Generation and Characterization of Vascular Smooth Muscle Cell Lines Derived from a Patient with a Bicuspid Aortic Valve
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture of Primary VSMCs
2.2. Generating the WG-59 Cell Line from Primary VSMC Culture
2.3. Fluorescence in Situ Hybridization Mapping of the SV-40 Early Region in VSMCs after Transfection
2.4. Immunofluorescence for Large T-Antigen in the WG-59 Cell Line
2.5. Chromosome Analysis with Spectral Karyotyping of the WG-59 Cell Line
2.6. Highly Polymorphic Short Tandem Repeat Profiling
2.7. Characterization of the WG-59 Cell Line
2.8. Collagen-Based Cell Contraction Assay
2.9. TGFβ Pathway and Data Analysis
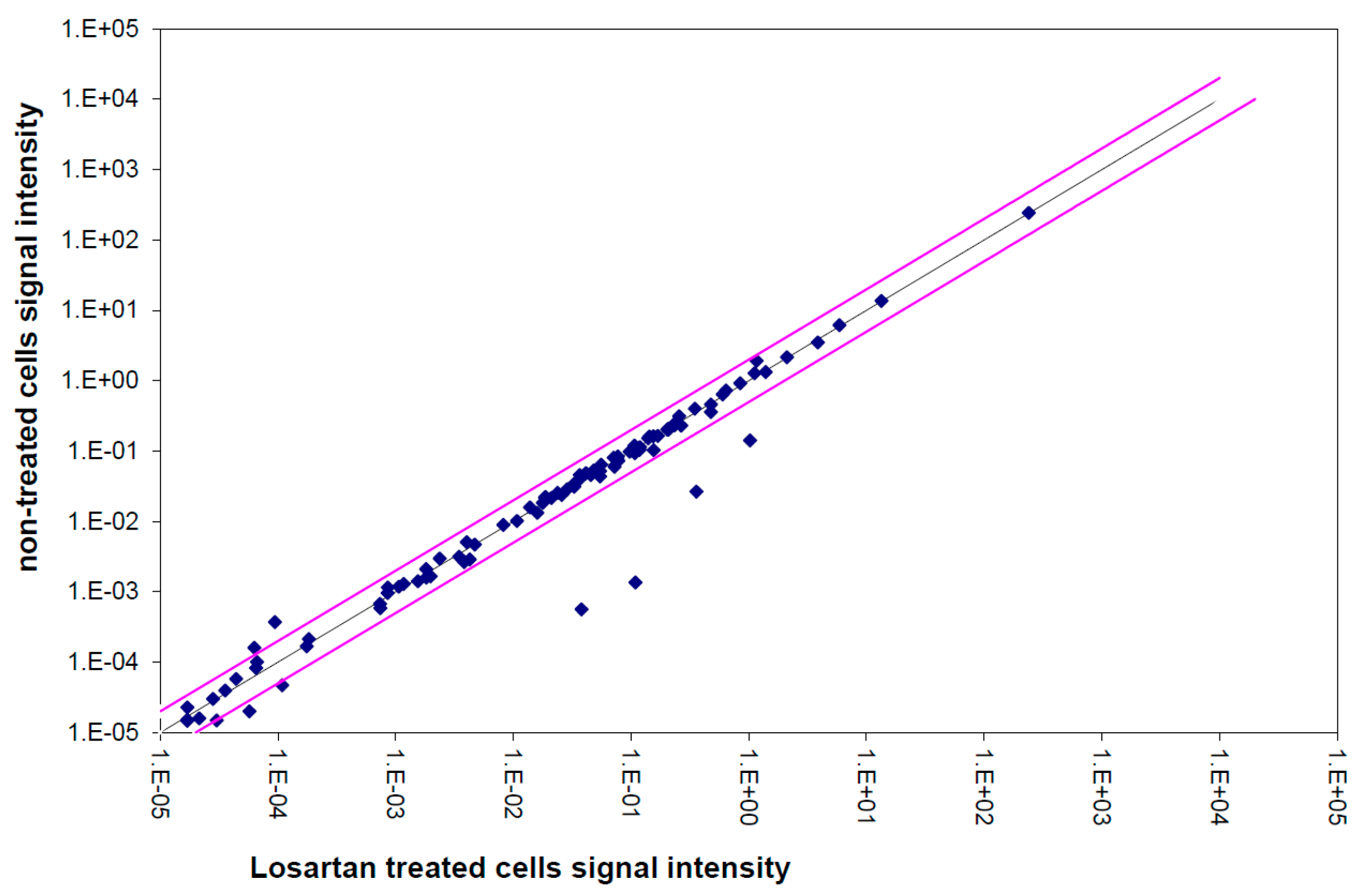
2.10. Single Gene Expression Analysis
3. Results
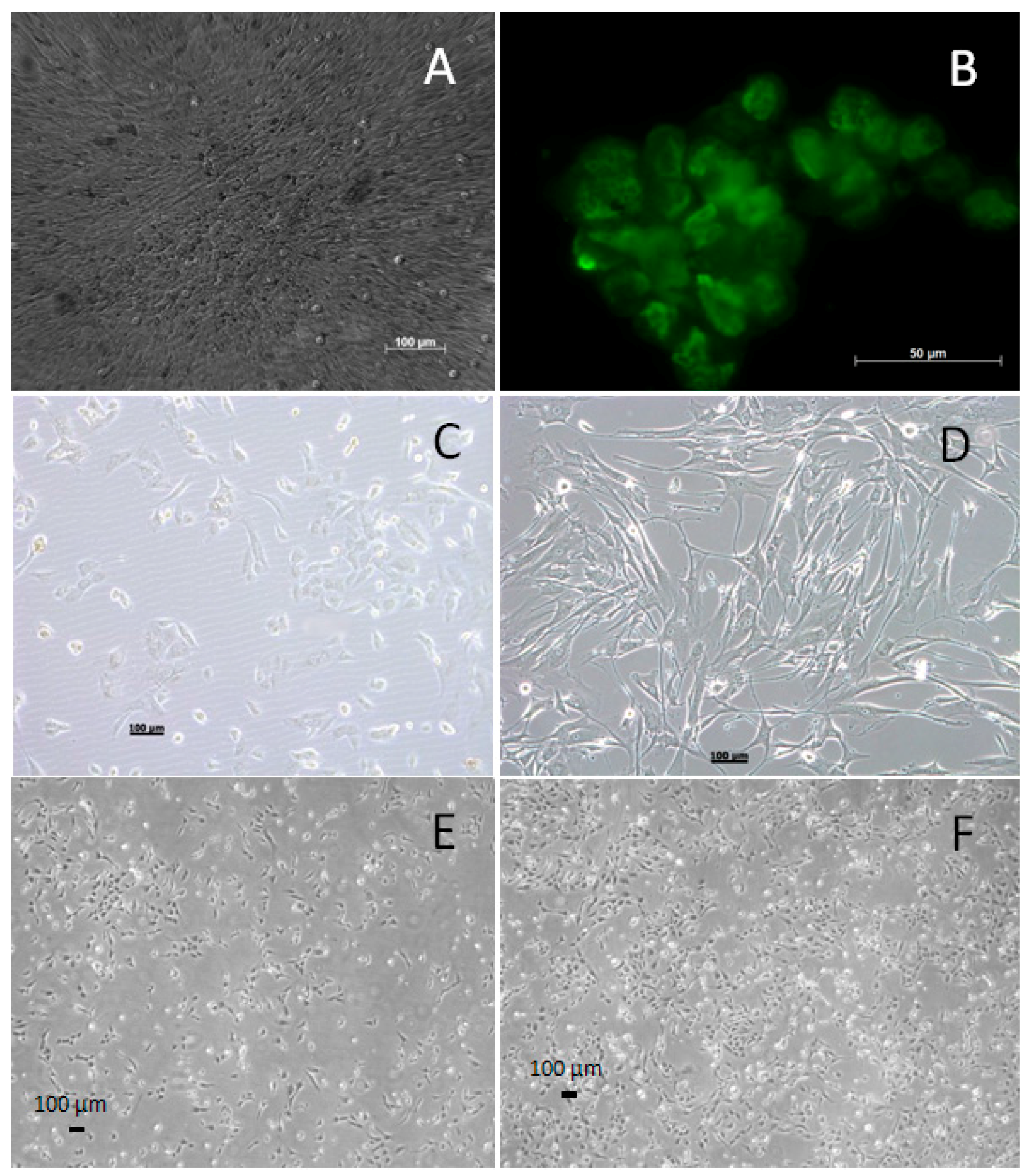
3.1. Generating and Characterizing the WG-59 Cell Line
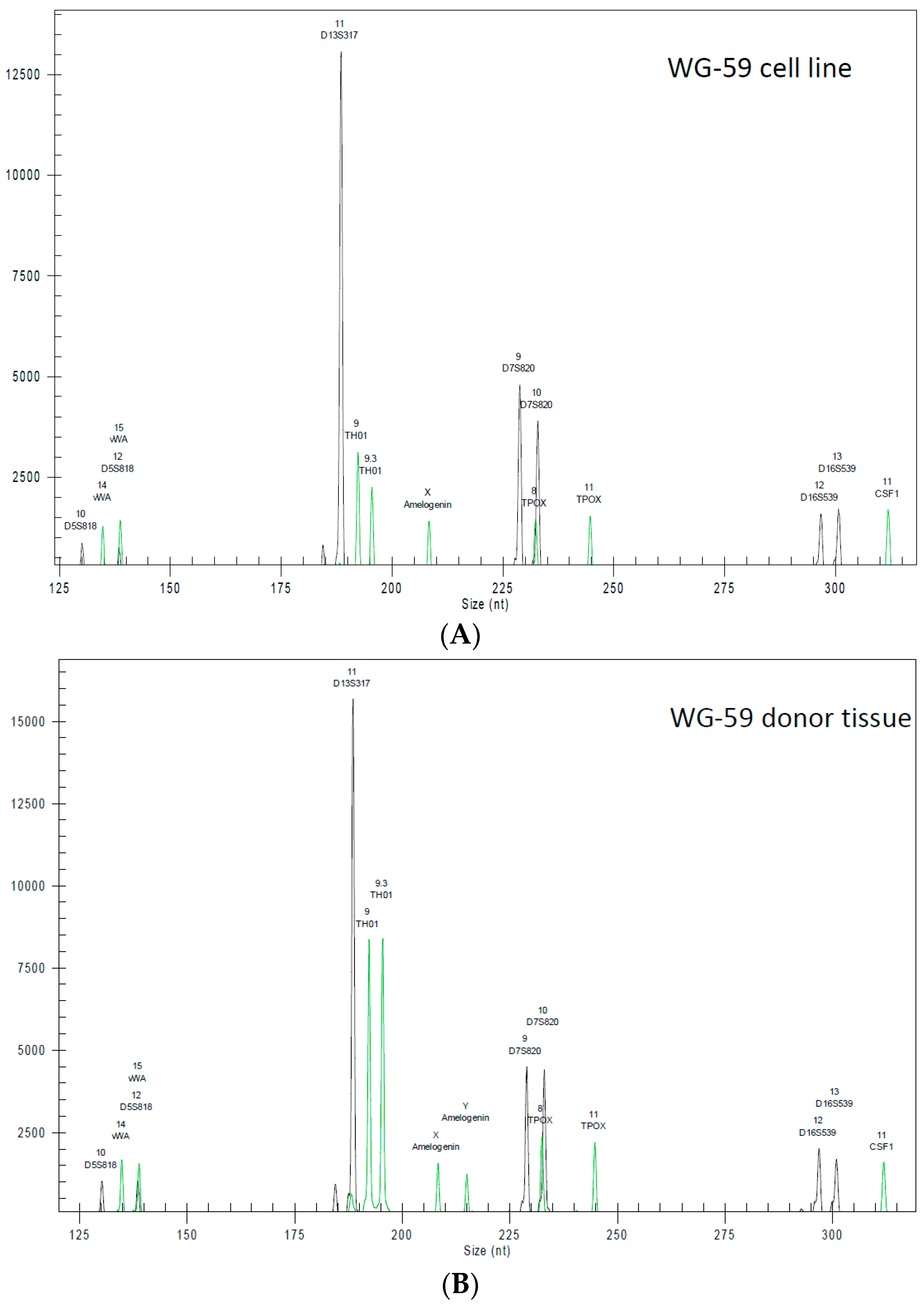
3.2. Cytogenic Characteristics and DNA Profile of the WG-59 Cell Line
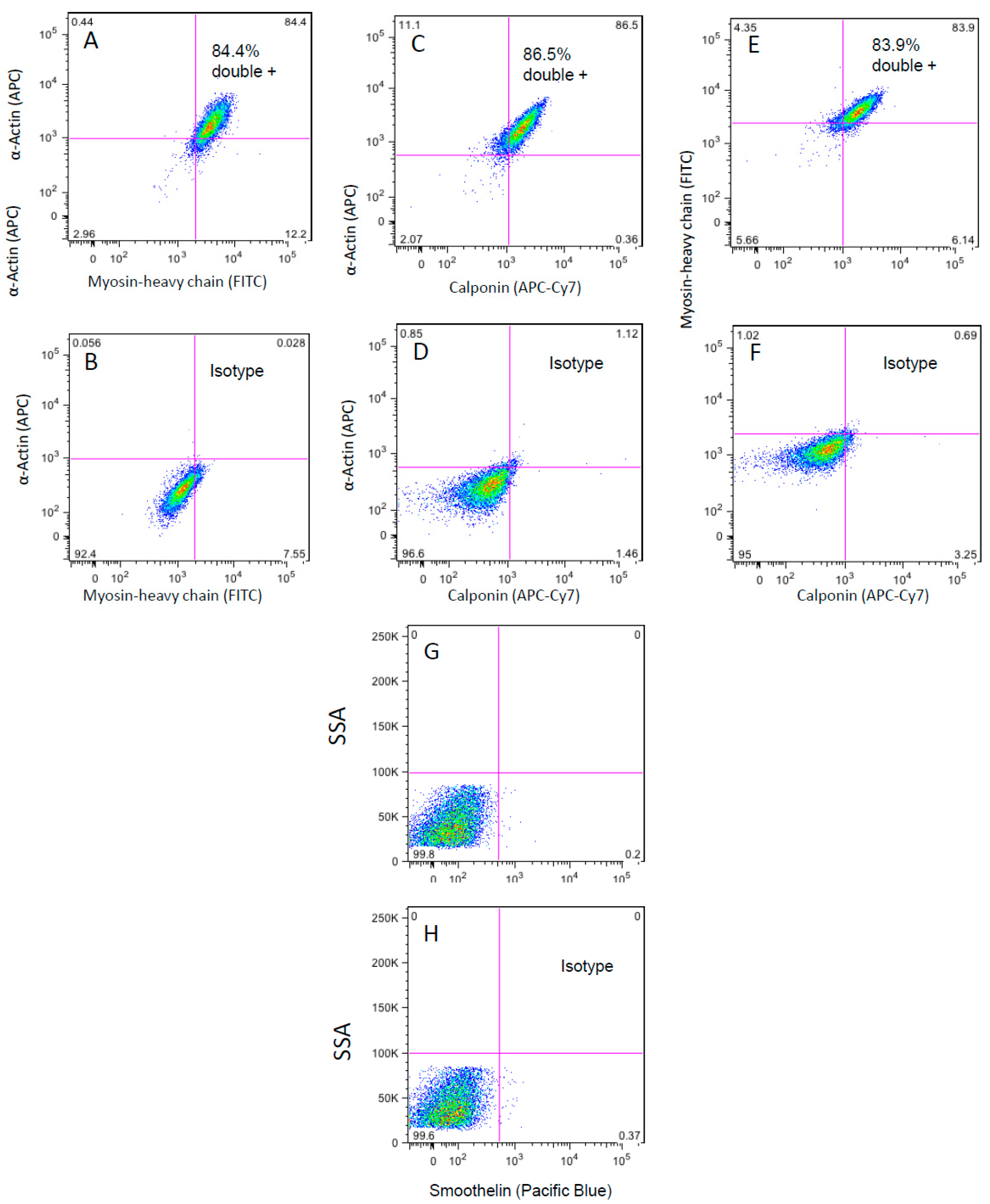
3.3. Flow Cytometry Analysis of the WG-59 Cell Line
3.4. Cell Contraction Assay
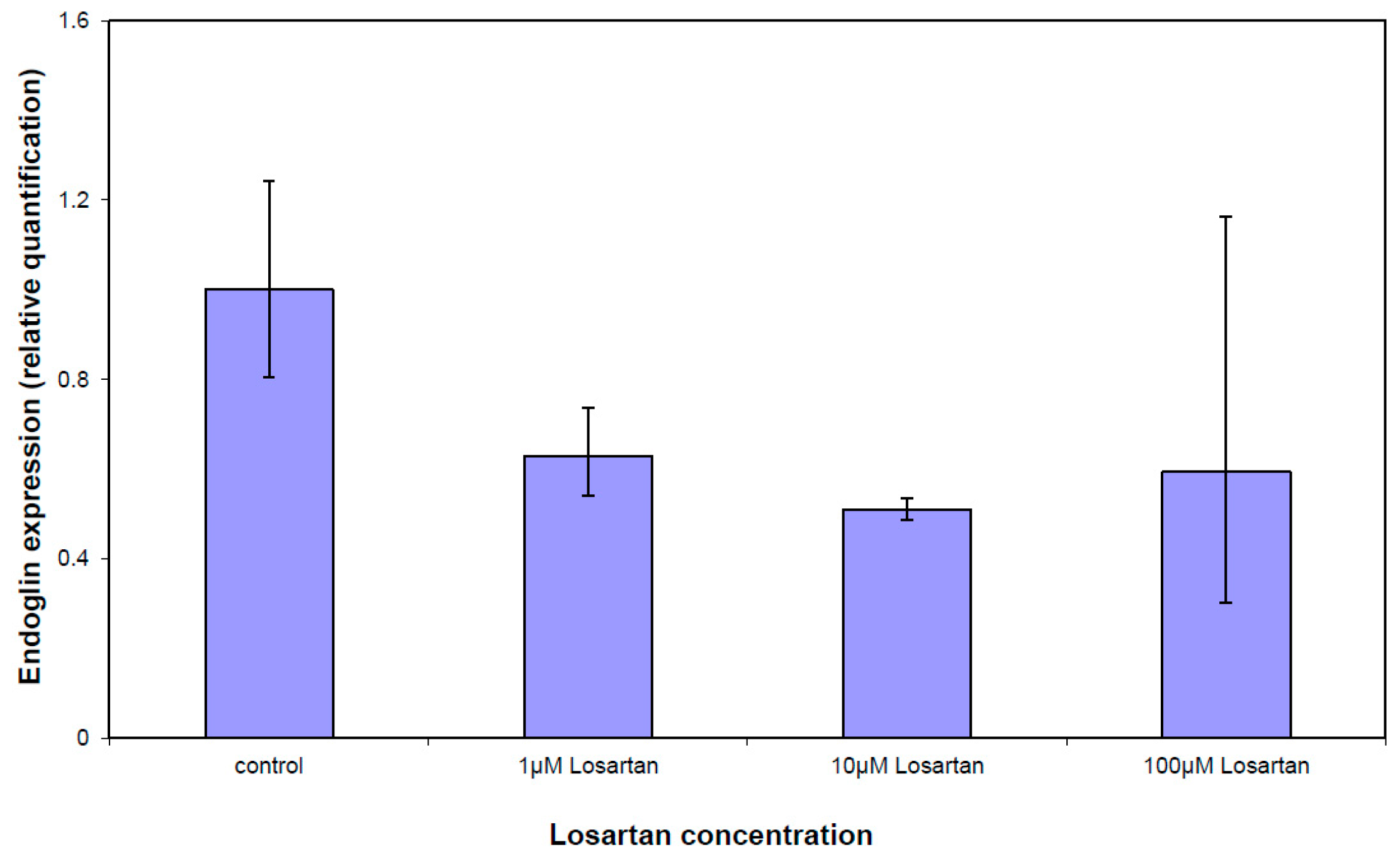
3.5. Gene Expression Analysis of the TGFβ Pathway
4. Discussion
5. Study Limitation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frid, M.G.; Dempsey, E.C.; Durmowicz, A.G.; Stenmark, K.R. Smooth muscle cell heterogeneity in pulmonary and systemic vessels. Importance in vascular disease. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Schaper, W.; Ito, W.D. Molecular mechanisms of coronary collateral vessel growth. Circ.Res. 1996, 79, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.F.; Zahradka, P. Vascular smooth muscle cell motility: From migration to invasion. Exp. Clin. Cardiol. 2010, 15, e75–e85. [Google Scholar] [PubMed]
- Lopez-Candales, A.; Holmes, D.R.; Liao, S.; Scott, M.J.; Wickline, S.A.; Thompson, R.W. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathol. 1997, 150, 993–1007. [Google Scholar] [PubMed]
- Milewicz, D.M.; Kwartler, C.S.; Papke, C.L.; Regalado, E.S.; Cao, J.; Reid, A.J. Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: Evidence for a hyperplastic vasculomyopathy. Genet. Med. 2010, 12, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Willing, M.C.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; Klautz, R.J.; Lindeman, J.H.; Goumans, M.J.; Palmen, M.; Mohamed, S.A.; Sievers, H.H.; Bogers, A.J.; et al. Ascending aorta dilation in association with bicuspid aortic valve: A maturation defect of the aortic wall. J. Thorac. Cardiovasc. Surg. 2014, 148, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A.; Body, S.C.; Booher, A.M.; Schaefers, H.J.; Milewski, R.K.; Michelena, H.I.; Evangelista, A.; Pibarot, P.; Mathieu, P.; Limongelli, G.; et al. Surgical treatment of bicuspid aortic valve disease: Knowledge gaps and research perspectives. J. Thorac Cardiovasc Surg. 2014, 147, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.; David, T.E.; Borger, M.; Verma, S.; Butany, J.; Weisel, R.D. Bicuspid aortic valve disease: Recent insights in pathophysiology and treatment. Expert. Rev. Cardiovasc Ther. 2005, 3, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Waldo, K.L. Role of neural crest in congenital heart disease. Circulation 1990, 82, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.E.; Dietz, H.C. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011, 473, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, B.; Bartnitzke, S.; Hartl, M.; Bullerdiek, J. In vitro transformation by the SV40 'early region' of cells from a human benign salivary gland tumor with a 12q13→q15 rearrangement. Cytogenet. Cell Genet. 1990, 53, 37–39. [Google Scholar] [CrossRef]
- Golledge, J.; Eagle, K.A. Acute aortic dissection. Lancet 2008, 372, 55–66. [Google Scholar] [CrossRef]
- He, R.; Guo, D.C.; Estrera, A.L.; Safi, H.J.; Huynh, T.T.; Yin, Z.; Cao, S.N.; Lin, J.; Kurian, T.; Buja, L.M.; et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006, 131, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Shein, H.M.; Enders, J.F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc. Soc. Exp. Biol. Med. 1962, 109, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Girardi, A.J.; Jensen, F.C.; Koprowski, H. SV40-Induced transformation of human diploid cells: Crisis and recovery. J. Cell Physiol. 1965, 65, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E.; Werbin, H. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta 1991, 1072, 1–7. [Google Scholar] [PubMed]
- Beamish, J.A.; He, P.; Kottke-Marchant, K.; Marchant, R.E. Molecular regulation of contractile smooth muscle cell phenotype: Implications for vascular tissue engineering. Tissue Eng Part B Rev. 2010, 16, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Misfeld, M.; Hanke, T.; Charitos, E.I.; Bullerdiek, J.; Belge, G.; Kuehnel, W.; Sievers, H.H. Inhibition of caspase-3 differentially affects vascular smooth muscle cell apoptosis in the concave versus convex aortic sites in ascending aneurysms with a bicuspid aortic valve. Ann. Anat. 2010, 192, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Sievers, H.H. Ascending Aneurysms in Bicuspid Aortic Valve; INTECH Open Access Publisher: Rijeka, Croatia, 2011; pp. 161–174. [Google Scholar]
- Bonderman, D.; Gharehbaghi-Schnell, E.; Wollenek, G.; Maurer, G.; Baumgartner, H.; Lang, I.M. Mechanisms underlying aortic dilatation in congenital aortic valve malformation. Circulation 1999, 99, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.X.; Bielenberg, K.; Schneider, A.; Haussler, A.; Keyser, A.; Birnbaum, D. Ascending aortic aneurysm associated with bicuspid and tricuspid aortic valve: Involvement and clinical relevance of smooth muscle cell apoptosis and expression of cell death-initiating proteins. Eur. J. Cardiothorac. Surg. 2003, 23, 537–543. [Google Scholar] [CrossRef]
- Nataatmadja, M.; West, M.; West, J.; Summers, K.; Walker, P.; Nagata, M.; Watanabe, T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation 2003, 108 (Suppl. S1), II329–II334. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.C.; Loeys, B.; Carta, L.; Ramirez, F. Recent progress towards a molecular understanding of Marfan syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2005, 139, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Habashi, J.P.; Judge, D.P.; Patel, N.; Loeys, B.; Dietz, H.C., III. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N. Engl. J. Med. 2008, 358, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat.Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Radtke, A.; Saraei, R.; Bullerdiek, J.; Sorani, H.; Nimzyk, R.; Karluss, A.; Sievers, H.H.; Belge, G. Locally different endothelial nitric oxide synthase protein levels in ascending aortic aneurysms of bicuspid and tricuspid aortic valve. Cardiol. Res. Pract. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.L.; Verdi, J.M.; Conley, B.A.; Nicola, T.; Spicer, D.B.; Oxburgh, L.H.; Vary, C.P. Endoglin is required for myogenic differentiation potential of neural crest stem cells. Dev. Biol. 2007, 308, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Kaschina, E.; Schrader, F.; Sommerfeld, M.; Kemnitz, U.R.; Grzesiak, A.; Krikov, M.; Unger, T. Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J. Hypertens. 2008, 26, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Nataatmadja, M.; West, J.; Prabowo, S.; West, M. Angiotensin II Receptor Antagonism Reduces Transforming Growth Factor Beta and Smad Signaling in Thoracic Aortic Aneurysm. Ochsner. J. 2013, 13, 42–48. [Google Scholar] [PubMed]
- Mohamed, S.A.; Yan, J.; Sievers, H.H. Studies on Aortic and Aortic Valve Diseases. Clin. Exp. Cardiol. 2013, 4, 2–5. [Google Scholar]
- Yamaguchi, K.; Wakatsuki, T.; Soeki, T.; Niki, T.; Taketani, Y.; Oeduka, H.; Kusunose, K.; Ise, T.; Iwase, T.; Yamada, H.; et al. Effects of telmisartan on inflammatory cytokines and coronary plaque component as assessed on integrated backscatter intravascular ultrasound in hypertensive patients. Circ. J. 2014, 78, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Franken, R.; Mulder, B.J.; Goumans, M.J.; Lindeman, J.H.; Jongbloed, M.R.; Deruiter, M.C.; Klautz, R.J.; Bogers, A.J.; Poelmann, R.E.; et al. Histopathology of aortic complications in bicuspid aortic valve versus Marfan syndrome: Relevance for therapy? Heart Vessels. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Lehsau, A.-C.; Lazar-Karsten, P.; Schult-Badusche, D.; Mertens, L.; Loeys, B.L.; Mohamed, S.A. Genetic Basis, Pathogenesis and Histopathology of Aortopathy in Bicuspid Aortic Valve and Marfan Syndrome. 2015, 3, 55–82. [Google Scholar]
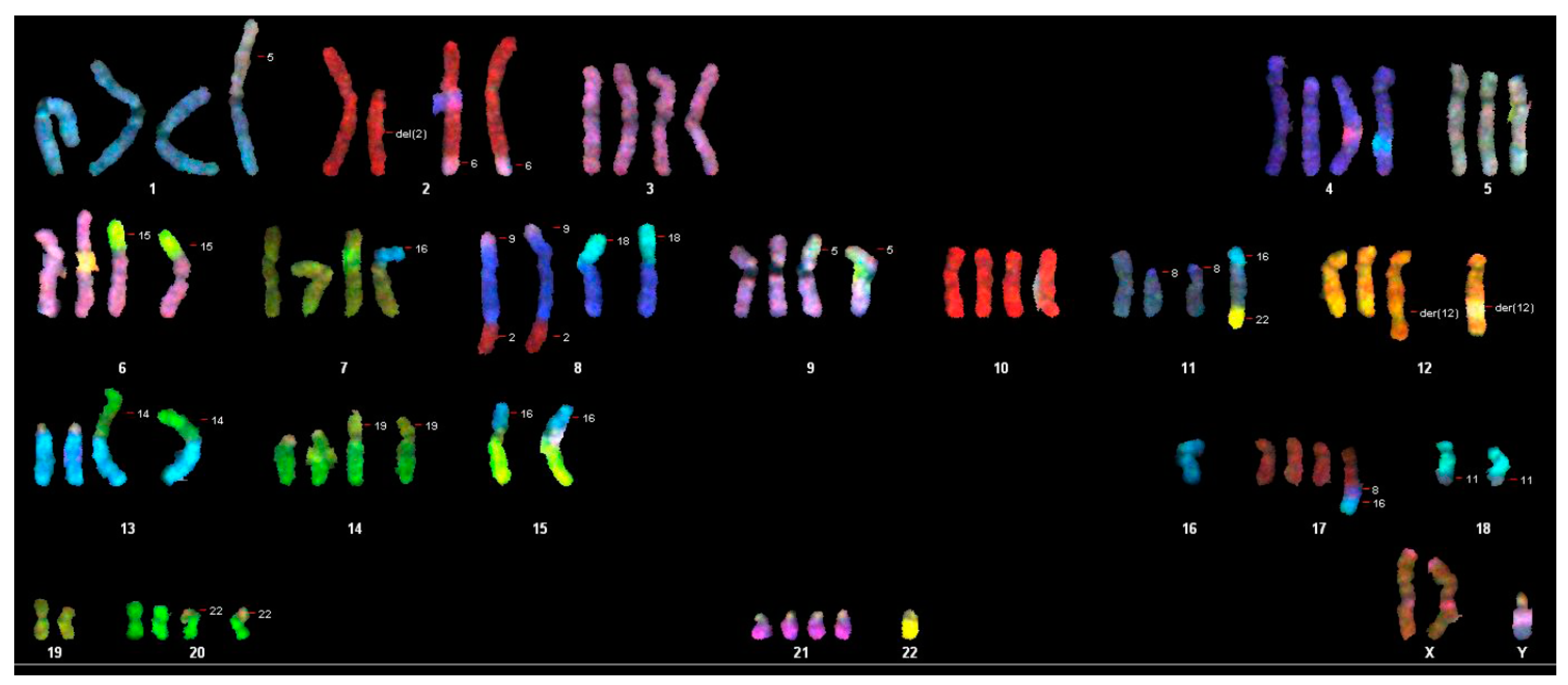
| Composite Karyotype SKY: |
|---|
| 70-91,XX,-Y[11],-Y[12],der(2)t(2;6)x2[11],-3[3],-4[4],der(6)t(6;15)[2],der(6)t(6;15der(8)t(9;8;2)x2[12],der(8)t(8;18)[7],der(8)t(8;18)x2[5],-9[4],+9[2],der(9)t(5;9)[5],der(9)t(5;9)x2[6],-10[4],-11[3],der(11)t(8;11)x2[7],der(12)t(5;12)x2[4],-13[7],-13[3],rob(13;14)[4],rob(13;14)x2[7],-14[5],-14[7],der(14)t(14,19)[4],der(14)t(14,19)x2[4],-15[12],-15[12], der(15)t(15;16)x2[9],-16[5], del(16)x2[8],der(16)t(16;20)x2[3],-17[4],-18[11],-18[7],der(18)t(5;18)[2],der(18)t(11;18)x2[7],-19[2]-19[10],-20[3],-20[2],der(20)t(20;22)x2[9],-21[3],-22[12],-22[12][cp12] |
| Marker | WG-59 Cells | WG-59 Tissue |
|---|---|---|
| D5 | 10 | 10 |
| D5’ | 12 | 12 |
| D13 | 11 | 11 |
| D13’ | 11 | 11 |
| D7 | 9 | 9 |
| D7’ | 10 | 10 |
| D16 | 12 | 12 |
| D16’ | 13 | 13 |
| vWA | 14 | 14 |
| vWA’ | 15 | 15 |
| TH01 | 9 | 9 |
| TH01’ | 9.3 | 9.3 |
| TPOX | 8 | 8 |
| TPOX’ | 11 | 11 |
| CFS1 | 11 | 11 |
| CSF1’ | 11 | 11 |
| Amel | X | X |
| Amel’ | X | Y |
| Gene | Description | Gene Bank | Fold Change | t-Test |
|---|---|---|---|---|
| 1 µM Losartan vs. Control | p-Value | |||
| ACVR2 | Activin A receptor, type IIA | NM_001616.3 | −1.16 | 0.0274 |
| BAMBI | BMP and activin membrane-bound inhibitor | NM_012342.2 | −1.10 | 0.0273 |
| BMP4 | Bone morphogenetic protein 4 | NM_001202.3 | −1.12 | 0.0325 |
| BMPER | BMP binding endothelial regulator | NM_133468 | −1.16 | 0.0121 |
| CDC25A | Cell division cycle 25A | NM_001789.2 | −1.24 | 0.0008 |
| CST3 | Cystatin C | NC_000020.10 | −1.11 | 0.0305 |
| ENG | Endoglin | NM_000118 | −1.14 | 0.0052 |
| FST | Follistatin | NM_013409 | −1.13 | 0.0099 |
| GDF5 | Growth Differentiation Factor 5 | NM_000557 | −1.26 | 0.0091 |
| JUN | Jun Proto-Oncogene | NM_002228 | −1.06 | 0.0479 |
| PLAU | Plasminogen Activator, urokinase | NM_002658 | −1.07 | 0.0052 |
| SMAD3 | SMAD family member 3 | NM_005902 | 1.01 | 0.0335 |
| SOX4 | SRY (sex determining region Y)-box 4 | NM_003107 | −1.25 | 0.0061 |
| STAT1 | Signal transducer and activator of transcription, 1.91 kD | NM_007315 | −1.08 | 0.0015 |
| TGFB1 | Transforming growth factor beta 1 | NM_000660 | 1.02 | 0.0460 |
| TGFBI | Transforming growth factor, beta-induced, 68 kDa | NM_000358 | −1.12 | 0.0316 |
| TGFBR1 | Transforming growth factor beta, receptor 1 | NM_004612 | −1.20 | 0.0028 |
| HPRT1 | Hypoxanthine phosphoribosyltransferase1 | NM_000194 | −1.04 | 0.0396 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar-Karsten, P.; Belge, G.; Schult-Badusche, D.; Focken, T.; Radtke, A.; Yan, J.; Ranabhat, P.; Mohamed, S.A. Generation and Characterization of Vascular Smooth Muscle Cell Lines Derived from a Patient with a Bicuspid Aortic Valve. Cells 2016, 5, 19. https://doi.org/10.3390/cells5020019
Lazar-Karsten P, Belge G, Schult-Badusche D, Focken T, Radtke A, Yan J, Ranabhat P, Mohamed SA. Generation and Characterization of Vascular Smooth Muscle Cell Lines Derived from a Patient with a Bicuspid Aortic Valve. Cells. 2016; 5(2):19. https://doi.org/10.3390/cells5020019
Chicago/Turabian StyleLazar-Karsten, Pamela, Gazanfer Belge, Detlev Schult-Badusche, Tim Focken, Arlo Radtke, Junfeng Yan, Pramod Ranabhat, and Salah A. Mohamed. 2016. "Generation and Characterization of Vascular Smooth Muscle Cell Lines Derived from a Patient with a Bicuspid Aortic Valve" Cells 5, no. 2: 19. https://doi.org/10.3390/cells5020019





