How Can Elispot Add Information to Improve Knowledge on Tropical Diseases?
Abstract
:1. Introduction
2. Intracellular Protozoa: Plasmodium spp., Toxoplasma gondii, Leishmania spp. and Trypanosoma cruzi
3. Tropical Arboviruses
4. Bacterial Infections in the Tropics: Mycobacterium tuberculosis, Mycobacterium leprae, Treponema pallidum, Vibrio cholerae
5. Helminths
6. Mycosis
7. Elispot as an Important Tool in Vaccine, Allergy and Adverse Reactions to Drugs
8. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Mosmann, T.R.; Coffman, R.L. Th1 and th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.K. Lymphocyte-mediated immune regulation in health and disease: The treg and gammadelta T cell co-conspiracy. Immunol. Investig. 2016, 45, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Karasawa, Y.; Harimoto, K.; Tanaka, A.; Shibata, M.; Sato, T.; Caspi, R.R.; Ito, M. Analysis of th cell-related cytokine production in behcet disease patients with uveitis before and after infliximab treatment. Ocul. Immunol. Inflam. 2017, 25, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kubo, R.; Muramatsu, S.; Sagawa, Y.; Saito, C.; Kasuya, S.; Nishioka, A.; Nishida, E.; Yamazaki, S.; Morita, A. Bath-puva therapy improves impaired resting regulatory T cells and increases activated regulatory T cells in psoriasis. J. Dermatol. Sci. 2017, 86, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rountree, W.; Vandergrift, N.; Bainbridge, J.; Sanchez, A.M.; Denny, T.N. Statistical methods for the assessment of eqapol proficiency testing: Elispot, luminex, and flow cytometry. J. Immunol. Methods 2014, 409, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Rosel, A.L.; Scheibenbogen, C.; Schliesser, U.; Sollwedel, A.; Hoffmeister, B.; Hanitsch, L.; von Bernuth, H.; Kruger, R.; Warnatz, K.; Volk, H.D.; et al. Classification of common variable immunodeficiencies using flow cytometry and a memory B-cell functionality assay. J. Allergy Clin. Immun. 2015, 135, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.A.; Hui, C.; Krawczyk, C.M. Detecting secreted analytes from immune cells: An overview of technologies. Methods Mol. Biol. 2016, 1458, 111–124. [Google Scholar] [PubMed]
- Slota, M.; Lim, J.B.; Dang, Y.; Disis, M.L. Elispot for measuring human immune responses to vaccines. Expert Rev. Vaccines 2011, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.V.; Zhang, W. Unique strengths of elispot for T cell diagnostics. Methods Mol. Biol. 2012, 792, 3–23. [Google Scholar] [PubMed]
- Strom, P.; Stoer, N.; Borthwick, N.; Dong, T.; Hanke, T.; Reilly, M. A statistical approach to determining responses to individual peptides from pooled-peptide elispot data. J. Immunol. Methods 2016, 435, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Holz, L.E.; Fernandez-Ruiz, D.; Heath, W.R. Protective immunity to liver-stage malaria. Clin. Transl. Immunol. 2016, 5, e105. [Google Scholar] [CrossRef] [PubMed]
- Kassegne, K.; Abe, E.M.; Chen, J.H.; Zhou, X.N. Immunomic approaches for antigen discovery of human parasites. Expert Rev. Proteomic. 2016, 13, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Sedegah, M.; Kim, Y.; Ganeshan, H.; Huang, J.; Belmonte, M.; Abot, E.; Banania, J.G.; Farooq, F.; McGrath, S.; Peters, B.; et al. Identification of minimal human mhc-restricted cd8+ T-cell epitopes within the plasmodium falciparum circumsporozoite protein (csp). Malar. J. 2013, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, D.; Hollingdale, M.R.; Anum, D.; Koram, K.A.; Gyan, B.; Akanmori, B.D.; Ocran, J.; Adu-Amankwah, S.; Geneshan, H.; Abot, E.; et al. Measuring naturally acquired immune responses to candidate malaria vaccine antigens in ghanaian adults. Malar. J. 2011, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Woodberry, T.; Pinzon-Charry, A.; Piera, K.A.; Panpisutchai, Y.; Engwerda, C.R.; Doolan, D.L.; Salwati, E.; Kenangalem, E.; Tjitra, E.; Price, R.N.; et al. Human T cell recognition of the blood stage antigen plasmodium hypoxanthine guanine xanthine phosphoribosyl transferase (hgxprt) in acute malaria. Malar. J. 2009, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Herrera, M.; Valencia, A.Z.; Vergara, J.; Bonelo, A.; Fleischhauer, K.; Gonzalez, J.M.; Restrepo, J.C.; Lopez, J.A.; Valmori, D.; Corradin, G.; et al. Identification of hla-a2 restricted cd8(+) t-lymphocyte responses to plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria. Parasite Immunol. 2002, 24, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.H.; Hafalla, J.C.; Zavala, F. Elispot assay to measure antigen-specific murine cd8(+) T cell responses. J. Immunol. Methods 2001, 252, 207–218. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Peter, K.; Esposito, F.; Nebie, I.; Tiercy, J.M.; Bonelo, A.; Arevalo-Herrera, M.; Valmori, D.; Romero, P.; Herrera, S.; et al. Hla-a * 0201 restricted cd8+ t-lymphocyte responses to malaria: Identification of new plasmodium falciparum epitopes by ifn-gamma elispot. Parasite Immunol. 2000, 22, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Reece, W.H.; Pinder, M.; Gothard, P.K.; Milligan, P.; Bojang, K.; Doherty, T.; Plebanski, M.; Akinwunmi, P.; Everaere, S.; Watkins, K.R.; et al. A cd4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural plasmodium falciparum infection and disease. Nat. Med. 2004, 10, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Weiss, W.; Tine, J.A.; Hoffman, S.L.; Rogers, W.O. Elispot assay for detection of peptide specific interferon-gamma secreting cells in rhesus macaques. J. Immunol. Methods 2001, 247, 49–60. [Google Scholar] [CrossRef]
- Lima-Junior, J.C.; Banic, D.M.; Tran, T.M.; Meyer, V.S.; De-Simone, S.G.; Santos, F.; Porto, L.C.; Marques, M.T.; Moreno, A.; Barnwell, J.W.; et al. Promiscuous T-cell epitopes of plasmodium merozoite surface protein 9 (pvmsp9) induces ifn-gamma and il-4 responses in individuals naturally exposed to malaria in the brazilian amazon. Vaccine 2010, 28, 3185–3191. [Google Scholar] [CrossRef] [PubMed]
- Wipasa, J.; Suphavilai, C.; Okell, L.C.; Cook, J.; Corran, P.H.; Thaikla, K.; Liewsaree, W.; Riley, E.M.; Hafalla, J.C. Long-lived antibody and B cell memory responses to the human malaria parasites, plasmodium falciparum and plasmodium vivax. PLoS Pathog. 2010, 6, e1000770. [Google Scholar] [CrossRef] [PubMed]
- Bottger, E.; Multhoff, G.; Kun, J.F.; Esen, M. Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-hsp70. PLoS ONE 2012, 7, e33774. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.M.; Okitsu, S.; Porter, D.W.; Duncan, C.; Amacker, M.; Pluschke, G.; Cavanagh, D.R.; Hill, A.V.; Todryk, S.M. Antibody and T-cell responses associated with experimental human malaria infection or vaccination show limited relationships. Immunology 2015, 145, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Cai, J.; Yin, H. Review on the identification and role of toxoplasma gondii antigenic epitopes. Parasitol. Res. 2016, 115, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Cardona, N.I.; Moncada, D.M.; Gomez-Marin, J.E. A rational approach to select immunogenic peptides that induce ifn-gamma response against toxoplasma gondii in human leukocytes. Immunobiology 2015, 220, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Jensen, M.R.; Rosenberg, C.A.; Zhu, X.Q.; Petersen, E.; Vorup-Jensen, T. In silico and in vivo analysis of toxoplasma gondii epitopes by correlating survival data with peptide-mhc-i binding affinities. Int. J. Infect. Dis. 2016, 48, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Ernst, M.; Meyer, P.; Wolf, E.; Rosenkranz, T.; Plettenberg, A.; Stoehr, A.; Horst, H.A.; Marienfeld, K.; Lange, C. Evolving characteristics of toxoplasmosis in patients infected with human immunodeficiency virus-1: Clinical course and toxoplasma gondii-specific immune responses. Clin. Microbiol. Infect. 2007, 13, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, C.S.; Van Aartsen, D.; Terry, F.E.; Meymandi, S.K.; Traina, M.M.; Hernandez, S.; Martin, W.D.; Moise, L.; De Groot, A.S.; Hoft, D.F. An immunoinformatic approach for identification of trypanosoma cruzi hla-a2-restricted cd8(+) T cell epitopes. Hum. Vacc. Immunother. 2015, 11, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Olivera, G.C.; Albareda, M.C.; Alvarez, M.G.; De Rissio, A.M.; Fichera, L.E.; Cooley, G.; Yachelini, P.; Hrellac, H.A.; Riboldi, H.; Laucella, S.A.; et al. Trypanosoma cruzi-specific immune responses in subjects from endemic areas of chagas disease of argentina. Microbes Infect. 2010, 12, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Diez, H.; Lopez, M.C.; Del Carmen Thomas, M.; Guzman, F.; Rosas, F.; Velazco, V.; Gonzalez, J.M.; Puerta, C. Evaluation of ifn-gamma production by cd8 t lymphocytes in response to the k1 peptide from kmp-11 protein in patients infected with trypanosoma cruzi. Parasite Immunol. 2006, 28, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Moins-Teisserenc, H.; Clave, E.; Ianni, B.; Nunes, V.L.; Mady, C.; Iwai, L.K.; Sette, A.; Sidney, J.; Marin, M.L.; et al. Identification of multiple hla-a * 0201-restricted cruzipain and fl-160 cd8+ epitopes recognized by T cells from chronically trypanosoma cruzi-infected patients. Microbes Infect. 2005, 7, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; Espinoza, A.G.; Taibi, A.; Ouaissi, A.; Minoprio, P. A 24,000 mw trypanosoma cruzi antigen is a B-cell activator. Immunology 1998, 94, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.G.; Bertocchi, G.L.; Cooley, G.; Albareda, M.C.; Viotti, R.; Perez-Mazliah, D.E.; Lococo, B.; Castro Eiro, M.; Laucella, S.A.; Tarleton, R.L. Treatment success in trypanosoma cruzi infection is predicted by early changes in serially monitored parasite-specific t and b cell responses. PLoS Neglect. Trop. Dis. 2016, 10, e0004657. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H.; Savioli, L.; Engels, D. Neglected tropical diseases: Progress towards addressing the chronic pandemic. Lancet 2017, 389, 312–325. [Google Scholar] [CrossRef]
- Engelman, D.; Fuller, L.C.; Solomon, A.W.; McCarthy, J.S.; Hay, R.J.; Lammie, P.J.; Steer, A.C. Opportunities for integrated control of neglected tropical diseases that affect the skin. Trends Parasitol. 2016, 32, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Seyed, N.; Taheri, T.; Vauchy, C.; Dosset, M.; Godet, Y.; Eslamifar, A.; Sharifi, I.; Adotevi, O.; Borg, C.; Rohrlich, P.S.; et al. Immunogenicity evaluation of a rationally designed polytope construct encoding hla-a * 0201 restricted epitopes derived from leishmania major related proteins in hla-a2/dr1 transgenic mice: Steps toward polytope vaccine. PLoS ONE 2014, 9, e108848. [Google Scholar] [CrossRef] [PubMed]
- Seyed, N.; Zahedifard, F.; Safaiyan, S.; Gholami, E.; Doustdari, F.; Azadmanesh, K.; Mirzaei, M.; Saeedi Eslami, N.; Khadem Sadegh, A.; Eslami Far, A.; et al. In silico analysis of six known leishmania major antigens and in vitro evaluation of specific epitopes eliciting hla-a2 restricted cd8 T cell response. PLoS Neglect. Trop. Dis. 2011, 5, e1295. [Google Scholar] [CrossRef] [PubMed]
- Stager, S.; Smith, D.F.; Kaye, P.M. Immunization with a recombinant stage-regulated surface protein from leishmania donovani induces protection against visceral leishmaniasis. J. Immunol. 2000, 165, 7064–7071. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.S.; Gomes-Silva, A.; Bittar, R.C.; Silva Mendonca, D.; Amato, V.S.; Da Silva Mattos, M.; Oliveira-Neto, M.P.; Coutinho, S.G.; Da-Cruz, A.M. Antigen-triggered interferon-gamma and interleukin-10 pattern in cured mucosal leishmaniasis patients is shaped during the active phase of disease. Clin. Exp. Immunol. 2014, 177, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Conceicao-Silva, F.; Morgado, F.N.; Pimentel, M.I.; e Vasconcellos, E.D.C.F.; Schubach, A.O.; Valete-Rosalino, C.M.; Kropf, P.; Muller, I. Two women presenting worsening cutaneous ulcers during pregnancy: Diagnosis, immune response, and follow-up. PLoS Neglect. Trop. Dis. 2013, 7, e2472. [Google Scholar] [CrossRef] [PubMed]
- Lakew, M.; Nordstrom, I.; Czerkinsky, C.; Quiding-Jarbrink, M. Combined immunomagnetic cell sorting and elispot assay for the phenotypic characterization of specific antibody-forming cells. J. Immunol. Methods 1997, 203, 193–198. [Google Scholar] [CrossRef]
- Linares, E.M.; Pannuti, C.S.; Kubota, L.T.; Thalhammer, S. Immunospot assay based on fluorescent nanoparticles for dengue fever detection. Biosens. Bioelectron. 2013, 41, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hadjilaou, A.; Green, A.M.; Coloma, J.; Harris, E. Single-cell analysis of B cell/antibody cross-reactivity using a novel multicolor fluorospot assay. J. Immunol. 2015, 195, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Zivny, J.; DeFronzo, M.; Jarry, W.; Jameson, J.; Cruz, J.; Ennis, F.A.; Rothman, A.L. Partial agonist effect influences the ctl response to a heterologous dengue virus serotype. J. Immunol. 1999, 163, 2754–2760. [Google Scholar] [PubMed]
- Loke, H.; Bethell, D.B.; Phuong, C.X.; Dung, M.; Schneider, J.; White, N.J.; Day, N.P.; Farrar, J.; Hill, A.V. Strong hla class I—Restricted T cell responses in dengue hemorrhagic fever: A double-edged sword? J. Infect. Dis. 2001, 184, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Dong, T.; Chau, N.V.; Dung, N.T.; Chau, T.N.; Thao, L.T.T.; Dung, N.T.; Hien, T.T.; Rowland-Jones, S.; Farrar, J. Early T-cell responses to dengue virus epitopes in vietnamese adults with secondary dengue virus infections. J. Virol. 2005, 79, 5665–5675. [Google Scholar] [CrossRef] [PubMed]
- Appanna, R.; Huat, T.L.; See, L.L.; Tan, P.L.; Vadivelu, J.; Devi, S. Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in malaysia. Clin. Vaccine Immunol. 2007, 14, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Jeewandara, C.; Alles, K.M.; Salimi, M.; Gomes, L.; Kamaladasa, A.; Jayaratne, S.D.; Ogg, G.S. Suppression of virus specific immune responses by IL-10 in acute dengue infection. PLoS Neglect. Trop. Dis. 2013, 7, e2409. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Adikari, T.N.; Gomes, L.; Fernando, S.; Fernando, R.H.; Perera, M.K.; Ariyaratne, D.; Kamaladasa, A.; Salimi, M.; Prathapan, S.; et al. Functionality of dengue virus specific memory T cell responses in individuals who were hospitalized or who had mild or subclinical dengue infection. PLoS Neglect. Trop. Dis. 2015, 9, e0003673. [Google Scholar] [CrossRef] [PubMed]
- Kamaladasa, A.; Wickramasinghe, N.; Adikari, T.N.; Gomes, L.; Shyamali, N.L.; Salio, M.; Cerundolo, V.; Ogg, G.S.; Malavige, G.N. Expansion of highly activated invariant natural killer T cells with altered phenotype in acute dengue infection. Clin. Exp. Immunol. 2016, 185, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.S.; Tang, A.; Chen, Z.; Horton, M.S.; Yan, H.; Wang, X.M.; Dubey, S.A.; DiStefano, D.J.; Ettenger, A.; Fong, R.H.; et al. Rapid isolation of dengue-neutralizing antibodies from single cell-sorted human antigen-specific memory B-cell cultures. mAbs 2016, 8, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; West, K.; Kalayanarooj, S.; Gibbons, R.V.; Srikiatkhachorn, A.; Green, S.; Libraty, D.; Jaiswal, S.; Rothman, A.L. B-cell responses during primary and secondary dengue virus infections in humans. J. Infect. Dis. 2011, 204, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Friberg, H.; Jaiswal, S.; West, K.; O’Ketch, M.; Rothman, A.L.; Mathew, A. Analysis of human monoclonal antibodies generated by dengue virus-specific memory B cells. Viral Immunol. 2012, 25, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.S.; Jiang, L.F.; Zhou, J.M.; Yan, H.J.; Fang, D.Y. Computational prediction and identification of dengue virus-specific cd4(+) T-cell epitopes. Virus Res. 2008, 132, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Duan, Z.; Jiang, L. Identification of a dengue virus-specific hla-a * 0201-restricted cd8+ T cell epitope. J. Med. Virol. 2010, 82, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; De Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; De Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an hla-linked protective role for cd8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.J.; Mailliard, R.B.; Khan, A.M.; Sidney, J.; Sette, A.; Guzman, N.; Paulaitis, M.; de Melo, A.B.; Cordeiro, M.T.; Gil, L.V.; et al. Identification of conserved and hla promiscuous denv3 T-cell epitopes. PLoS Neglect. Trop. Dis. 2013, 7, e2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Peng, L.; Zhao, W.; Zhong, H.; Zhang, F.; Yan, Z.; Cao, H. Synthetic peptides containing B- and T-cell epitope of dengue virus-2 e domain iii provoked B- and T-cell responses. Vaccine 2011, 29, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Guo, J.; Huang, X.; Liu, H.; Chen, X.; Jiang, M.; Wen, J. Identification of cytotoxic t lymphocyte epitopes in dengue virus serotype 1. J. Med. Virol. 2015, 87, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.N.; Nascimento, E.J.; Cordeiro, M.T.; Gil, L.H.; Abath, F.G.; Montenegro, S.M.; Marques, E.T. Identification of continuous human B-cell epitopes in the envelope glycoprotein of dengue virus type 3 (denv-3). PLoS ONE 2009, 4, e7425. [Google Scholar] [CrossRef]
- Sun, P.; Beckett, C.; Danko, J.; Burgess, T.; Liang, Z.; Kochel, T.; Porter, K. A dendritic cell-based assay for measuring memory T cells specific to dengue envelope proteins in human peripheral blood. J. Virol. Methods 2011, 173, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; De Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The human cd8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, S.H.; Kim, J.; Lee, K.Y.; Jang, Y.S. A microneutralization assay for dengue viruses using mosquito c6/36 cells. Acta Virol. 2013, 57, 379–381. [Google Scholar] [PubMed]
- Kim, S.H.; Yang, I.Y.; Jang, S.H.; Kim, J.; Truong, T.T.; Van Pham, T.; Truong, N.U.; Lee, K.Y.; Jang, Y.S. C5a receptor-targeting ligand-mediated delivery of dengue virus antigen to M cells evokes antigen-specific systemic and mucosal immune responses in oral immunization. Microbes Infect. 2013, 15, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Chen, J.; Huang, Y.F.; Ding, X.X.; Liu, L.D.; Qiu, L.W.; Pan, Y.X.; Deng, Y.Q.; Hu, D.M.; Di, B.; et al. Evaluation and analysis of dengue virus enhancing and neutralizing activities using simple high-throughput assays. Appl. Microbiol. Biot. 2013, 97, 6503–6511. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wen, K.; Li, J.; Hu, D.; Huang, Y.; Qiu, L.; Cai, J.; Che, X. Comparison of plaque- and enzyme-linked immunospot-based assays to measure the neutralizing activities of monoclonal antibodies specific to domain iii of dengue virus envelope protein. Clin. Vaccine Immunol. 2012, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, L.D.; Agudelo, I.Y.; Trujillo, A.I.; Ramirez, R.E.; Osorio, J.E.; Restrepo, B.N. Evaluation of commercially available assays for diagnosis of acute dengue in schoolchildren during an epidemic period in medellin, colombia. Am. J. Trop. Med. Hyg. 2016, 95, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, W.W.; Alcena, D.C.; Rose, R.C.; Jin, X.; Schlesinger, J.J. An automated dengue virus microneutralization plaque assay performed in human fc{gamma} receptor-expressing cv-1 cells. Am. J. Trop. Med. Hyg. 2009, 80, 61–65. [Google Scholar] [PubMed]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive cd8+ T cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.H.; Lum, F.M.; Claser, C.; Lulla, V.; Lulla, A.; Merits, A.; Renia, L.; Ng, L.F. A pathogenic role for cd4+ T cells during chikungunya virus infection in mice. J. Immunol. 2013, 190, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.J.; Gay, F.; Pelle, O.; Samri, A.; Jaffar-Bandjee, M.C.; Gasque, P.; Autran, B. Identical strength of the T cell responses against e2, nsp1 and capsid chikv proteins in recovered and chronic patients after the epidemics of 2005–2006 in la reunion island. PLoS ONE 2013, 8, e84695. [Google Scholar] [CrossRef] [PubMed]
- Co, M.D.; Terajima, M.; Cruz, J.; Ennis, F.A.; Rothman, A.L. Human cytotoxic t lymphocyte responses to live attenuated 17d yellow fever vaccine: Identification of hla-b35-restricted ctl epitopes on nonstructural proteins ns1, ns2b, ns3, and the structural protein e. Virology 2002, 293, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Matos, D.C.; Bertho, A.L.; Mendonca, S.C.; Marcovistz, R. Detection of th1/th2 cytokine signatures in yellow fever 17dd first-time vaccinees through elispot assay. Cytokine 2008, 42, 152–155. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.B.; Nascimento, E.J.; Braga-Neto, U.; Dhalia, R.; Silva, A.M.; Oelke, M.; Schneck, J.P.; Sidney, J.; Sette, A.; Montenegro, S.M.; et al. T-cell memory responses elicited by yellow fever vaccine are targeted to overlapping epitopes containing multiple hla-i and -ii binding motifs. PLoS Neglect. Trop. Dis. 2013, 7, e1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Z.; Qiao, J.; Shen, T.; Link, H. Early diagnosis of tuberculous meningitis by detection of anti-bcg secreting cells in cerebrospinal fluid. Lancet 1990, 336, 10–13. [Google Scholar] [CrossRef]
- Santin Cerezales, M.; Dominguez Benitez, J. Diagnosis of tuberculosis infection using interferon-gamma-based assays. Enferm. Infec. Microbiol. Clin. 2011, 29 (Suppl. 1), 26–33. [Google Scholar] [CrossRef]
- Sester, M.; Sotgiu, G.; Lange, C.; Giehl, C.; Girardi, E.; Migliori, G.B.; Bossink, A.; Dheda, K.; Diel, R.; Dominguez, J.; et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Rangaka, M.X.; Wilkinson, K.A.; Glynn, J.R.; Ling, D.; Menzies, D.; Mwansa-Kambafwile, J.; Fielding, K.; Wilkinson, R.J.; Pai, M. Predictive value of interferon-gamma release assays for incident active tuberculosis: A systematic review and meta-analysis. Lancet. Infect. Dis. 2012, 12, 45–55. [Google Scholar] [CrossRef]
- Millington, K.A.; Innes, J.A.; Hackforth, S.; Hinks, T.S.; Deeks, J.J.; Dosanjh, D.P.; Guyot-Revol, V.; Gunatheesan, R.; Klenerman, P.; Lalvani, A. Dynamic relationship between ifn-gamma and IL-2 profile of mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 2007, 178, 5217–5226. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.S.; Adetifa, I.M.; Hill, P.C.; Adegbola, R.A.; Ota, M.O. Pattern and diversity of cytokine production differentiates between mycobacterium tuberculosis infection and disease. European J. Immunol. 2009, 39, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Rozot, V.; Bellutti Enders, F.; Perreau, M.; Stalder, J.M.; Nicod, L.P.; Cavassini, M.; Calandra, T.; Blanchet, C.L.; Jaton, K.; et al. Dominant tnf-alpha+ mycobacterium tuberculosis-specific cd4+ T cell responses discriminate between latent infection and active disease. Nat. Med. 2011, 17, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Sester, U.; Fousse, M.; Dirks, J.; Mack, U.; Prasse, A.; Singh, M.; Lalvani, A.; Sester, M. Whole-blood flow-cytometric analysis of antigen-specific cd4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLoS ONE 2011, 6, e17813. [Google Scholar] [CrossRef] [PubMed]
- Chesov, D.; Lange, C.; Daduna, F.; Crudu, V.; Preyer, R.; Ernst, M.; Kalsdorf, B. Combined antigen-specific interferon-gamma and interleukin-2 release assay (fluorospot) for the diagnosis of mycobacterium tuberculosis infection. PLoS ONE 2015, 10, e0120006. [Google Scholar] [CrossRef] [PubMed]
- Kifayet, A.; Shahid, F.; Lucas, S.; Hussain, R. Erythema nodosum leprosum is associated with up-regulation of polyclonal igg1 antibody synthesis. Clin. Exp. Immunol. 1996, 106, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Geluk, A.; van Meijgaarden, K.E.; Franken, K.L.; Subronto, Y.W.; Wieles, B.; Arend, S.M.; Sampaio, E.P.; De Boer, T.; Faber, W.R.; Naafs, B.; et al. Identification and characterization of the esat-6 homologue of mycobacterium leprae and T-cell cross-reactivity with mycobacterium tuberculosis. Infect. Immun. 2002, 70, 2544–2548. [Google Scholar] [CrossRef] [PubMed]
- Geluk, A.; Van Meijgaarden, K.E.; Franken, K.L.; Wieles, B.; Arend, S.M.; Faber, W.R.; Naafs, B.; Ottenhoff, T.H. Immunological crossreactivity of the mycobacterium leprae cfp-10 with its homologue in mycobacterium tuberculosis. Scand. J. Immunol. 2004, 59, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Tabidze, I.L.; Lee, F.K.; Tambe, P.; Rocha, E.; Larsen, S.A.; Stoll, B.J.; St Louis, M.E.; Nahmias, A.J. Enzyme-linked immunospot assay for the diagnosis of active treponema pallidum infection during the various stages of syphilis. Sex. Transm. Dis. 1999, 26, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Lee, F.K.; Larsen, S.; Hale, E.; Schwartz, D.; Rice, R.J.; Ashby, R.; Holmes, R.; Nahmias, A.J. Clinical and serologic evaluation of neonates for congenital syphilis: A continuing diagnostic dilemma. J. Infect. Dis. 1993, 167, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Jayasekera, C.R.; Harris, J.B.; Bhuiyan, S.; Chowdhury, F.; Khan, A.I.; Faruque, A.S.; Larocque, R.C.; Ryan, E.T.; Ahmed, R.; Qadri, F.; et al. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by vibrio cholerae o1. J. Infect. Dis. 2008, 198, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Ryan, E.T.; Faruque, A.S.; Ahmed, F.; Khan, A.I.; Islam, M.M.; Akramuzzaman, S.M.; Sack, D.A.; Calderwood, S.B. Antigen-specific immunoglobulin a antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: Comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 2003, 71, 4808–4814. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Andros, T.S.; Campbell, C.H., Jr. Schistosomiasis is more prevalent than previously thought: What does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect. Dis. Poverty 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- King, C.L.; Low, C.C.; Nutman, T.B. Ige production in human helminth infection. Reciprocal interrelationship between IL-4 and ifn-gamma. J. Immunol. 1993, 150, 1873–1880. [Google Scholar] [PubMed]
- Williams, M.E.; Montenegro, S.; Domingues, A.L.; Wynn, T.A.; Teixeira, K.; Mahanty, S.; Coutinho, A.; Sher, A. Leukocytes of patients with schistosoma mansoni respond with a th2 pattern of cytokine production to mitogen or egg antigens but with a th0 pattern to worm antigens. J. Infect. Dis. 1994, 170, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Farid, A.; Al-Sherbiny, M.; Osman, A.; Mohamed, N.; Saad, A.; Shata, M.T.; Lee, D.H.; Prince, A.M.; Strickland, G.T. Schistosoma infection inhibits cellular immune responses to core hcv peptides. Parasite Immunol. 2005, 27, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Zhang, W.; Torben, W.; Damian, R.T.; Wolf, R.F.; White, G.L.; Chavez-Suarez, M.; Kennedy, R.C.; Siddiqui, A.A. Protective and antifecundity effects of sm-p80-based DNA vaccine formulation against schistosoma mansoni in a nonhuman primate model. Vaccine 2009, 27, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Wajja, A.; Kizito, D.; Nassanga, B.; Nalwoga, A.; Kabagenyi, J.; Kimuda, S.; Galiwango, R.; Mutonyi, G.; Vermaak, S.; Satti, I.; et al. The effect of current schistosoma mansoni infection on the immunogenicity of a candidate tb vaccine, mva85a, in bcg-vaccinated adolescents: An open-label trial. PLoS Neglect. Trop. Dis. 2017, 11, e0005440. [Google Scholar] [CrossRef] [PubMed]
- Gebreegziabiher, D.; Desta, K.; Desalegn, G.; Howe, R.; Abebe, M. The effect of maternal helminth infection on maternal and neonatal immune function and immunity to tuberculosis. PLoS ONE 2014, 9, e93429. [Google Scholar] [CrossRef] [PubMed]
- Abate, E.; Belayneh, M.; Idh, J.; Diro, E.; Elias, D.; Britton, S.; Aseffa, A.; Stendahl, O.; Schon, T. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and th-2 responses and a lower sputum smear positivity. PLoS Neglect. Trop. Dis. 2015, 9, e0003994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabeerdoss, J.; Pugazhendhi, S.; Subramanian, V.; Binder, H.J.; Ramakrishna, B.S. Exposure to hookworms in patients with crohn’s disease: A case-control study. Aliment. Pharmacol. Therapeut. 2011, 34, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Daveson, A.J.; Jones, D.M.; Gaze, S.; McSorley, H.; Clouston, A.; Pascoe, A.; Cooke, S.; Speare, R.; Macdonald, G.A.; Anderson, R.; et al. Effect of hookworm infection on wheat challenge in celiac disease—A randomised double-blinded placebo controlled trial. PLoS ONE 2011, 6, e17366. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.; Makristathis, A.; Rotter, M.; Hirschl, A.M. Gene expression profiling in ags cells stimulated with helicobacter pylori isogenic strains (caga positive or caga negative). Infect. Immun. 2002, 70, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Urban, J.J.; Argo, C.K.; Weinstock, J.V. Does the failure to acquire helminthic parasites predispose to crohn’s disease? FASEB J. 2000, 14, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Vehling, D.; Schunk, K.; Holtmann, M.; Brockmann, H.; Helisch, A.; Orth, T.; Schreckenberger, M.; Galle, P.R.; Bartenstein, P. Noninvasive assessment of crohn’s disease activity: A comparison of 18f-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am. J. Gastroenterol. 2002, 97, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Blaszkowska, J.; Wojcik, A. Current problems concerning parasitology and mycology with regard to diseases of the skin and its appendages. Ann. Parasitol. 2012, 58, 111–123. [Google Scholar] [PubMed]
- Davenport, C.; Bottazzo, G.F.; Todd, I. Stimulation of human b cells specific for candida albicans for monoclonal antibody production. FEMS Microbiol. Immunol. 1992, 4, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, E.; Boccanera, M.; Giordani, L.; Cassone, A.; Luzzati, A.L. Induction of antibody forming cells with specificity for candida albicans mannoproteins in cultures of human peripheral blood lymphocytes. J. Immunol. Methods 1993, 164, 203–211. [Google Scholar] [CrossRef]
- Li, S.P.; Lee, S.I.; Domer, J.E. Alterations in frequency of interleukin-2 (IL-2)-, gamma interferon-, or IL-4-secreting splenocytes induced by candida albicans mannan and/or monophosphoryl lipid A. Infect. Immun. 1998, 66, 1392–1399. [Google Scholar] [PubMed]
- Zhou, M.; Yang, B.; Ma, R.; Wu, C. Memory th-17 cells specific for C. albicans are persistent in human peripheral blood. Immunol. Lett. 2008, 118, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Tongchusak, S.; Brusic, V.; Chaiyaroj, S.C. Promiscuous T cell epitope prediction of candida albicans secretory aspartyl protienase family of proteins. Infect. Genet. Evol. 2008, 8, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Tongchusak, S.; Leelayuwat, C.; Brusic, V.; Chaiyaroj, S.C. In silico prediction and immunological validation of common hla-drb1-restricted T cell epitopes of candida albicans secretory aspartyl proteinase 2. Microbiol. Immunol. 2008, 52, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Paulovicova, L.; Paulovicova, E.; Karelin, A.A.; Tsvetkov, Y.E.; Nifantiev, N.E.; Bystricky, S. Effect of branched alpha-oligomannoside structures on induction of anti-candida humoral immune response. Scand. J. Immunol. 2013, 77, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Dillenbeck, T.; Gelius, E.; Fohlstedt, J.; Ahlborg, N. Triple cytokine fluorospot analysis of human antigen-specific ifn-gamma, IL-17a and IL-22 responses. Cells 2014, 3, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, M.; Schroppel, K.; Taylor, B.; Bergmann, S.; Schmitt-Haendle, M.; Harrer, T. Analysis of the candida albicans—Specific T-cell response and oropharyngeal candida colonization in a cohort of hiv-1-infected patients. Eur. J. Med. Res. 2006, 11, 479–484. [Google Scholar] [PubMed]
- Burgess, K.; Price, P.; James, I.R.; Stone, S.F.; Keane, N.M.; Lim, A.Y.; Warmington, J.R.; French, M.A. Interferon-gamma responses to candida recover slowly or remain low in immunodeficient hiv patients responding to art. J. Clin. Immunol. 2006, 26, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Hamagami, S.; Ohhata, I.; Ochi, T.; Kishimoto, S. [specific antibody-forming cells in bronchoalveolar lavage fluid of patients with summer-type hypersensitivity pneumonitis—Detection by enzyme-linked immunospot (elispot)]. Nihon Kyobu Shikkan Gakkai Zasshi 1993, 31, 840–847. [Google Scholar] [PubMed]
- Tan, D.B.; Yong, Y.K.; Tan, H.Y.; Kamarulzaman, A.; Tan, L.H.; Lim, A.; James, I.; French, M.; Price, P. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008, 9, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; Schubach, A.O.; Pimentel, M.I.; Lyra, M.R.; Vasconcellos, E.C.; Valete-Rosalino, C.M.; Conceicao-Silva, F. Is there any difference between the in situ and systemic il-10 and ifn-gamma production when clinical forms of cutaneous sporotrichosis are compared? PLoS ONE 2016, 11, e0162764. [Google Scholar] [CrossRef] [PubMed]
- Potenza, L.; Vallerini, D.; Barozzi, P.; Riva, G.; Gilioli, A.; Forghieri, F.; Candoni, A.; Cesaro, S.; Quadrelli, C.; Maertens, J.; et al. Mucorales-specific t cells in patients with hematologic malignancies. PLoS ONE 2016, 11, e0149108. [Google Scholar] [CrossRef] [PubMed]
- Saade, F.; Gorski, S.A.; Petrovsky, N. Pushing the frontiers of T-cell vaccines: Accurate measurement of human T-cell responses. Expert Rev. Vaccines 2012, 11, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Matthews, Q.L.; Farrow, A.L.; Rachakonda, G.; Gu, L.; Nde, P.; Krendelchtchikov, A.; Pratap, S.; Sakhare, S.S.; Sabbaj, S.; Lima, M.F.; et al. Epitope capsid-incorporation: New effective approach for vaccine development for chagas disease. Pathogens Immun. 2016, 1, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Sullivan, M.; Narvaez, C.F.; Holmes, T.H.; Furman, D.; Zheng, N.Y.; Nishtala, M.; Wrammert, J.; Smith, K.; James, J.A.; et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J. Clin. Invest. 2011, 121, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Oshansky, C.M.; Gartland, A.J.; Wong, S.S.; Jeevan, T.; Wang, D.; Roddam, P.L.; Caniza, M.A.; Hertz, T.; Devincenzo, J.P.; Webby, R.J.; et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am. J. Respir. Crit. Care. Med. 2014, 189, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Winokur, P.L.; Salata, R.A.; El-Kamary, S.S.; Turley, C.B.; Walter, E.B., Jr.; Hay, C.M.; Newman, F.K.; Hill, H.R.; Zhang, Y.; et al. Safety and immunogenicity of imvamune(r) smallpox vaccine using different strategies for a post event scenario. Vaccine 2013, 31, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, U.N.; Costner, P.; Enama, M.E.; Berkowitz, N.; Hu, Z.; Hendel, C.S.; Sitar, S.; Plummer, S.; Mulangu, S.; Bailer, R.T.; et al. Safety and immunogenicity of DNA vaccines encoding ebolavirus and marburgvirus wild-type glycoproteins in a phase i clinical trial. J. Infect. Dis. 2015, 211, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Sedegah, M.; Peters, B.; Hollingdale, M.R.; Ganeshan, H.D.; Huang, J.; Farooq, F.; Belmonte, M.N.; Belmonte, A.D.; Limbach, K.J.; Diggs, C.; et al. Vaccine strain-specificity of protective hla-restricted class 1 p. Falciparum epitopes. PLoS ONE 2016, 11, e0163026. [Google Scholar]
- Lima-Junior, J.C.; Tran, T.M.; Meyer, E.V.; Singh, B.; De-Simone, S.G.; Santos, F.; Daniel-Ribeiro, C.T.; Moreno, A.; Barnwell, J.W.; Galinski, M.R.; et al. Naturally acquired humoral and cellular immune responses to plasmodium vivax merozoite surface protein 9 in northwestern amazon individuals. Vaccine 2008, 26, 6645–6654. [Google Scholar] [CrossRef] [PubMed]
- Pollard, R.B.; Rockstroh, J.K.; Pantaleo, G.; Asmuth, D.M.; Peters, B.; Lazzarin, A.; Garcia, F.; Ellefsen, K.; Podzamczer, D.; van Lunzen, J.; et al. Safety and efficacy of the peptide-based therapeutic vaccine for hiv-1, vacc-4x: A phase 2 randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis 2014, 14, 291–300. [Google Scholar] [CrossRef]
- Minassian, A.M.; Rowland, R.; Beveridge, N.E.; Poulton, I.D.; Satti, I.; Harris, S.; Poyntz, H.; Hamill, M.; Griffiths, K.; Sander, C.R.; et al. A phase i study evaluating the safety and immunogenicity of mva85a, a candidate tb vaccine, in hiv-infected adults. BMJ Open 2011, 1, e000223. [Google Scholar] [CrossRef] [PubMed]
- Scriba, T.J.; Tameris, M.; Smit, E.; van der Merwe, L.; Hughes, E.J.; Kadira, B.; Mauff, K.; Moyo, S.; Brittain, N.; Lawrie, A.; et al. A phase iia trial of the new tuberculosis vaccine, mva85a, in hiv- and/or mycobacterium tuberculosis-infected adults. Am. J. Respir. Crit. Care. Med. 2012, 185, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Oseroff, C.; Sidney, J.; Vita, R.; Tripple, V.; McKinney, D.M.; Southwood, S.; Brodie, T.M.; Sallusto, F.; Grey, H.; Alam, R.; et al. T cell responses to known allergen proteins are differently polarized and account for a variable fraction of total response to allergen extracts. J. Immunol. 2012, 189, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Oseroff, C.; Pham, J.; Frazier, A.; Hinz, D.; Sidney, J.; Paul, S.; Greenbaum, J.A.; Vita, R.; Peters, B.; Schulten, V.; et al. Immunodominance in allergic T-cell reactivity to japanese cedar in different geographic cohorts. Ann. Allergy Asthma Immunol. 2016, 117, 680–689.e1. [Google Scholar] [CrossRef] [PubMed]
- Michaud, B.; Aroulandom, J.; Baiz, N.; Amat, F.; Gouvis-Echraghi, R.; Candon, S.; Foray, A.P.; Couderc, R.; Bach, J.F.; Chatenoud, L.; et al. Casein-specific IL-4- and IL-13-secreting T cells: A tool to implement diagnosis of cow’s milk allergy. Allergy 2014, 69, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Schulten, V.; Tripple, V.; Aasbjerg, K.; Backer, V.; Lund, G.; Wurtzen, P.A.; Sette, A.; Peters, B. Distinct modulation of allergic T cell responses by subcutaneous vs. Sublingual allergen-specific immunotherapy. Clin. Exp. Allergy 2016, 46, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, B.; Rozieres, A.; Nosbaum, A.; Nicolas, J.F.; Berard, F. Amikacin-induced drug reaction with eosinophilia and systemic symptoms syndrome: Delayed skin test and elispot assay results allow the identification of the culprit drug. J. Allergy Clin. Immun. 2012, 130, 1413–1414. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Gao, Y.; Pan, Y.; Li, W.; Liao, W.; Wang, G.; Li, C.; Li, C.; Gao, T.; Liu, Y. Recovered patients with stevens-johson syndrome and toxic epidermal necrolysis maintain long-lived ifn-gamma and sfasl memory response. PLoS ONE 2012, 7, e45516. [Google Scholar] [CrossRef] [PubMed]
- Phatharacharukul, P.; Klaewsongkram, J. A case of sulfasalazine-induced hypersensitivity syndrome confirmed by enzyme-linked immunospot assay. Allergy Asthma Immunol. Res. 2013, 5, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Tanvarasethee, B.; Buranapraditkun, S.; Klaewsongkram, J. The potential of using enzyme-linked immunospot to diagnose cephalosporin-induced maculopapular exanthems. Acta Derm. Venereol. 2013, 93, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Amali, M.O.; Sullivan, A.; Jenkins, R.E.; Farrell, J.; Meng, X.; Faulkner, L.; Whitaker, P.; Peckham, D.; Park, B.K.; Naisbitt, D.J. Detection of drug-responsive b lymphocytes and antidrug igg in patients with beta-lactam hypersensitivity. Allergy 2017, 72, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Klaewsongkram, J.; Thantiworasit, P.; Suthumchai, N.; Rerknimitr, P.; Sukasem, C.; Tuchinda, P.; Chularojanamontri, L.; Srinoulprasert, Y.; Rerkpattanapipat, T.; Chanprapaph, K.; et al. In vitro test to confirm diagnosis of allopurinol-induced severe cutaneous adverse reactions. Br. J. Dermatol. 2016, 175, 994–1002. [Google Scholar] [CrossRef] [PubMed]

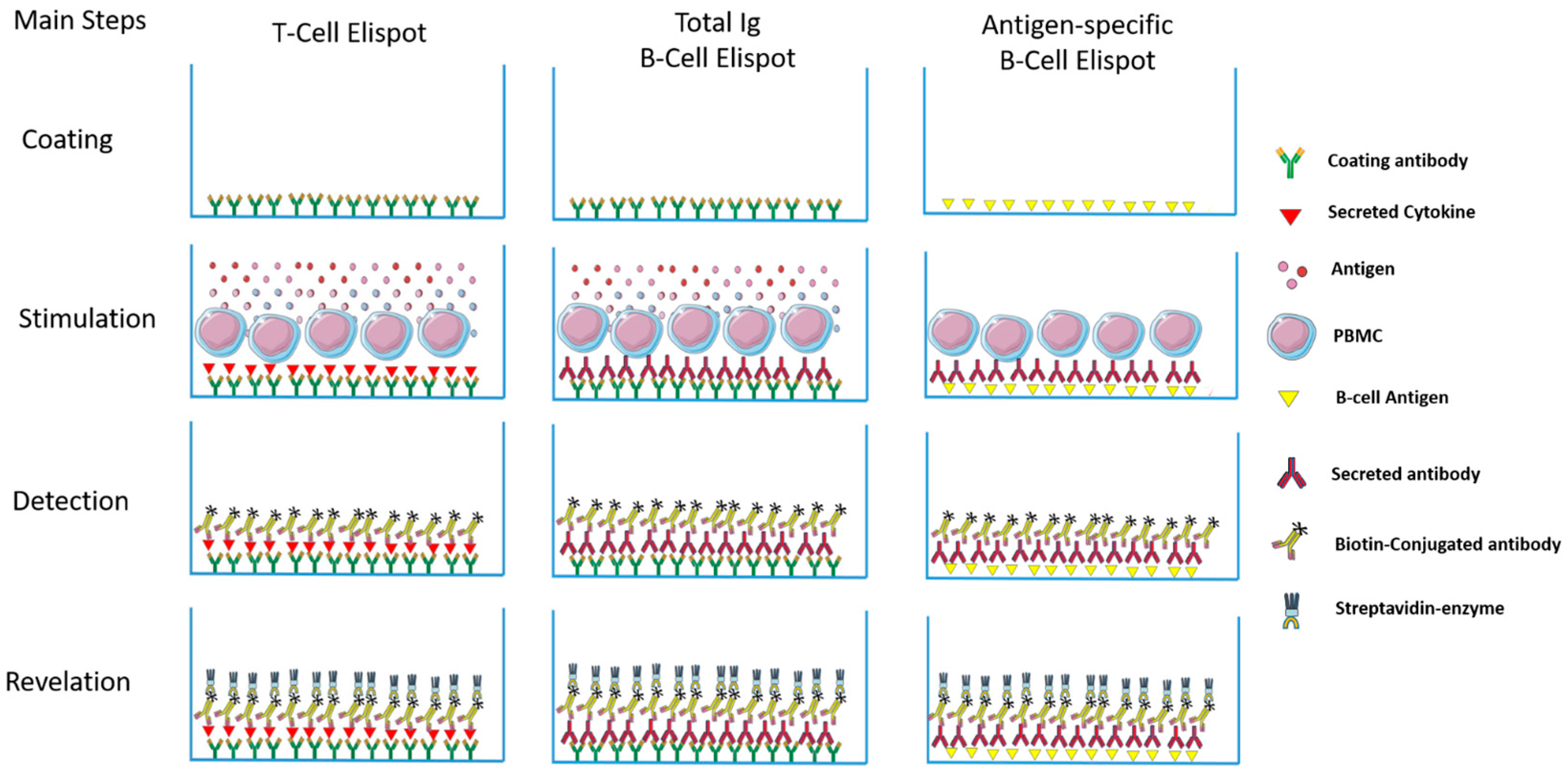
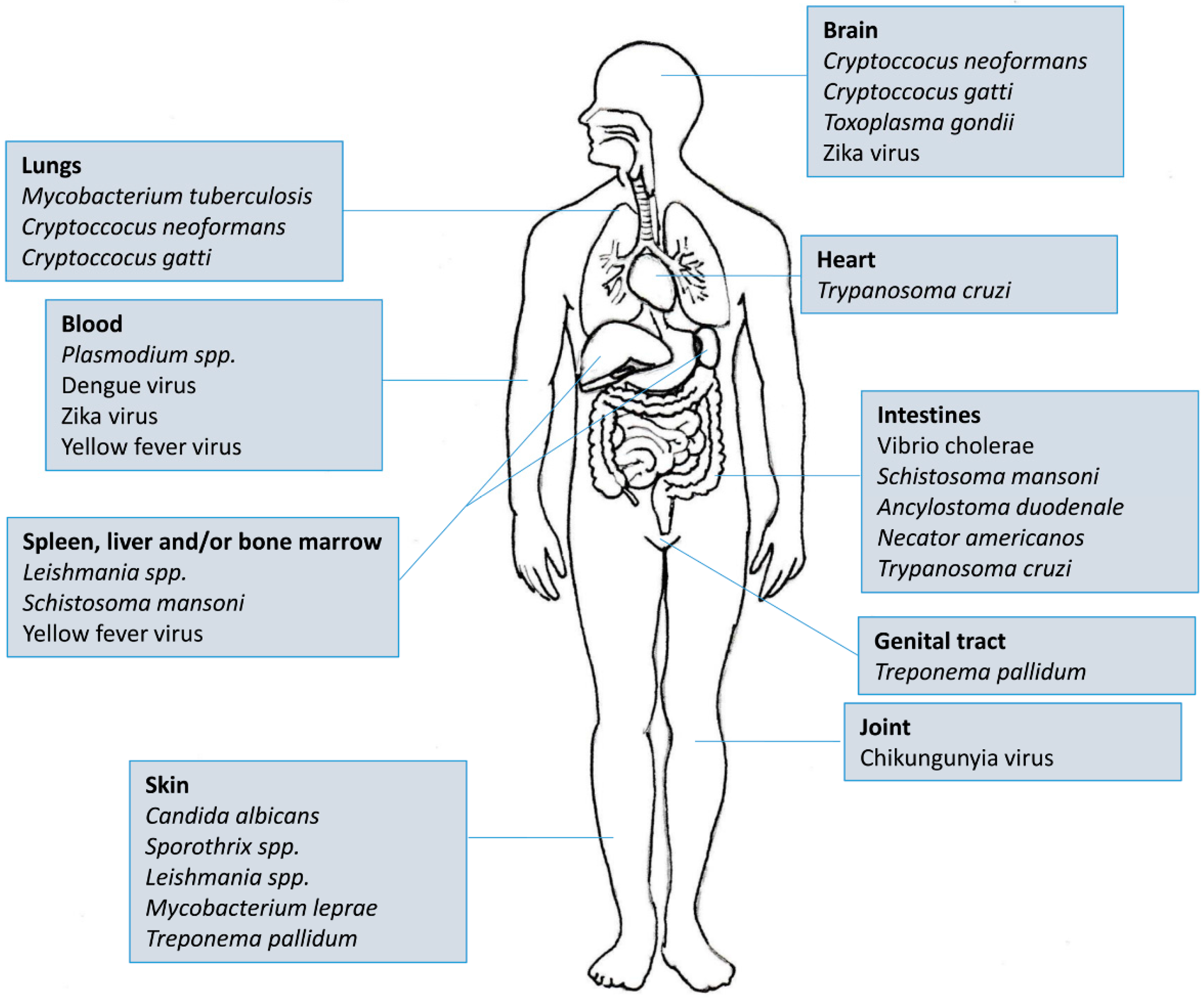

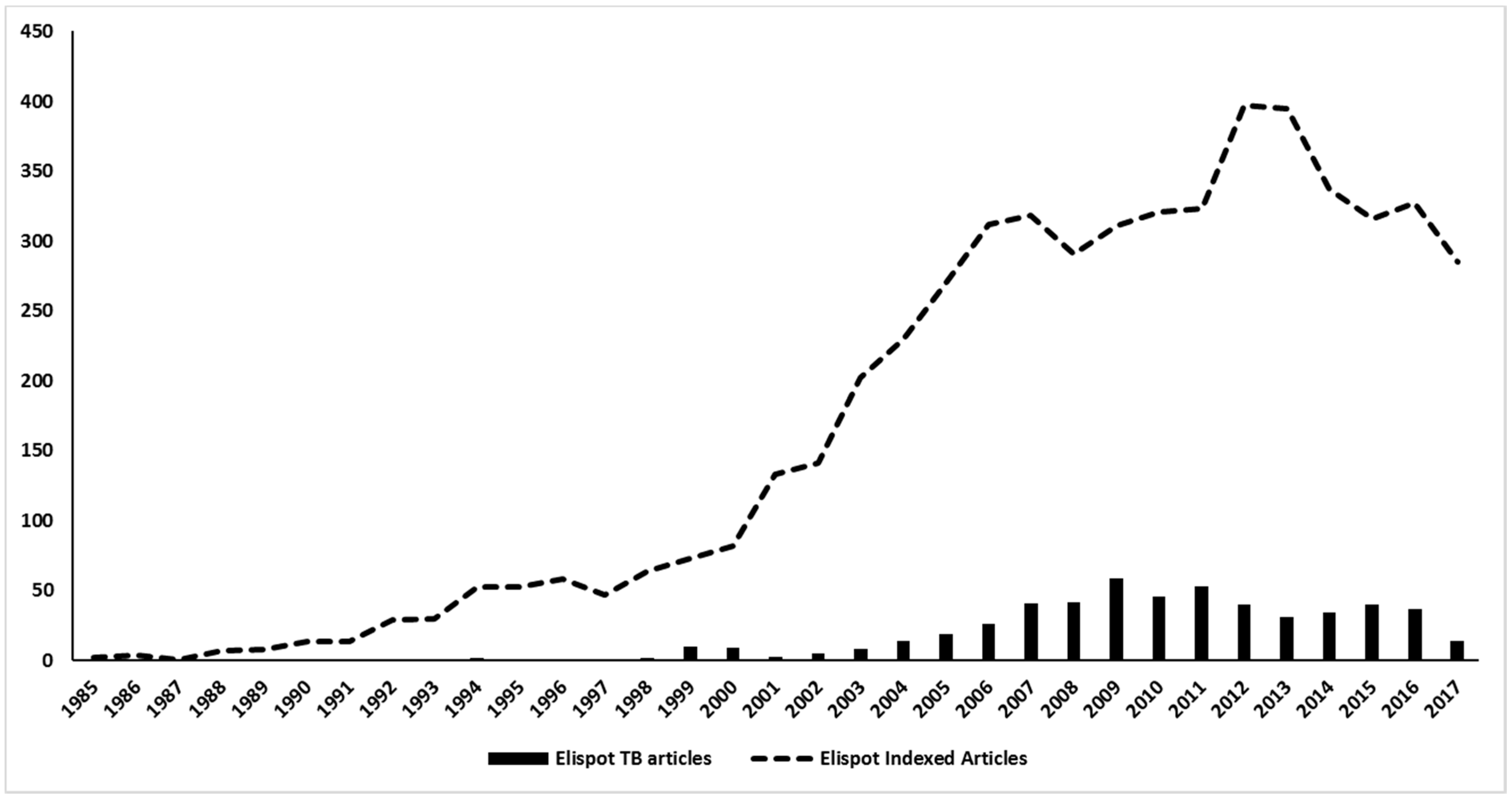
| Diagnosis/Prognosis | Pathogenesis | Vaccine development | Epitope identification | |
|---|---|---|---|---|
| Protozoan diseases | ||||
| Malaria | - | [12,13,14,25,26] | [15,16,17,18,19,20,21,22] | [23,24] |
| Toxoplasmosis | - | [27,30] | - | [28,29] |
| Chagas disease | - | [36] | - | [31,32,33,34,35] |
| Leishmaniasis | [43] | [42] | [39,41] | [40] |
| Tropical Arboviroses | ||||
| Dengue fever | [45,52,55,56,70] | [47,48,49,50,51,53] | [65,68] | [57,58,59,60,61,62,63,69] |
| Zika | - | - | - | [72] |
| Chikungunya | - | [73,74] | - | - |
| Yellow fever | - | - | [75,76,77] | [77] |
| Bacterial diseases | ||||
| Tuberculosis | [80,81,85,87,89,90] | [83,84] | [83] | [83] |
| Leprosy | [89,90] | [88] | - | - |
| Yaws | [91,92] | - | - | - |
| Cholera | [94] | - | - | [93] |
| Helminthiasis | ||||
| Schistosomiasis | - | [102] | [99,100] | - |
| Ancylostomiasis | - | [103,104] | - | - |
| Mycosis | ||||
| Candidiasis | [117,118] | [109,110,111,112,116] | [115] | [113,114] |
| Cryptoccocosis | [119,120] | - | - | - |
| Sporotrichosis | - | [121] | - | - |
| Mucormycosis | - | [122] | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima-Junior, J.D.C.; Morgado, F.N.; Conceição-Silva, F. How Can Elispot Add Information to Improve Knowledge on Tropical Diseases? Cells 2017, 6, 31. https://doi.org/10.3390/cells6040031
Lima-Junior JDC, Morgado FN, Conceição-Silva F. How Can Elispot Add Information to Improve Knowledge on Tropical Diseases? Cells. 2017; 6(4):31. https://doi.org/10.3390/cells6040031
Chicago/Turabian StyleLima-Junior, Josué Da Costa, Fernanda Nazaré Morgado, and Fátima Conceição-Silva. 2017. "How Can Elispot Add Information to Improve Knowledge on Tropical Diseases?" Cells 6, no. 4: 31. https://doi.org/10.3390/cells6040031





