago/Turabian StyleLiu, Jiangtao, Yuelong Zhou, Changxin Luo, Yun Xiang, and Lizhe An. 2016. "De Novo Transcriptome Sequencing of Desert Herbaceous Achnatherum splendens (Achnatherum) Seedlings and Identification of Salt Tolerance Genes" Genes 7, no. 4: 12. https://doi.org/10.3390/genes7040012





