Mood Disorders, Accelerated Aging, and Inflammation: Is the Link Hidden in Telomeres?
Abstract
:1. Introduction
2. Telomeres
2.1. Telomeres and Inflammation
2.2. Inflammation and Mood Disorders
3. Telomeres and Mood Disorders
3.1. Telomeres and Bipolar Disorder
3.2. Telomeres and Major Depressive Disorder
4. Telomeres and Psychotropic Medications
4.1. Lithium and Telomeres
4.2. Antidepressants and Telomeres
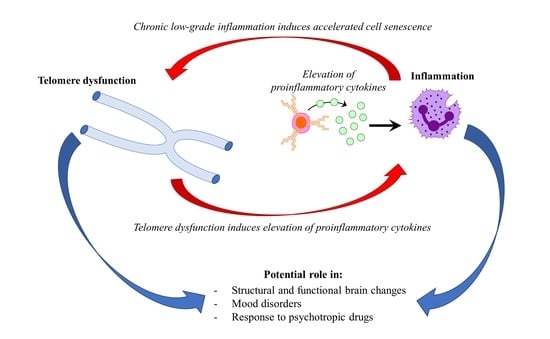
5. Mood Disorders, Telomeres, and Inflammation
6. Longitudinal Studies Exploring Telomere Length and Inflammation in Mood Disorders
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: http://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 13 December 2018).
- Chesney, E.; Goodwin, G.M.; Fazel, S. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry 2014, 13, 153–160. [Google Scholar] [CrossRef]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Kisely, S.; Pais, J. The epidemiology of excess mortality in people with mental illness. Can. J. Psychiatry 2010, 55, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Wilson, S.J. Psychiatric Disorders, Morbidity, and Mortality: Tracing Mechanistic Pathways to Accelerated Aging. Psychosom. Med. 2016, 78, 772–775. [Google Scholar] [CrossRef]
- Leboyer, M.; Oliveira, J.; Tamouza, R.; Groc, L. Is it time for immunopsychiatry in psychotic disorders? Psychopharmacology 2016, 233, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Epel, E.S.; Mellon, S.H.; Penninx, B.W.; Revesz, D.; Verhoeven, J.E.; Reus, V.I.; Lin, J.; Mahan, L.; Hough, C.M.; et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 2015, 55, 333–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Darrow, S.M.; Verhoeven, J.E.; Revesz, D.; Lindqvist, D.; Penninx, B.W.; Delucchi, K.L.; Wolkowitz, O.M.; Mathews, C.A. The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosom. Med. 2016, 78, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Giardini, M.A.; Segatto, M.; da Silva, M.S.; Nunes, V.S.; Cano, M.I. Telomere and telomerase biology. Prog. Mol. Biol. Transl. Sci. 2014, 125, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010, 584, 3819–3825. [Google Scholar] [CrossRef] [Green Version]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Bojesen, S.E. Telomeres and human health. J. Intern. Med. 2013, 274, 399–413. [Google Scholar] [CrossRef] [Green Version]
- Codd, V.; Nelson, C.P.; Albrecht, E.; Mangino, M.; Deelen, J.; Buxton, J.L.; Hottenga, J.J.; Fischer, K.; Esko, T.; Surakka, I.; et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 2013, 45, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangino, M.; Hwang, S.J.; Spector, T.D.; Hunt, S.C.; Kimura, M.; Fitzpatrick, A.L.; Christiansen, L.; Petersen, I.; Elbers, C.C.; Harris, T.; et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum. Mol. Genet. 2012, 21, 5385–5394. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.S.; Bendickova, K.; Kepak, T.; Krenova, Z.; Fric, J. Chronic Inflammation in Immune Aging: Role of Pattern Recognition Receptor Crosstalk with the Telomere Complex? Front. Immunol. 2017, 8, 1078. [Google Scholar] [CrossRef]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 2, 4172. [Google Scholar] [CrossRef]
- Amsellem, V.; Gary-Bobo, G.; Marcos, E.; Maitre, B.; Chaar, V.; Validire, P.; Stern, J.B.; Noureddine, H.; Sapin, E.; Rideau, D.; et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 184, 1358–1366. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, K.; Chen, H.; Zhao, X.; Wang, J.; Li, L.; Cong, Y.; Ju, Z.; Xu, D.; Williams, B.R.; et al. Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs. J. Biol. Chem. 2015, 290, 30813–30829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Criscuolo, F.; Sorci, G.; Behaim-Delarbre, M.; Zahn, S.; Faivre, B.; Bertile, F. Age-related response to an acute innate immune challenge in mice: Proteomics reveals a telomere maintenance-related cost. Proc. Biol. Sci. 2018, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, L.; Swartz, K.R.; Bruemmer, D.; Eum, S.Y.; Huang, W.; Seelbach, M.; Choi, Y.J.; Hennig, B.; Toborek, M. Deficiency of telomerase activity aggravates the blood-brain barrier disruption and neuroinflammatory responses in a model of experimental stroke. J. Neurosci. Res. 2010, 88, 2859–2868. [Google Scholar] [CrossRef]
- Masi, S.; Nightingale, C.M.; Day, I.N.; Guthrie, P.; Rumley, A.; Lowe, G.D.; von Zglinicki, T.; D’Aiuto, F.; Taddei, S.; Klein, N.; et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arter. Thromb. Vasc. Biol. 2012, 32, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Revesz, D.; Verhoeven, J.E.; Milaneschi, Y.; de Geus, E.J.; Wolkowitz, O.M.; Penninx, B.W. Dysregulated physiological stress systems and accelerated cellular aging. Neurobiol. Aging 2014, 35, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Lopizzo, N.; Tosato, S.; Begni, V.; Tomassi, S.; Cattane, N.; Barcella, M.; Turco, G.; Ruggeri, M.; Riva, M.A.; Pariante, C.M.; et al. Transcriptomic analyses and leukocyte telomere length measurement in subjects exposed to severe recent stressful life events. Transl. Psychiatry 2017, 7, e1042. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.C.; Satterfield, S.; et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, J.; Wang, S.; Milush, J.M.; Baker, C.A.R.; Coccia, M.; Effros, R.B.; Puterman, E.; Blackburn, E.; Prather, A.A.; et al. In vitro proinflammatory gene expression predicts in vivo telomere shortening: A preliminary study. Psychoneuroendocrinology 2018, 96, 179–187. [Google Scholar] [CrossRef]
- Lustig, A.; Liu, H.B.; Metter, E.J.; An, Y.; Swaby, M.A.; Elango, P.; Ferrucci, L.; Hodes, R.J.; Weng, N.P. Telomere Shortening, Inflammatory Cytokines, and Anti-Cytomegalovirus Antibody Follow Distinct Age-Associated Trajectories in Humans. Front. Immunol. 2017, 8, 1027. [Google Scholar] [CrossRef]
- Mechawar, N.; Savitz, J. Neuropathology of mood disorders: Do we see the stigmata of inflammation? Transl. Psychiatry 2016, 6, e946. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, K.; Vinberg, M.; Vedel Kessing, L. Cytokines in bipolar disorder: A systematic review and meta-analysis. J. Affect. Disord. 2013, 144, 16–27. [Google Scholar] [CrossRef]
- Chen, M.H.; Chang, W.C.; Hsu, J.W.; Huang, K.L.; Tu, P.C.; Su, T.P.; Li, C.T.; Lin, W.C.; Bai, Y.M. Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. J. Affect. Disord. 2018, 245, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Neurauter, G.; Schrocksnadel, K.; Scholl-Burgi, S.; Sperner-Unterweger, B.; Schubert, C.; Ledochowski, M.; Fuchs, D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 2008, 9, 622–627. [Google Scholar] [CrossRef]
- Zhu, C.B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, L.A.; Torre, J.P.; Papadopoulos, A.S.; Poon, L.; Juruena, M.F.; Markopoulou, K.; Cleare, A.J.; Pariante, C.M. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J. Affect. Disord. 2013, 148, 136–140. [Google Scholar] [CrossRef]
- Cattaneo, A.; Gennarelli, M.; Uher, R.; Breen, G.; Farmer, A.; Aitchison, K.J.; Craig, I.W.; Anacker, C.; Zunsztain, P.A.; McGuffin, P.; et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: Differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology 2013, 38, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Tansey, K.E.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry 2014, 171, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.R.; Schalkwyk, L.C.; Heffernan, A.L.; Breen, G.; Lawrence, T.; Price, T.; Farmer, A.E.; Aitchison, K.J.; Craig, I.W.; Danese, A.; et al. Tumor necrosis factor and its targets in the inflammatory cytokine pathway are identified as putative transcriptomic biomarkers for escitalopram response. Eur Neuropsychopharmacol 2013, 23, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Wiedlocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jazdzik, M.; Janus, M.; Debowska, W.; Mosiolek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; Barros, A.; Rosa, D.V.; Albuquerque, M.; Malloy-Diniz, L.; Neves, F.S.; Romano-Silva, M.A.; de Miranda, D.M. Analysis of telomere attrition in bipolar disorder. J. Affect. Disord. 2015, 172, 43–47. [Google Scholar] [CrossRef]
- Martinsson, L.; Wei, Y.; Xu, D.; Melas, P.A.; Mathe, A.A.; Schalling, M.; Lavebratt, C.; Backlund, L. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl. Psychiatry 2013, 3, e261. [Google Scholar] [CrossRef]
- Simon, N.M.; Smoller, J.W.; McNamara, K.L.; Maser, R.S.; Zalta, A.K.; Pollack, M.H.; Nierenberg, A.A.; Fava, M.; Wong, K.K. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 2006, 60, 432–435. [Google Scholar] [CrossRef]
- Squassina, A.; Pisanu, C.; Congiu, D.; Caria, P.; Frau, D.; Niola, P.; Melis, C.; Baggiani, G.; Lopez, J.P.; Cruceanu, C.; et al. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur. Neuropsychopharmacol. 2016, 26, 1241–1247. [Google Scholar] [CrossRef]
- Elvsashagen, T.; Vera, E.; Boen, E.; Bratlie, J.; Andreassen, O.A.; Josefsen, D.; Malt, U.F.; Blasco, M.A.; Boye, B. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J. Affect. Disord. 2011, 135, 43–50. [Google Scholar] [CrossRef]
- Rizzo, L.B.; Do Prado, C.H.; Grassi-Oliveira, R.; Wieck, A.; Correa, B.L.; Teixeira, A.L.; Bauer, M.E. Immunosenescence is associated with human cytomegalovirus and shortened telomeres in type I bipolar disorder. Bipolar Disord. 2013, 15, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.R.; Dima, D.; Frangou, S.; Breen, G. Telomere Length and Bipolar Disorder. Neuropsychopharmacology 2018, 43, 454. [Google Scholar] [CrossRef] [PubMed]
- Kose Cinar, R. Telomere length and hTERT in mania and subsequent remission. Braz. J. Psychiatry 2018, 40, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Wang, L.J.; Tseng, P.T.; Hung, C.F.; Lin, P.Y. Leukocyte telomere length in patients with bipolar disorder: An updated meta-analysis and subgroup analysis by mood status. Psychiatry Res. 2018, 270, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Barbe-Tuana, F.M.; Parisi, M.M.; Panizzutti, B.S.; Fries, G.R.; Grun, L.K.; Guma, F.T.; Kapczinski, F.; Berk, M.; Gama, C.S.; Rosa, A.R. Shortened telomere length in bipolar disorder: A comparison of the early and late stages of disease. Braz. J. Psychiatry 2016, 38, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kapczinski, F.; Magalhaes, P.V.; Balanza-Martinez, V.; Dias, V.V.; Frangou, S.; Gama, C.S.; Gonzalez-Pinto, A.; Grande, I.; Ha, K.; Kauer-Sant’Anna, M.; et al. Staging systems in bipolar disorder: An International Society for Bipolar Disorders Task Force Report. Acta Psychiatr. Scand. 2014, 130, 354–363. [Google Scholar] [CrossRef]
- Wei, Y.B.; Martinsson, L.; Liu, J.J.; Forsell, Y.; Schalling, M.; Backlund, L.; Lavebratt, C. hTERT genetic variation in depression. J. Affect. Disord. 2016, 189, 62–69. [Google Scholar] [CrossRef]
- Palmos, A.B.; Breen, G.; Goodwin, L.; Frissa, S.; Hatch, S.L.; Hotopf, M.; Thuret, S.; Lewis, C.M.; Powell, T.R. Genetic Risk for Psychiatric Disorders and Telomere Length. Front. Genet. 2018, 9, 468. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, L.; Craig, D.W.; Redman, M.; Liu, C. Cerebellar telomere length and psychiatric disorders. Behav. Genet. 2010, 40, 250–254. [Google Scholar] [CrossRef]
- Mamdani, F.; Rollins, B.; Morgan, L.; Myers, R.M.; Barchas, J.D.; Schatzberg, A.F.; Watson, S.J.; Akil, H.; Potkin, S.G.; Bunney, W.E.; et al. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl. Psychiatry 2016, 6, e969. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Mellon, S.H.; Lindqvist, D.; Epel, E.S.; Blackburn, E.H.; Lin, J.; Reus, V.I.; Burke, H.; Rosser, R.; Mahan, L.; et al. PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res. 2015, 232, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Powell, T.R.; De Jong, S.; Breen, G.; Lewis, C.M.; Dima, D. Telomere length as a predictor of emotional processing in the brain. Hum. Brain Mapp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ridout, K.K.; Ridout, S.J.; Price, L.H.; Sen, S.; Tyrka, A.R. Depression and telomere length: A meta-analysis. J. Affect. Disord. 2016, 191, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, M.C.; Bui, E.; Hoeppner, S.S.; Kovachy, B.; Prescott, J.; Mischoulon, D.; Walton, Z.E.; Dong, M.; Nadal, M.F.; Worthington, J.J.; et al. Prospective association between major depressive disorder and leukocyte telomere length over two years. Psychoneuroendocrinology 2018, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Revesz, D.; Verhoeven, J.E.; Milaneschi, Y.; Penninx, B.W. Depressive and anxiety disorders and short leukocyte telomere length: Mediating effects of metabolic stress and lifestyle factors. Psychol. Med. 2016, 46, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.E.; Kepa, A.; Vincent, J.; Frissa, S.; Goodwin, L.; Hotopf, M.; Hatch, S.L.; Breen, G.; Powell, T.R. Genetic predisposition to advanced biological ageing increases risk for childhood-onset recurrent major depressive disorder in a large UK sample. J. Affect. Disord. 2017, 213, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooley, K.A.; Bojesen, S.E.; Weischer, M.; Nielsen, S.F.; Thompson, D.; Amin Al Olama, A.; Michailidou, K.; Tyrer, J.P.; Benlloch, S.; Brown, J.; et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: Identified loci show little association with hormone-related cancer risk. Hum. Mol. Genet. 2013, 22, 5056–5064. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Prescott, J.; De Vivo, I.; Kraft, P.; Okereke, O.I. Polygenic risk score of shorter telomere length and risk of depression and anxiety in women. J. Psychiatr. Res. 2018, 103, 182–188. [Google Scholar] [CrossRef]
- Szebeni, A.; Szebeni, K.; DiPeri, T.; Chandley, M.J.; Crawford, J.D.; Stockmeier, C.A.; Ordway, G.A. Shortened telomere length in white matter oligodendrocytes in major depression: Potential role of oxidative stress. Int. J. Neuropsychopharmacol. 2014, 17, 1579–1589. [Google Scholar] [CrossRef]
- Wei, Y.B.; Backlund, L.; Wegener, G.; Mathe, A.A.; Lavebratt, C. Telomerase dysregulation in the hippocampus of a rat model of depression: Normalization by lithium. Int. J. Neuropsychopharmacol. 2015, 18, pyv002. [Google Scholar] [CrossRef]
- Cardillo, G.M.; De-Paula, V.J.R.; Ikenaga, E.H.; Costa, L.R.; Catanozi, S.; Schaeffer, E.L.; Gattaz, W.F.; Kerr, D.S.; Forlenza, O.V. Chronic Lithium Treatment Increases Telomere Length in Parietal Cortex and Hippocampus of Triple-Transgenic Alzheimer’s Disease Mice. J. Alzheimers Dis. 2018, 63, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Coutts, F.; Palmos, A.B.; Duarte, R.R.R.; de Jong, S.; Lewis, C.M.; Dima, D.; Powell, T.R. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacology 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.G.; Hu, Y.; Wu, D.L.; Zhu, L.J.; Chen, C.; Jin, X.; Luo, C.X.; Wu, H.Y.; Zhang, J.; Zhu, D.Y. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci. 2011, 31, 12258–12269. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Reus, V.I.; Rosser, R.; Burke, H.; Compagnone, M.; Nelson, J.C.; Dhabhar, F.S.; et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol. Psychiatry 2012, 17, 164–172. [Google Scholar] [CrossRef]
- Hough, C.M.; Bersani, F.S.; Mellon, S.H.; Epel, E.S.; Reus, V.I.; Lindqvist, D.; Lin, J.; Mahan, L.; Rosser, R.; Burke, H.; et al. Leukocyte telomere length predicts SSRI response in major depressive disorder: A preliminary report. Mol. Neuropsychiatry 2016, 2, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.; Lin, K.W.; Lin, J.; Epel, E.; Blackburn, E. Telomere length as a predictor of response to Pioglitazone in patients with unremitted depression: A preliminary study. Transl. Psychiatry 2016, 6, e709. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Dhabhar, F.S.; Su, Y.; Reus, V.I.; Rosser, R.; Burke, H.M.; Kupferman, E.; et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress—Preliminary findings. PLoS ONE 2011, 6, e17837. [Google Scholar] [CrossRef]
- Crawford, B.; Craig, Z.; Mansell, G.; White, I.; Smith, A.; Spaull, S.; Imm, J.; Hannon, E.; Wood, A.; Yaghootkar, H.; et al. DNA methylation and inflammation marker profiles associated with a history of depression. Hum. Mol. Genet. 2018, 27, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Osler, M.; Bendix, L.; Rask, L.; Rod, N.H. Stressful life events and leucocyte telomere length: Do lifestyle factors, somatic and mental health, or low grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain Behav. Immun. 2016, 58, 248–253. [Google Scholar] [CrossRef]
- Vasconcelos-Moreno, M.P.; Fries, G.R.; Gubert, C.; Dos Santos, B.; Fijtman, A.; Sartori, J.; Ferrari, P.; Grun, L.K.; Parisi, M.M.; Guma, F.; et al. Telomere Length, Oxidative Stress, Inflammation and BDNF Levels in Siblings of Patients with Bipolar Disorder: Implications for Accelerated Cellular Aging. Int. J. Neuropsychopharmacol. 2017, 20, 445–454. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry 2014, 71, 1121–1128. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squassina, A.; Pisanu, C.; Vanni, R. Mood Disorders, Accelerated Aging, and Inflammation: Is the Link Hidden in Telomeres? Cells 2019, 8, 52. https://doi.org/10.3390/cells8010052
Squassina A, Pisanu C, Vanni R. Mood Disorders, Accelerated Aging, and Inflammation: Is the Link Hidden in Telomeres? Cells. 2019; 8(1):52. https://doi.org/10.3390/cells8010052
Chicago/Turabian StyleSquassina, Alessio, Claudia Pisanu, and Roberta Vanni. 2019. "Mood Disorders, Accelerated Aging, and Inflammation: Is the Link Hidden in Telomeres?" Cells 8, no. 1: 52. https://doi.org/10.3390/cells8010052