Nutrient Limitation Inactivates Mrc1-to-Cds1 Checkpoint Signalling in Schizosaccharomyces pombe
Abstract
:1. Introduction
2. Results
2.1. Glucose Starvation Transiently Activates Cds1
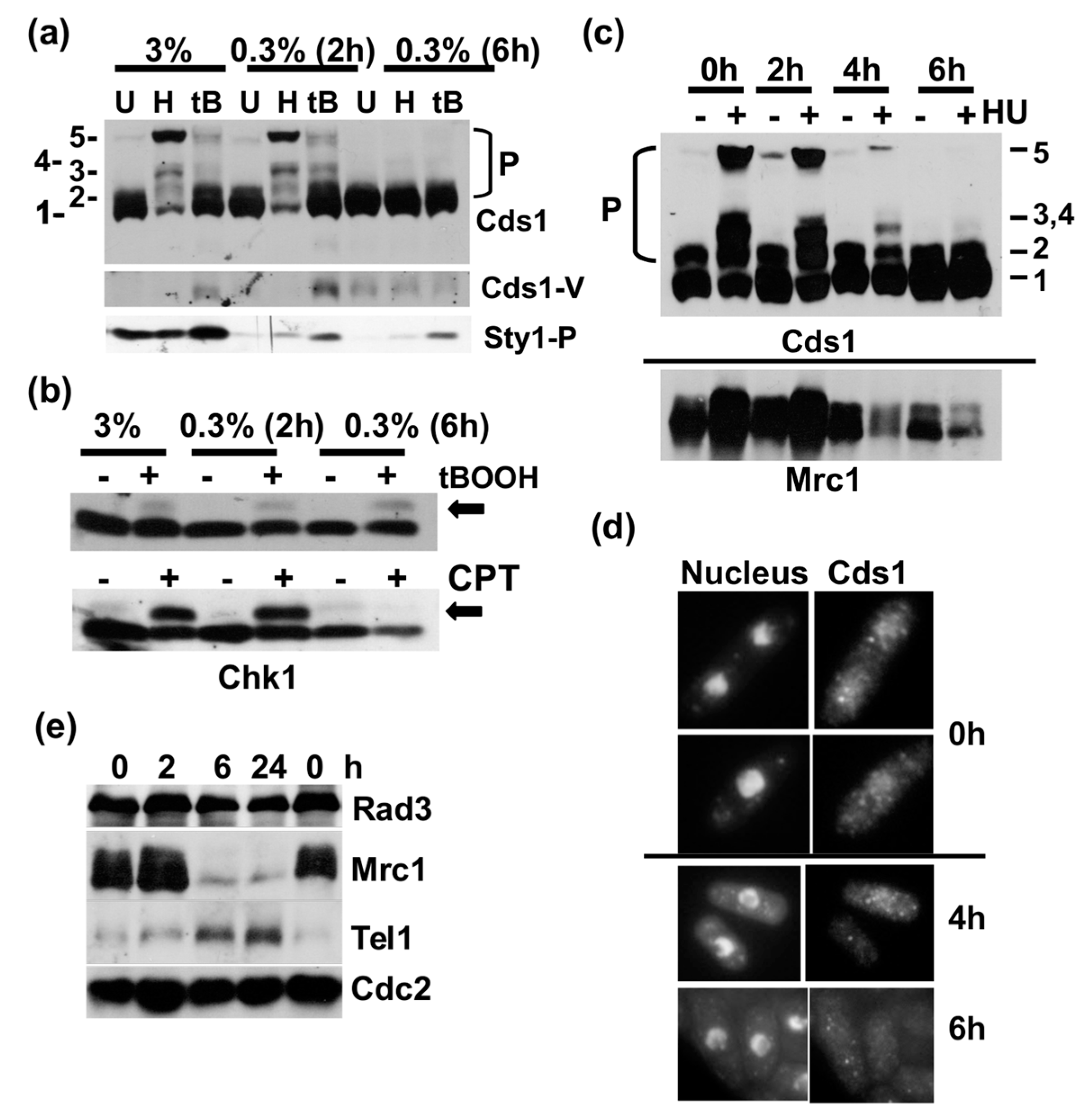
2.2. Low Glucose Concentrations Terminate Cds1 Activation
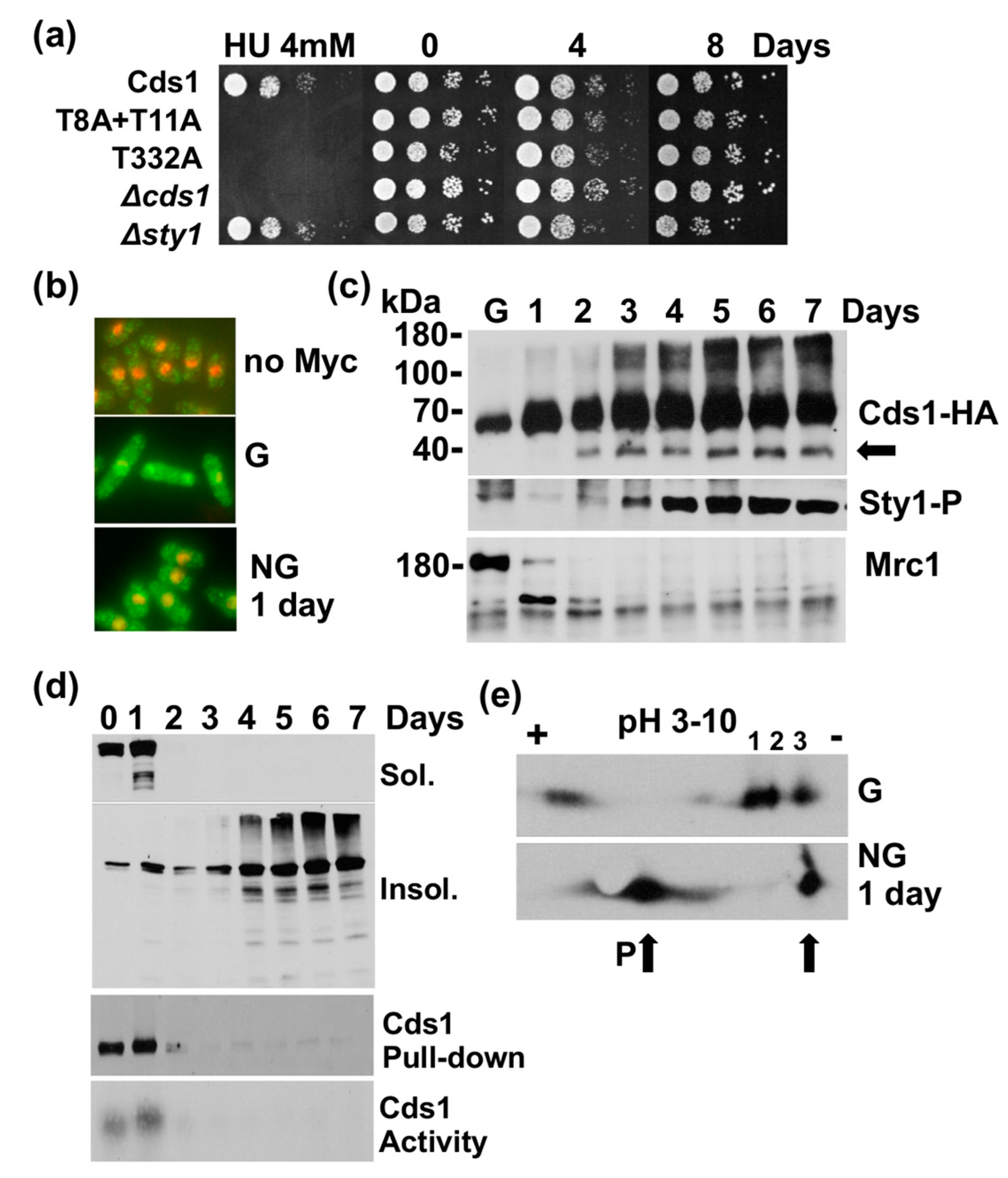
2.3. Cds1 Undergoes a Transformation in the Stationary Phase
2.4. Nitrogen Starvation Causes Similar Changes to Cds1 and Mrc1
3. Discussion
4. Materials and Methods
4.1. Yeast Strains
4.2. Media
4.3. Phos-tag SDS Page
4.4. Isoelectric Focusing (IF)
4.5. In Vitro Kinase Assay
4.6. Survival Assays
4.7. Indirect Immunofluorescence Microscopy
4.8. Cell Synchronisation
4.9. Antibodies
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirayama, A.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; Ochiai, A.; Tomita, M.; et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009, 69, 4918–4925. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Ciszewski, W.M.; Kania, K.D. l- and d-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell. Commun. Signal. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.L.; Rodríguez, C.; Petit, T.; Gancedo, C. Carbohydrate and energy-yielding metabolism in non-conventional yeasts. FEMS Microbiol. Rev. 2000, 24, 507–529. [Google Scholar] [CrossRef]
- Masuda, F.; Ishii, M.; Mori, A.; Uehara, L.; Yanagida, M.; Takeda, K.; Saitoh, S. Glucose restriction induces transient G2 cell cycle arrest extending cellular chronological lifespan. Sci. Rep. 2016, 6, 19629. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Galoforo, S.S.; Berns, C.M.; Chen, J.C.; Davis, B.H.; Sim, J.E.; Corry, P.M.; Spitz, D.R. Glucose Deprivation-induced Cytotoxicity and Alterations in Mitogen-activated Protein Kinase Activation Are Mediated by Oxidative Stress in Multidrug-resistant Human Breast Carcinoma Cells. J. Biol. Chem. 1998, 273, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Madrid, M.; Soto, T.; Franco, A.; Paredes, V.; Vicente, J.; Hidalgo, E.; Gacto, M.; Cansado, J. A Cooperative Role for Atf1 and Pap1 in the Detoxification of the Oxidative Stress Induced by Glucose Deprivation in Schizosaccharomyces pombe. J. Biol. Chem. 2004, 279, 41594–41602. [Google Scholar] [CrossRef] [PubMed]
- Zuin, A.; Carmona, M.; Morales-Ivorra, I.; Gabrielli, N.; Vivancos, A.P.; Ayté, J.; Hidalgo, E. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J. 2010, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Dal Santo, P.; Blanchard, B.; Hoffman, C.S. The Schizosaccharomyces pombe pyp1 protein tyrosine phosphatase negatively regulates nutrient monitoring pathways. J. Cell Sci. 1996, 109, 1919–1925. [Google Scholar]
- Stettler, S.; Warbrick, E.; Prochnik, S.; Mackie, S.; Fantes, P. The wis1 signal transduction pathway is required for expression of cAMP-repressed genes in fission yeast. J. Cell Sci. 1996, 109, 1927–1935. [Google Scholar] [PubMed]
- Caspari, T. Onset of gluconate-H+ symport in Schizosaccharomyces pombe is regulated by the kinases Wis1 and Pka1, and requires the gti1+ gene product. J. Cell. Sci. 1997, 110, 2599–2608. [Google Scholar] [PubMed]
- Madrid, M.; Fernández-Zapata, J.; Sánchez-Mir, L.; Soto, T.; Franco, A.; Vicente-Soler, J.; Gacto, M.; Cansado, J. Role of the fission yeast cell integrity MAPK pathway in response to glucose limitation. BMC Microbiol. 2013, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, Y.; Sugiura, R.; Kobayashi, D.; Suzuki, M.; Deng, L.; Kuno, T. MAP kinase kinase kinase (MAPKKK)-dependent and -independent activation of Sty1 stress MAPK in fission yeast. J. Biol. Chem. 2010, 285, 32818–32823. [Google Scholar] [CrossRef] [PubMed]
- Hannig, G.; Ottilie, S.; Schievella, A.R.; Erikson, R.L. Comparison of the biochemical and biological functions of tyrosine phosphatases from fission yeast, budding yeast and animal cells. Yeast 1993, 9, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Ottilie, S.; Chernoff, J.; Hannig, G.; Hoffman, C.S.; Erikson, R.L. The fission yeast genes pyp1+ and pyp2+ encode protein tyrosine phosphatases that negatively regulate mitosis. Mol. Cell. Biol. 1992, 12, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Davenport, M.; Kelly, T.J. Two-stage mechanism for activation of the DNA replication checkpoint kinase Cds1 in fission yeast. Genes Dev. 2006, 20, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Capasso, H.; Palermo, C.; Wan, S.; Rao, H.; John, U.P.; O’Connell, M.J.; Walworth, N.C. Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J. Cell Sci. 2002, 115, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Krohn, N.G.; Brown, N.A.; Colabardini, A.C.; Reis, T.; Savoldi, M.; Dinamarco, T.M.; Goldman, M.H.S.; Goldman, G.H. The Aspergillus nidulans ATM kinase regulates mitochondrial function, glucose uptake and the carbon starvation response. G3 2014, 4, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Halaby, M.-J.; Hibma, J.C.; He, J.; Yang, D.-Q. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell. Signal. 2008, 20, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Lavy, K.J.; Bronstein, A.; Kupiec, M.; Johnston, M. Cross-Talk between Carbon Metabolism and the DNA Damage Response in S. cerevisiae. Cell Rep. 2015, 12, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Costello, G.; Rodgers, L.; Beach, D. Fission yeast enters the stationary phase G0 state from either mitotic G1 or G2. Curr. Genet. 1986, 11, 119–125. [Google Scholar] [CrossRef]
- Boddy, M.N.; Lopez-Girona, A.; Shanahan, P.; Interthal, H.; Heyer, W.-D.; Russell, P. Damage Tolerance Protein Mus81 Associates with the FHA1 Domain of Checkpoint Kinase Cds1. Mol. Cell. Biol. 2000, 20, 8758–8766. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yoshimura, K.; Iida, H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat. Commun. 2012, 3, 1020. [Google Scholar] [CrossRef] [PubMed]
- Janes, S.; Schmidt, U.; Ashour Garrido, K.; Ney, N.; Concilio, S.; Zekri, M.; Caspari, T. Heat induction of a novel Rad9 variant from a cryptic translation initiation site reduces mitotic commitment. J. Cell Sci. 2012, 125, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.T.; Garcia, V.; Bone, N.; Carr, A.M.; Armstrong, J. Gene tagging and gene replacement using recombinase-mediated cassette exchange in Schizosaccharomyces pombe. Gene 2008, 407, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Rozenzhak, S.; Mejía-Ramírez, E.; Williams, J.S.; Schaffer, L.; Hammond, J.A.; Head, S.R.; Russell, P. Rad3 decorates critical chromosomal domains with gammaH2A to protect genome integrity during S-Phase in fission yeast. PLoS Genet. 2010, 6, e1001032. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, Y.; Toda, T.; Yanagida, M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell 1984, 39, 349–358. [Google Scholar] [CrossRef]
- Fornalewicz, K.; Wieczorek, A.; Węgrzyn, G.; Łyżeń, R. Silencing of the pentose phosphate pathway genes influences DNA replication in human fibroblasts. Gene 2017, 635, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Brondello, J.-M.; Boddy, M.N.; Furnari, B.; Russell, P. Basis for the Checkpoint Signal Specificity That Regulates Chk1 and Cds1 Protein Kinases. Mol. Cell. Biol. 1999, 19, 4262–4269. [Google Scholar] [CrossRef] [PubMed]
- Egel, R. Genes involved in mating type expression of fission yeast. Mol. Gen. Genet. 1973, 122, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S.; Yanagida, M. Distinct modes of DNA damage response in S. pombe G0 and vegetative cells. Genes Cells 2006, 11, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Nurse, P. Regulation of premeiotic S phase and recombination-related double-strand DNA breaks during meiosis in fission yeast. Nat. Genet. 2001, 28, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Degols, G.; Shiozaki, K.; Russell, P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 1996, 16, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, H.D.; Griffiths, D.J.; Edwards, R.J.; Christensen, P.U.; Murray, J.M.; Osman, F.; Walworth, N.; Carr, A.M. S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev. 1998, 12, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Sanders, G.M.; Grossman, A.D. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 2007, 128, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.W.; Kang, C.M. Induction of S. cerevisiae filamentous differentiation by slowed DNA synthesis involves Mec1, Rad53 and Swe1 checkpoint proteins. Mol. Biol. Cell 2003, 14, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Mori, A.; Uehara, L.; Masuda, F.; Soejima, S.; Yanagida, M. Mechanisms of expression and translocation of major fission yeast glucose transporters regulated by CaMKK/phosphatases, nuclear shuttling, and TOR. Mol. Biol. Cell 2015, 26, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Russell, P. Mrc1 channels the DNA replication arrest signal to checkpoint kinase Cds1. Nat. Cell Biol. 2001, 3, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Song, Y.-H. Genetic screen for genes involved in Chk2 signaling in Drosophila. Mol. Cells 2008, 26, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.V.; Das, J.; Meyer, M.J.; Cordero, N.A.; Akturk, N.; Wei, X.; Fair, B.J.; Degatano, A.G.; Fragoza, R.; Liu, L.G.; et al. A Proteome-wide Fission Yeast Interactome Reveals Network Evolution Principles from Yeasts to Human. Cell 2016, 164, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Andrisse, S.; Koehler, R.M.; Chen, J.E.; Patel, G.D.; Vallurupalli, V.R.; Ratliff, B.A.; Warren, D.E.; Fisher, J.S. Role of GLUT1 in regulation of reactive oxygen species. Redox Biol. 2014, 2, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Tong, J.; Lu, P.; Wang, Y.; Zhang, J.; Sun, C.; Yuan, K.; Xue, R.; Zou, B.; Li, N.; et al. Formation of a Snf1-Mec1-Atg1 Module on Mitochondria Governs Energy Deprivation-Induced Autophagy by Regulating Mitochondrial Respiration. Dev. Cell 2017, 41, 59–71.e4. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.; Hall, M.N. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 1999, 402, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Weisman, R.; Roitburg, I.; Schonbrun, M.; Harari, R.; Kupiec, M. Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 2007, 175, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Colman, M.J.; Sorolla, M.A.; Vall-Llaura, N.; Tamarit, J.; Ros, J.; Cabiscol, E. The FOX transcription factor Hcm1 regulates oxidative metabolism in response to early nutrient limitation in yeast. Role of Snf1 and Tor1/Sch9 kinases. Biochim. Biophys. Acta 2013, 1833, 2004–2015. [Google Scholar] [CrossRef] [PubMed]
- Boddy, M.N.; Furnari, B.; Mondesert, O.; Russell, P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science 1998, 280, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Walworth, N.C.; Bernards, R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 1996, 271, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.J.; Bentley, N.J.; Carr, A.M. A Rad3-Rad26 complex responds to DNA damage independently of other checkpoint proteins. Nat. Cell Biol. 1999, 1, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.W.; Kelly, T.J. Cell cycle regulation of Dfp1, an activator of the Hsk1 protein kinase. Proc. Natl. Acad. Sci. USA 1999, 96, 8443–8448. [Google Scholar] [CrossRef] [PubMed]
- Caspari, T.; Hilditch, V. Two Distinct Cdc2 Pools Regulate Cell Cycle Progression and the DNA Damage Response in the Fission Yeast S. pombe. PLoS ONE 2015, 10, e0130748. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Taricani, L.; Wang, T.S.-F. Methods for studying mutagenesis and checkpoints in Schizosaccharomyces pombe. Meth. Enzymol. 2006, 409, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Caspari, T.; Dahlen, M.; Kanter-Smoler, G.; Lindsay, H.D.; Hofmann, K.; Papadimitriou, K.; Sunnerhagen, P.; Carr, A.M. Characterization of Schizosaccharomyces pombe Hus1: A PCNA-related protein that associates with Rad1 and Rad9. Mol. Cell. Biol. 2000, 20, 1254–1262. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletcher, J.; Griffiths, L.; Caspari, T. Nutrient Limitation Inactivates Mrc1-to-Cds1 Checkpoint Signalling in Schizosaccharomyces pombe. Cells 2018, 7, 15. https://doi.org/10.3390/cells7020015
Fletcher J, Griffiths L, Caspari T. Nutrient Limitation Inactivates Mrc1-to-Cds1 Checkpoint Signalling in Schizosaccharomyces pombe. Cells. 2018; 7(2):15. https://doi.org/10.3390/cells7020015
Chicago/Turabian StyleFletcher, Jessica, Liam Griffiths, and Thomas Caspari. 2018. "Nutrient Limitation Inactivates Mrc1-to-Cds1 Checkpoint Signalling in Schizosaccharomyces pombe" Cells 7, no. 2: 15. https://doi.org/10.3390/cells7020015





