Segmental Aging Underlies the Development of a Parkinson Phenotype in the AS/AGU Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Collection and Processing
2.2. Immunohistochemistry
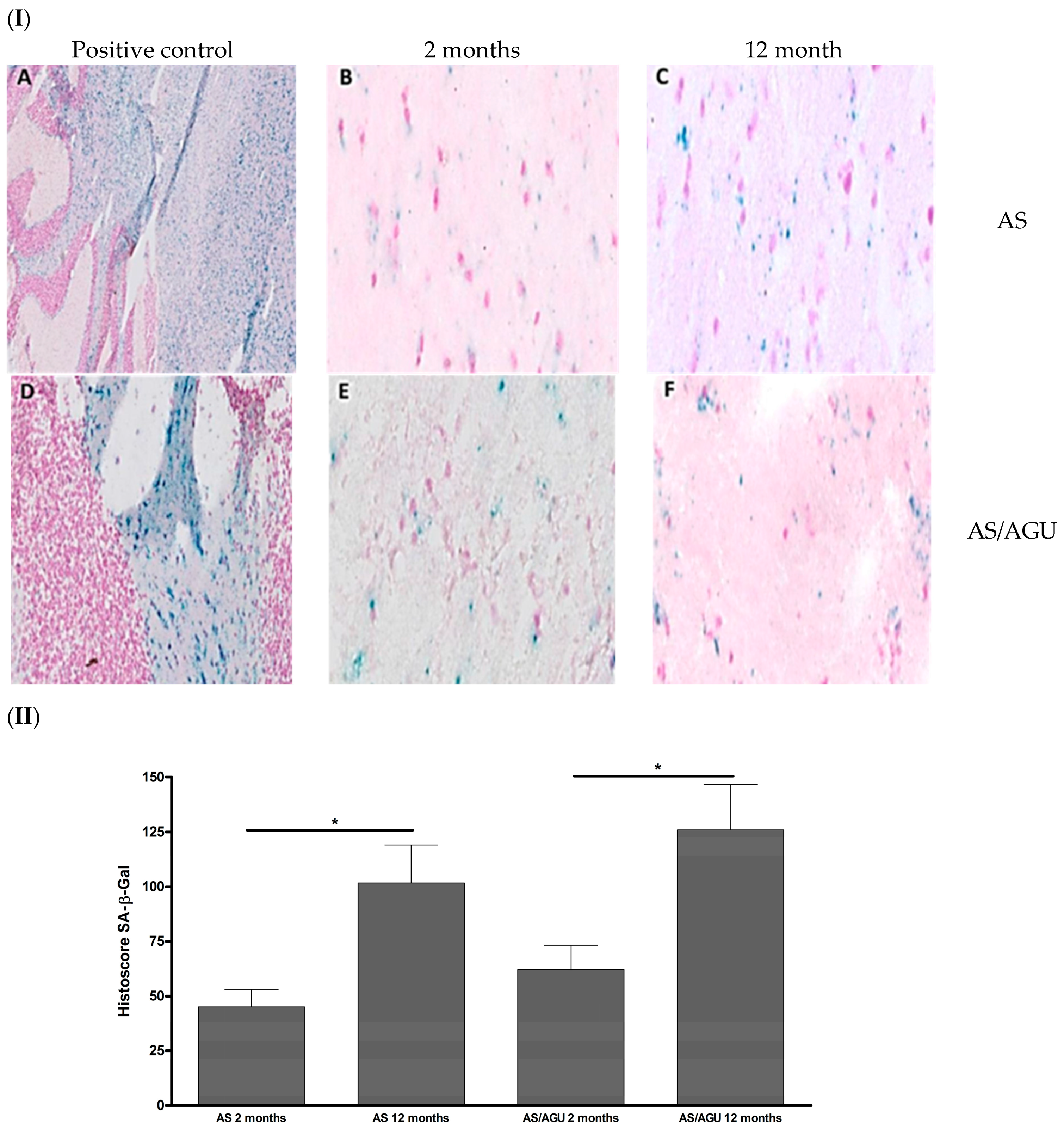
2.3. Senescence-Associated β-Galactosidase (SA-β-gal) Staining
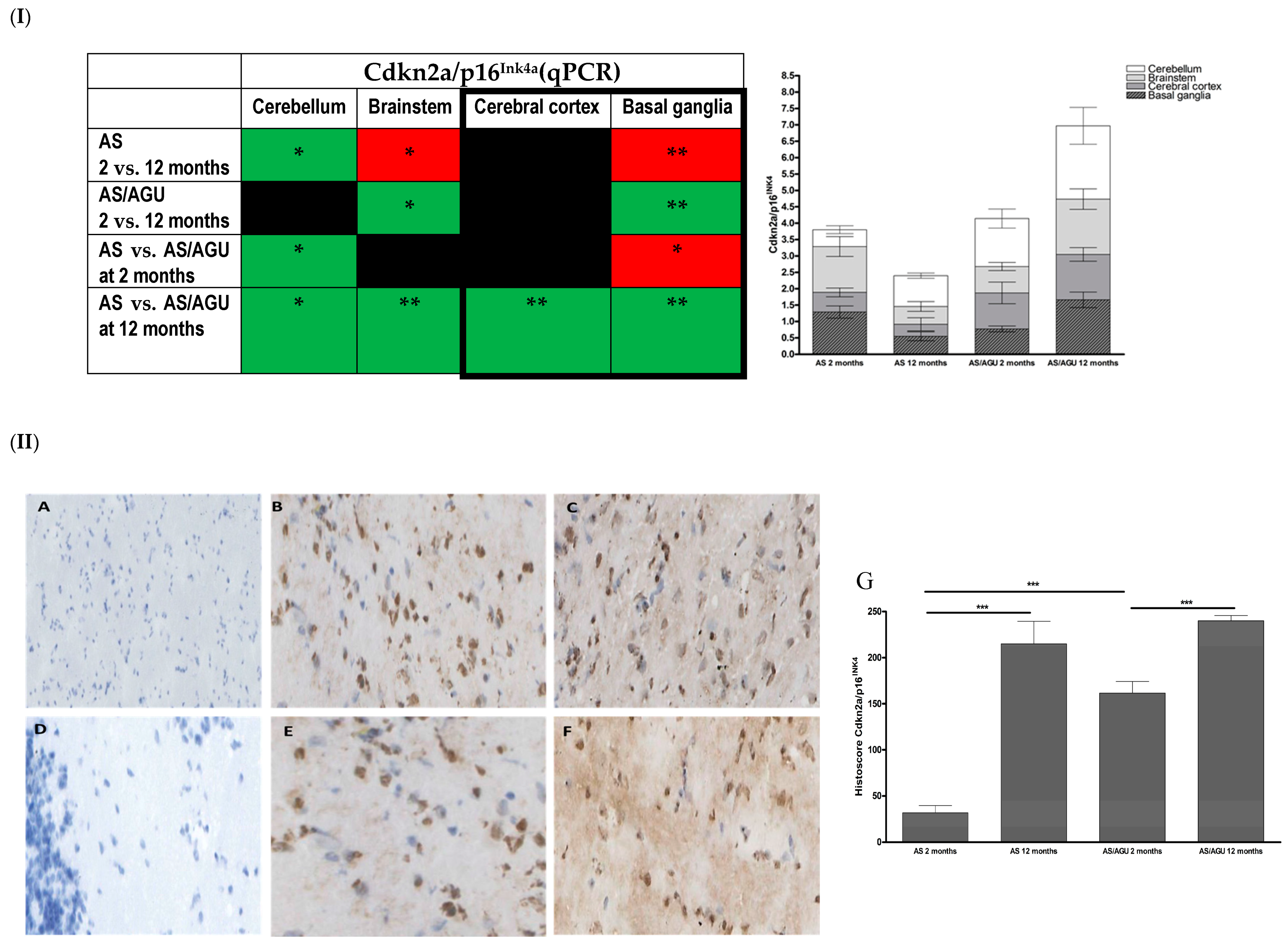
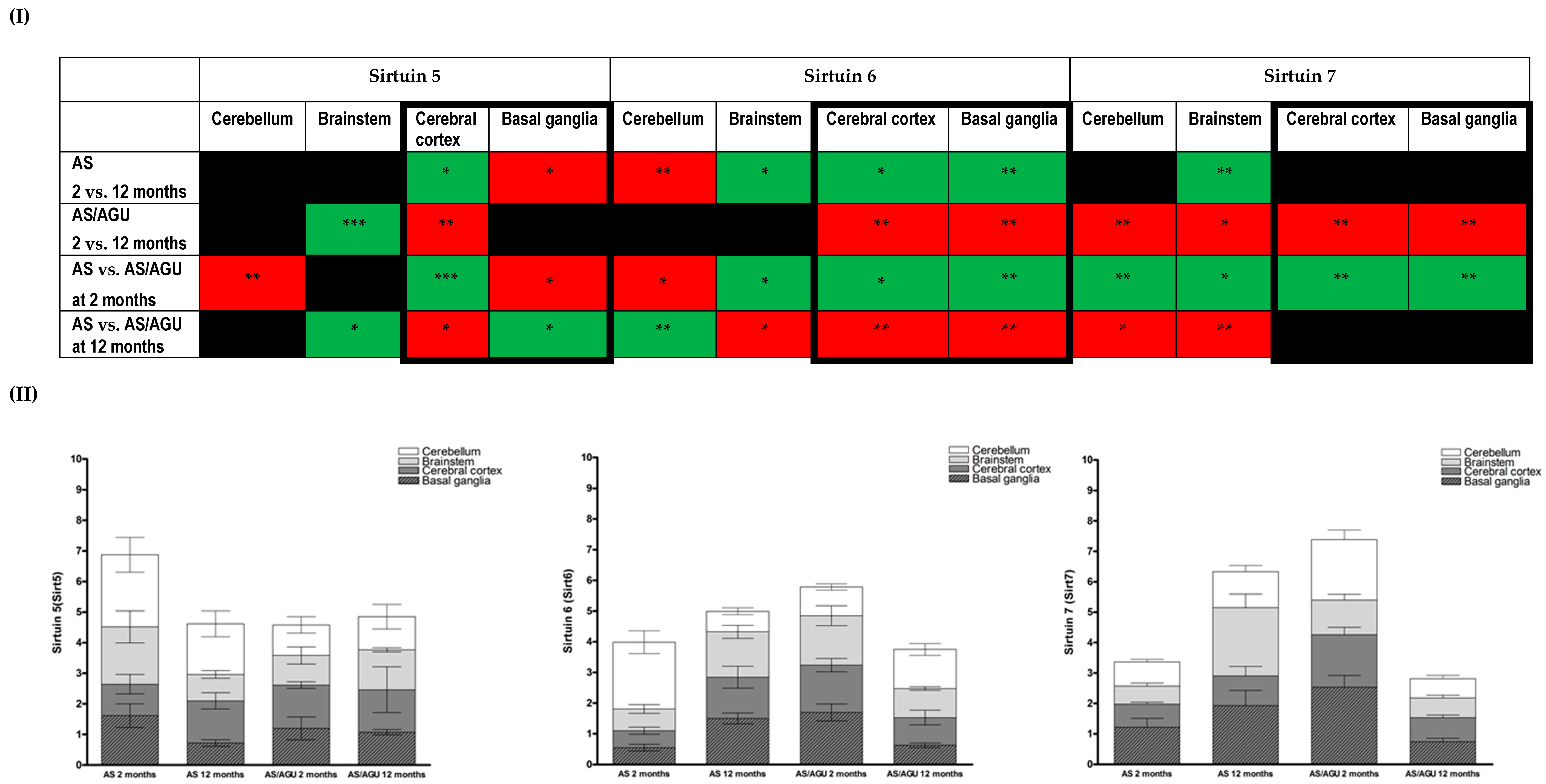
2.4. RNA Isolation and qPCR Analysis
2.5. Statistics
3. Results
3.1. AS/AGU Rat Brains Exhibit Features Consistent with Accelerated Aging
3.2. Age-Related Changes in Cell Stress in the Brain Are Reflected in Differing Expressions of Members of the Sirtuin Family
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clarke, D.J.; Payne, A.P. Neuroanatomical characterization of a new mutant rat with dopamine depletion in the substantia nigra. Eur. J. Neurosci. 1994, 6, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Gilmore, D.P.; Russell, D.; Growney, C.A.; Favor, G.; Kennedy, A.K.; Davies, R.W.; Payne, A.P.; Stone, T.W. Pharmacological analysis of extracellular dopamine and metabolites in the striatum of conscious as/agu rats, mutants with locomotor disorder. Neuroscience 2000, 100, 45–52. [Google Scholar] [CrossRef]
- Campbell, J.M.; Gilmore, D.P.; Russell, D.; Growney, C.A.; Favor, G.; Weir, J.; Stone, T.W.; Payne, A.P. Extracellular levels of dopamine and its metabolite 3,4-dihydroxy-phenylacetic acid measured by microdialysis in the corpus striatum of conscious as/agu mutant rats. Neuroscience 1998, 85, 323–325. [Google Scholar] [CrossRef]
- Campbell, J.M.; Payne, A.P.; Gilmore, D.P.; Russell, D.; McGadey, J.; Clarke, D.J.; Branton, R.; Davies, R.W.; Sutcliffe, R.G. Age changes in dopamine levels in the corpus striatum of albino swiss (as) and as/agu mutant rats. Neurosci. Lett. 1997, 239, 54–56. [Google Scholar] [CrossRef]
- Campbell, J.M.; Payne, A.P.; Gilmore, D.P.; Byrne, J.E.; Russell, D.; McGadey, J.; Clarke, D.J.; Davies, R.W.; Sutcliffe, R.G. Neostriatal dopamine depletion and locomotor abnormalities due to the albino swiss rat agu mutation. Neurosci. Lett. 1996, 213, 173–176. [Google Scholar] [CrossRef]
- Al-Fayez, M.; Russell, D.; Wayne Davies, R.; Shiels, P.G.; Baker, P.J.; Payne, A.P. Deficits in the mid-brain raphe nuclei and striatum of the as/agu rat, a protein kinase c-gamma mutant. Eur. J. Neurosci. 2005, 22, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.G.; Campbell, J.M.; Bennett, N.K.; Payne, A.P.; Davies, R.W.; Sutcliffe, R.G.; McCulloch, J. Local cerebral glucose utilization in the as/agu rat: A mutant with movement disorders. Eur. J. Neurosci. 1998, 10, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kano, M.; Abeliovich, A.; Chen, L.; Bao, S.; Kim, J.J.; Hashimoto, K.; Thompson, R.F.; Tonegawa, S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in pkc gamma mutant mice. Cell 1995, 83, 1233–1242. [Google Scholar] [CrossRef]
- Shirai, Y.; Segawa, S.; Kuriyama, M.; Goto, K.; Sakai, N.; Saito, N. Subtype-specific translocation of diacylglycerol kinase alpha and gamma and its correlation with protein kinase c. J. Biol. Chem. 2000, 275, 24760–24766. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, T.; Ueyama, T.; Yoshino, K.; Takahashi, H.; Adachi, N.; Ago, Y.; Koda, K.; Nashida, T.; Hiramatsu, N.; Matsuda, T.; et al. The role of pak-interacting exchange factor-beta phosphorylation at serines 340 and 583 by pkcgamma in dopamine release. J. Neurosci. 2014, 34, 9268–9280. [Google Scholar] [CrossRef] [PubMed]
- Shiels, P.G.; Ritzau-Reid, K. Biological aging, inflammation and nutrition: How might they impact on systemic sclerosis? Curr. Aging Sci. 2015, 8, 123–130. [Google Scholar] [CrossRef]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature aging. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Shiels, P.S.; Jeroen, P. Kooman, Dagmara McGuinness, Circulating markers of aging and allostatic load: A slow train coming. Pract. Lab. Med. 2016, 4, 1–88. [Google Scholar]
- Ferreira, L.K.; Busatto, G.F. Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 2013, 37, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Strong, R. Neurochemical changes in the aging human brain: Implications for behavioral impairment and neurodegenerative disease. Geriatrics 1998, 53 (Suppl. S1), S9–S12. [Google Scholar] [PubMed]
- Cunnane, S.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain fuel metabolism, aging, and alzheimer’s disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Aging and parkinson’s disease: Why is advancing age the biggest risk factor? Aging Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Gingell-Littlejohn, M.; McGuinness, D.; McGlynn, L.M.; Kingsmore, D.; Stevenson, K.S.; Koppelstaetter, C.; Clancy, M.J.; Shiels, P.G. Pre-transplant cdkn2a expression in kidney biopsies predicts renal function and is a future component of donor scoring criteria. PLoS ONE 2013, 8, e68133. [Google Scholar] [CrossRef] [PubMed]
- Shiels, P.G.; Davies, R.W. Ageing and death. In Neurons in Molecular Biology of the Neuron, 2nd ed.; Davies, R.W., Morris, B.J., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 436–464. [Google Scholar]
- McGuinness, D.; McGuinness, D.H.; McCaul, J.A.; Shiels, P.G. Sirtuins, bioaging, and cancer. J. Aging Res. 2011, 2011, 235754. [Google Scholar] [CrossRef] [PubMed]
- Sidorova-Darmos, E.; Wither, R.G.; Shulyakova, N.; Fisher, C.; Ratnam, M.; Aarts, M.; Lilge, L.; Monnier, P.P.; Eubanks, J.H. Differential expression of sirtuin family members in the developing, adult, and aged rat brain. Front. Aging Neurosci. 2014, 6, 333. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential expression of sirtuins in the aging rat brain. Front. Cell. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Peritore, C.; Ginsberg, J.; Shih, J.; Arun, S.; Donmez, G. Protective role of sirt5 against motor deficit and dopaminergic degeneration in mptp-induced mice model of parkinson’s disease. Behav. Brain Res. 2015, 281, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Schwer, B.; Schumacher, B.; Lombard, D.B.; Xiao, C.; Kurtev, M.V.; Gao, J.; Schneider, J.I.; Chai, H.; Bronson, R.T.; Tsai, L.H.; et al. Neural sirtuin 6 (sirt6) ablation attenuates somatic growth and causes obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 21790–21794. [Google Scholar] [CrossRef] [PubMed]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian sirt6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C.; Zwaans, B.M.; Silberman, D.M.; Gymrek, M.; Goren, A.; Zhong, L.; Ram, O.; Truelove, J.; Guimaraes, A.R.; Toiber, D.; et al. The histone deacetylase sirt6 is a tumor suppressor that controls cancer metabolism. Cell 2012, 151, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Michishita, E.; McCord, R.A.; Boxer, L.D.; Barber, M.F.; Hong, T.; Gozani, O.; Chua, K.F. Cell cycle-dependent deacetylation of telomeric histone h3 lysine k56 by human sirt6. Cell. Cycle 2009, 8, 2664–2666. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian sir2 homolog sirt7 is an activator of rna polymerase i transcription. Gene. Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Germain, D. Sirtuins and the estrogen receptor as regulators of the mammalian mitochondrial upr in cancer and aging. Adv. Cancer Res. 2016, 130, 211–256. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time pcr data by the comparative c(t) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Kooman, J.P.; Shiels, P.G. Nutrients and aging: What can we learn about aging interactions from animal biology? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.P.; Shiels, P.G.; Stenvinkel, P. Premature aging in chronic kidney disease and chronic obstructive pulmonary disease: Similarities and differences. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; McGuinness, D.; Swann, R.; Barclay, S.; Mills, P.R.; Patel, A.H.; McLauchlan, J.; Shiels, P.G. Non cell autonomous upregulation of cdkn2 transcription linked to progression of chronic hepatitis c disease. Aging Cell 2013, 12, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Pathai, S.; Lawn, S.D.; Gilbert, C.E.; McGuinness, D.; McGlynn, L.; Weiss, H.A.; Port, J.; Christ, T.; Barclay, K.; Wood, R.; et al. Accelerated biological aging in hiv-infected individuals in south africa: A case-control study. Aids 2013, 27, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative aging of human endothelial cells. J. Cell Sci. 2000, 113 ( Pt. 20), 3613–3622. [Google Scholar] [PubMed]
- Satyanarayana, A.; Greenberg, R.A.; Schaetzlein, S.; Buer, J.; Masutomi, K.; Hahn, W.C.; Zimmermann, S.; Martens, U.; Manns, M.P.; Rudolph, K.L. Mitogen stimulation cooperates with telomere shortening to activate DNA damage responses and senescence signaling. Mol. Cell. Biol. 2004, 24, 5459–5474. [Google Scholar] [CrossRef] [PubMed]
- Fanton, C.P.; McMahon, M.; Pieper, R.O. Dual growth arrest pathways in astrocytes and astrocytic tumors in response to raf-1 activation. J. Biol. Chem. 2001, 276, 18871–18877. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte senescence as a component of alzheimer’s disease. PLoS ONE 2012, 7, e45069. [Google Scholar] [CrossRef] [PubMed]
- Aureli, M.; Loberto, N.; Lanteri, P.; Chigorno, V.; Prinetti, A.; Sonnino, S. Cell surface sphingolipid glycohydrolases in neuronal differentiation and aging in culture. J. Neurochem. 2011, 116, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.Q.; Guan, J.T.; Xu, X.H.; Fu, Y.C. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochem. Biophys. Res. Commun. 2010, 396, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wang, C.; Miwa, S.; Maddick, M.; Korolchuk, V.; Tsolou, A.; Gonos, E.S.; Thrasivoulou, C.; Saffrey, M.J.; Cameron, K.; et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 2012, 11, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Abdouh, M.; Chatoo, W.; El Hajjar, J.; David, J.; Ferreira, J.; Bernier, G. Bmi1 is down-regulated in the aging brain and displays antioxidant and protective activities in neurons. PLoS ONE 2012, 7, e31870. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Podlutsky, A.; Sosnowska, D.; Tucsek, Z.; Toth, P.; Deak, F.; Gautam, T.; Csiszar, A.; Sonntag, W.E. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: Role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J. Gerontol. Biol. Med. Sci. 2013, 68, 1443–1457. [Google Scholar]
- Li, G.; Cheng, H.; Zhang, X.; Shang, X.; Xie, H.; Zhang, X.; Yu, J.; Han, J. Hippocampal neuron loss is correlated with cognitive deficits in samp8 mice. Neurol. Sci. 2013, 34, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Gomis-Coloma, C.; Morenilla-Palao, C.; Peiro, G.; Serra, E.; Serrano, M.; Cabedo, H. Epigenetic induction of the ink4a/arf locus prevents schwann cell overproliferation during nerve regeneration and after tumorigenic challenge. Brain 2013, 136, 2262–2278. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.L.; Misuraca, K.; Cordero, F.; Dobrikova, E.; Min, H.D.; Gromeier, M.; Kirsch, D.G.; Becher, O.J. Pd-0332991, a cdk4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS ONE 2013, 8, e77639. [Google Scholar] [CrossRef] [PubMed]
- Oshikawa, M.; Okada, K.; Nakajima, K.; Ajioka, I. Cortical excitatory neurons become protected from cell division during neurogenesis in an rb family-dependent manner. Development 2013, 140, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Checler, F.; Alves da Costa, C. P53 in neurodegenerative diseases and brain cancers. Pharmacol. Ther. 2014, 142, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Rachmany, L.; Tweedie, D.; Rubovitch, V.; Yu, Q.S.; Li, Y.; Wang, J.Y.; Pick, C.G.; Greig, N.H. Cognitive impairments accompanying rodent mild traumatic brain injury involve p53-dependent neuronal cell death and are ameliorated by the tetrahydrobenzothiazole pft-alpha. PLoS ONE 2013, 8, e79837. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. Nad(+) in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Cives, M.; Della-Morte, D.; Capuani, B.; Lauro, D.; Guadagni, F.; Silvestris, F. Sirtuins and cancer: Role in the epithelial-mesenchymal transition. Oxidative Med. Cell. Longev. 2016, 2016, 3031459. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Goligorsky, M.S. Sirtuins, cell senescence, and vascular aging. Canadian J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shan, T.Z.; Zhu, L.N.; Wu, T.; Guo, J.; Wang, Y.Z. Effect of breed on the expression of sirtuins (sirt1–7) and antioxidant capacity in porcine brain. Animal 2013, 7, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Li, Q.; Feng, R.L.; Wen, T.Q. Transcription levels of sirtuin family in neural stem cells and brain tissues of adult mice. Cell. Mol. Biol. 2012, 58, OL1737–O1743. [Google Scholar] [PubMed]
- Glorioso, C.; Oh, S.; Douillard, G.G.; Sibille, E. Brain molecular aging, promotion of neurological disease and modulation by sirtuin 5 longevity gene polymorphism. Neurobiol. Diss 2011, 41, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Glorioso, C.; Sibille, E. Between destiny and disease: Genetics and molecular pathways of human central nervous system aging. Prog. Neurobiol. 2011, 93, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.; Makela, J.; Di Liberto, V.; Mudo, G.; Belluardo, N.; Eriksson, O.; Saarma, M. Current disease modifying approaches to treat parkinson’s disease. Cell. Mol. Life Sci. 2016, 73, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Rardin, M.J.; He, W.; Nishida, Y.; Newman, J.C.; Carrico, C.; Danielson, S.R.; Guo, A.; Gut, P.; Sahu, A.K.; Li, B.; et al. Sirt5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013, 18, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Rardin, M.J.; Carrico, C.; He, W.; Sahu, A.K.; Gut, P.; Najjar, R.; Fitch, M.; Hellerstein, M.; Gibson, B.W.; et al. Sirt5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol. Cell 2015, 59, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, C.; Gertz, M.; Papatheodorou, P.; Kachholz, B.; Becker, C.F.; Steegborn, C. Substrates and regulation mechanisms for the human mitochondrial sirtuins sirt3 and sirt5. J. Mol. Biol. 2008, 382, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.; Steegborn, C. Using mitochondrial sirtuins as drug targets: Disease implications and available compounds. Cell. Mol. Life Sci. 2016, 73, 2871–2896. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Lomb, D.J.; Haigis, M.C.; Guarente, L. Sirt5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009, 137, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin sirt6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Peshti, V.; Gil, R.; Naiman, S.; Nahum, L.; Levin, E.; Kronfeld-Schor, N.; Cohen, H.Y. Sirt6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010, 9, 162–173. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khojah, S.M.; Payne, A.P.; McGuinness, D.; Shiels, P.G. Segmental Aging Underlies the Development of a Parkinson Phenotype in the AS/AGU Rat. Cells 2016, 5, 38. https://doi.org/10.3390/cells5040038
Khojah SM, Payne AP, McGuinness D, Shiels PG. Segmental Aging Underlies the Development of a Parkinson Phenotype in the AS/AGU Rat. Cells. 2016; 5(4):38. https://doi.org/10.3390/cells5040038
Chicago/Turabian StyleKhojah, Sohair M., Anthony P. Payne, Dagmara McGuinness, and Paul G. Shiels. 2016. "Segmental Aging Underlies the Development of a Parkinson Phenotype in the AS/AGU Rat" Cells 5, no. 4: 38. https://doi.org/10.3390/cells5040038




