Functions of Rab Proteins at Presynaptic Sites
Abstract
:1. Introduction
2. An Overview of Membrane Traffic in Presynaptic Nerve Terminals
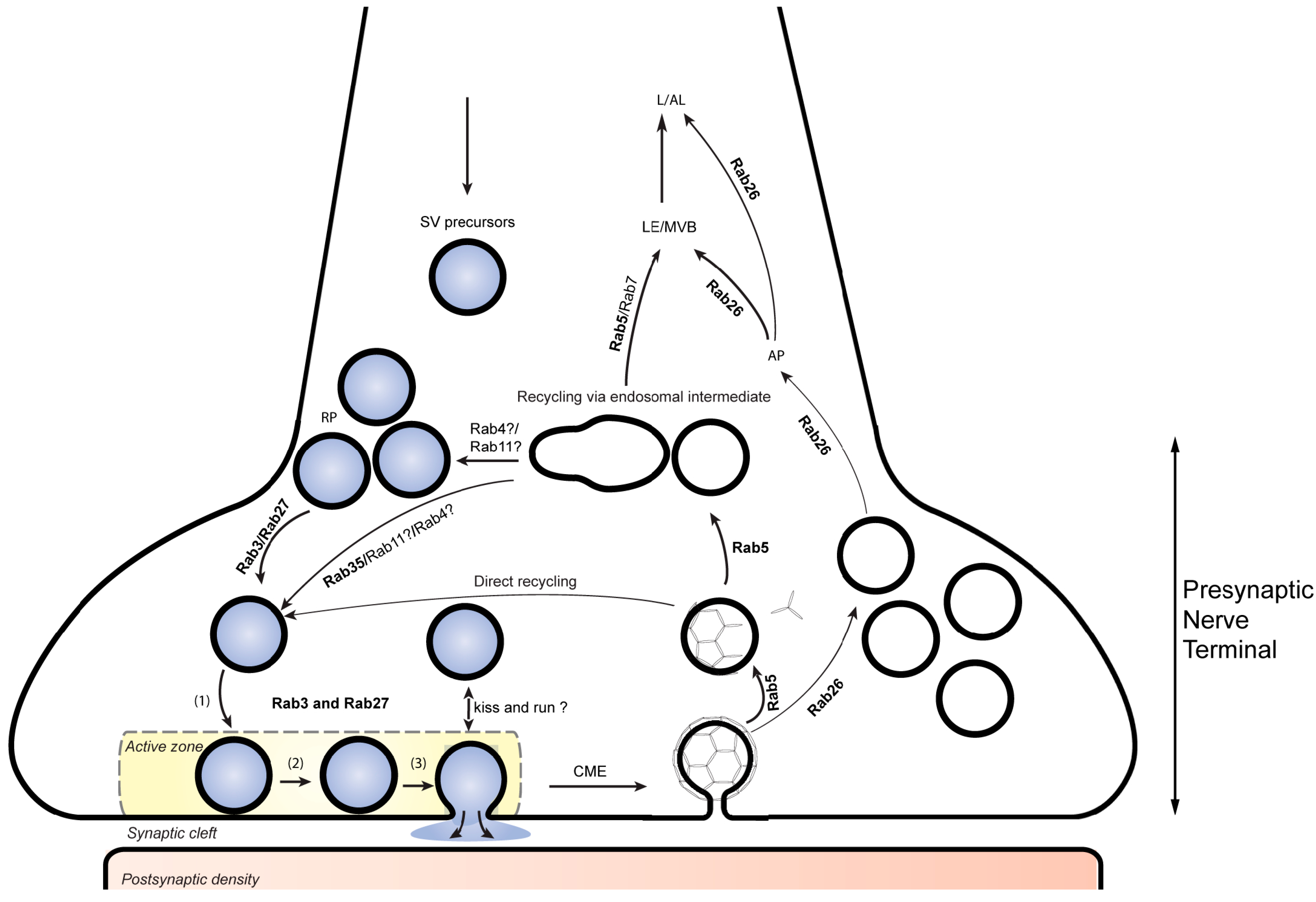
3. Synaptic Vesicle Docking and Exocytosis by Rab3-Related Proteins
4. GTPases Involved in Recycling of SVs
5. Degradation and Turnover of SVs
6. Presynaptic Rabs and Their Involvement in the Neuronal-Associated Disorders
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stenmark, H.; Olkkonen, V.M. The rab gtpase family. Genome Biol. 2001, 2, S3007. [Google Scholar] [CrossRef]
- Gallwitz, D.; Donath, C.; Sander, C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature 1983, 306, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Zahraoui, A.; Touchot, N.; Chardin, P.; Tavitian, A. The human rab genes encode a family of gtp-binding proteins related to yeast ypt1 and sec4 products involved in secretion. J. Biol. Chem. 1989, 264, 12394–12401. [Google Scholar] [PubMed]
- Cherfils, J.; Zeghouf, M. Regulation of small gtpases by gefs, gaps, and gdis. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef] [PubMed]
- Goody, R.S.; Rak, A.; Alexandrov, K. The structural and mechanistic basis for recycling of rab proteins between membrane compartments. Cell. Mol. Life Sci. 2005, 62, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Barr, F.; Lambright, D.G. Rab gefs and gaps. Curr. Opin. Cell. Biol. 2010, 22, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.; Aivazian, D. Targeting rab gtpases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 2004, 5, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.R.; Wittinghofer, A. The guanine nucleotide-binding switch in three dimensions. Science 2001, 294, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, C.; Brunger, A.T. Structural basis of rab effector specificity: Crystal structure of the small g protein rab3a complexed with the effector domain of rabphilin-3a. Cell 1999, 96, 363–374. [Google Scholar] [CrossRef]
- Barr, F.A. Review series: Rab gtpases and membrane identity: Causal or inconsequential? J. Cell Biol. 2013, 202, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.J. Macromolecular complexes at active zones: Integrated nano-machineries for neurotransmitter release. Cell. Mol. Life Sci. 2014, 71, 3903–3916. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature 2012, 490, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; De Camilli, P. Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 2012, 4, a005645. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Liu, Q.; Davis, M.W.; Hollopeter, G.; Thomas, N.; Jorgensen, N.B.; Jorgensen, E.M. Ultrafast endocytosis at caenorhabditis elegans neuromuscular junctions. Elife 2013, 2, e00723. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, S.O.; Jahn, R. Kiss-and-run, collapse and 'readily retrievable' vesicles. Traffic 2007, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Jahne, S.; Rizzoli, S.O.; Helm, M.S. The structure and function of presynaptic endosomes. Exp. Cell Res. 2015, 335, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.J.; Schmidt, A.A.; Huttner, W.B. Synaptic vesicle biogenesis. Annu. Rev. Cell Dev. Biol. 1999, 15, 733–798. [Google Scholar] [CrossRef] [PubMed]
- Shupliakov, O.; Brodin, L. Recent insights into the building and cycling of synaptic vesicles. Exp. Cell Res. 2010, 316, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Pavlos, N.J.; Gronborg, M.; Riedel, D.; Chua, J.J.; Boyken, J.; Kloepper, T.H.; Urlaub, H.; Rizzoli, S.O.; Jahn, R. Quantitative analysis of synaptic vesicle rabs uncovers distinct yet overlapping roles for rab3a and rab27b in ca2+-triggered exocytosis. J. Neurosci. 2010, 30, 13441–13453. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Sasaki, T.; Shirataki, H.; Nakanishi, H. Rab3a small gtp-binding protein in ca2+-dependent exocytosis. Genes Cells 1996, 1, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Geppert, M.; Goda, Y.; Stevens, C.F.; Sudhof, T.C. The small gtp-binding protein rab3a regulates a late step in synaptic vesicle fusion. Nature 1997, 387, 810–814. [Google Scholar] [PubMed]
- Fischer von Mollard, G.; Sudhof, T.C.; Jahn, R. A small gtp-binding protein dissociates from synaptic vesicles during exocytosis. Nature 1991, 349, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Stahl, B.; von Mollard, G.F.; Walch-Solimena, C.; Jahn, R. Gtp cleavage by the small gtp-binding protein rab3a is associated with exocytosis of synaptic vesicles induced by alpha-latrotoxin. J. Biol. Chem. 1994, 269, 24770–24776. [Google Scholar] [PubMed]
- Schluter, O.M.; Schmitz, F.; Jahn, R.; Rosenmund, C.; Sudhof, T.C. A complete genetic analysis of neuronal rab3 function. J. Neurosci. 2004, 24, 6629–6637. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Distinct rab binding specificity of rim1, rim2, rabphilin, and noc2. Identification of a critical determinant of rab3a/rab27a recognition by rim2. J. Biol. Chem. 2003, 278, 15373–15380. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013, 14, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Pavlos, N.J.; Jahn, R. Distinct yet overlapping roles of rab gtpases on synaptic vesicles. Small GTPases 2011, 2, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Staunton, J.; Saifee, O.; Nonet, M.; Thomas, J.H. Aex-3 encodes a novel regulator of presynaptic activity in c. Elegans. Neuron 1997, 18, 613–622. [Google Scholar] [CrossRef]
- Nonet, M.L.; Staunton, J.E.; Kilgard, M.P.; Fergestad, T.; Hartwieg, E.; Horvitz, H.R.; Jorgensen, E.M.; Meyer, B.J. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 1997, 17, 8061–8073. [Google Scholar] [PubMed]
- Figueiredo, A.C.; Wasmeier, C.; Tarafder, A.K.; Ramalho, J.S.; Baron, R.A.; Seabra, M.C. Rab3gep is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J. Biol. Chem. 2008, 283, 23209–23216. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, T.R.; Liu, Q.; Itoh, T.; Luo, S.; Hadwiger, G.; Vincent, R.; Wang, Z.W.; Fukuda, M.; Nonet, M.L. Regulation of synaptic transmission by rab-3 and rab-27 in caenorhabditis elegans. Mol. Boil. Cell 2006, 17, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Itzen, A.; Goody, R.S. Key determinants of rab specificity. Structure 2008, 16, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Handley, M.T.; Haynes, L.P.; Burgoyne, R.D. Differential dynamics of rab3a and rab27a on secretory granules. J. Cell Sci. 2007, 120, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Membrane traffic in the secretory pathway. Cell. Mol. Life Sci. 2008, 65, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Schluter, O.M.; Schnell, E.; Verhage, M.; Tzonopoulos, T.; Nicoll, R.A.; Janz, R.; Malenka, R.C.; Geppert, M.; Sudhof, T.C. Rabphilin knock-out mice reveal that rabphilin is not required for rab3 function in regulating neurotransmitter release. J. Neurosci. 1999, 19, 5834–5846. [Google Scholar] [PubMed]
- Schoch, S.; Mittelstaedt, T.; Kaeser, P.S.; Padgett, D.; Feldmann, N.; Chevaleyre, V.; Castillo, P.E.; Hammer, R.E.; Han, W.; Schmitz, F.; et al. Redundant functions of rim1alpha and rim2alpha in ca2+-triggered neurotransmitter release. EMBO J. 2006, 25, 5852–5863. [Google Scholar] [CrossRef] [PubMed]
- Schoch, S.; Castillo, P.E.; Jo, T.; Mukherjee, K.; Geppert, M.; Wang, Y.; Schmitz, F.; Malenka, R.C.; Sudhof, T.C. Rim1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 2002, 415, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kaeser, P.S.; Xu, W.; Südhof, T.C. Rim proteins activate vesicle priming by reversing autoinhibitory homodimerization of munc13. Neuron 2011, 69, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Dulubova, I.; Lou, X.; Lu, J.; Huryeva, I.; Alam, A.; Schneggenburger, R.; Sudhof, T.C.; Rizo, J. A munc13/rim/rab3 tripartite complex: From priming to plasticity? EMBO J. 2005, 24, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Okamoto, M.; Schmitz, F.; Hofmann, K.; Sudhof, T.C. Rim is a putative rab3 effector in regulating synaptic-vesicle fusion. Nature 1997, 388, 593–598. [Google Scholar] [PubMed]
- Imig, C.; Min, S.-W.; Krinner, S.; Arancillo, M.; Rosenmund, C.; Südhof, T.C.; Rhee, J.; Brose, N.; Cooper, B.H. The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron 2014, 84, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Novick, P.; Ferro, S.; Schekman, R. Order of events in the yeast secretory pathway. Cell 1981, 25, 461–469. [Google Scholar] [CrossRef]
- Novick, P.; Schekman, R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1979, 76, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Villarroel-Campos, D.; Gastaldi, L.; Conde, C.; Caceres, A.; Gonzalez-Billault, C. Rab-mediated trafficking role in neurite formation. J. Neurochem. 2014, 129, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.; Kiger, A.A. Coordination between rab gtpase and phosphoinositide regulation and functions. Nat. Rev. Mol. Cell Biol. 2012, 13, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab gtpases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Fischer von Mollard, G.; Stahl, B.; Walch-Solimena, C.; Takei, K.; Daniels, L.; Khoklatchev, A.; De Camilli, P.; Sudhof, T.C.; Jahn, R. Localization of rab5 to synaptic vesicles identifies endosomal intermediate in synaptic vesicle recycling pathway. Eur. J. Cell. Biol. 1994, 65, 319–326. [Google Scholar] [PubMed]
- de Hoop, M.J.; Huber, L.A.; Stenmark, H.; Williamson, E.; Zerial, M.; Parton, R.G.; Dotti, C.G. The involvement of the small gtp-binding protein rab5a in neuronal endocytosis. Neuron 1994, 13, 11–22. [Google Scholar] [CrossRef]
- Wucherpfennig, T.; Wilsch-Brauninger, M.; Gonzalez-Gaitan, M. Role of drosophila rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Boil. 2003, 161, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Kawamura, S.; Ozaki, K. An essential role of Rab5 in uniformity of synaptic vesicle size. J. Cell Sci. 2003, 116, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Uytterhoeven, V.; Kuenen, S.; Kasprowicz, J.; Miskiewicz, K.; Verstreken, P. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell 2011, 145, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.F.; Broadie, K. Roles of ubiquitination at the synapse. Biochim. Biophys. Acta 2008, 1779, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Cajigas, I.J.; Will, T.; Schuman, E.M. Protein homeostasis and synaptic plasticity. EMBO J. 2010, 29, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- Von Bartheld, C.S.; Altick, A.L. Multivesicular bodies in neurons: Distribution, protein content, and trafficking functions. Prog. Neurobiol. 2011, 93, 313–340. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Yue, Z. Autophagy and its normal and pathogenic states in the brain. Annu. Rev. Neurosci. 2014, 37, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.L.; Cuervo, A.M. Autophagy and human disease: Emerging themes. Curr. Opin. Genet. Dev. 2014, 26, 16–23. [Google Scholar]
- Cheng, X.T.; Zhou, B.; Lin, M.Y.; Cai, Q.; Sheng, Z.H. Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J. Cell Biol. 2015, 209, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Maday, S.; Holzbaur, E.L. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev. Cell 2014, 30, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Szatmari, Z.; Sass, M. The autophagic roles of rab small gtpases and their upstream regulators: A review. Autophagy 2014, 10, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Binotti, B.; Pavlos, N.J.; Riedel, D.; Wenzel, D.; Vorbruggen, G.; Schalk, A.M.; Kuhnel, K.; Boyken, J.; Erck, C.; Martens, H.; Chua, J.J.; et al. The gtpase rab26 links synaptic vesicles to the autophagy pathway. Elife 2015, 4, e00597. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Itoh, T. Direct link between atg protein and small gtpase rab: Atg16l functions as a potential rab33 effector in mammals. Autophagy 2008, 4, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Uemura, T.; Waguri, S.; Fukuda, M. Atg16l1, an essential factor for canonical autophagy, participates in hormone secretion from pc12 cells independently of autophagic activity. Mol. Biol. Cell 2012, 23, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Sada, T.; Toriyama, M.; Tago, K.; Sugiura, T.; Fukuda, M.; Inagaki, N. Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J. Neurosci. 2012, 32, 12712–12725. [Google Scholar] [PubMed]
- Dalfo, E.; Barrachina, M.; Rosa, J.L.; Ambrosio, S.; Ferrer, I. Abnormal alpha-synuclein interactions with rab3a and rabphilin in diffuse lewy body disease. Neurobiol. Dis. 2004, 16, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Aligianis, I.A.; Johnson, C.A.; Gissen, P.; Chen, D.; Hampshire, D.; Hoffmann, K.; Maina, E.N.; Morgan, N.V.; Tee, L.; Morton, J.; et al. Mutations of the catalytic subunit of rab3gap cause warburg micro syndrome. Nat. Genet. 2005, 37, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Aligianis, I.A.; Morgan, N.V.; Mione, M.; Johnson, C.A.; Rosser, E.; Hennekam, R.C.; Adams, G.; Trembath, R.C.; Pilz, D.T.; Stoodley, N.; et al. Mutation in Rab3 GTPase-activating protein (rab3gap) noncatalytic subunit in a kindred with martsolf syndrome. Am. J. Hum. Genet. 2006, 78, 702–707. [Google Scholar] [CrossRef] [PubMed]
- D'Adamo, P.; Menegon, A.; Lo Nigro, C.; Grasso, M.; Gulisano, M.; Tamanini, F.; Bienvenu, T.; Gedeon, A.K.; Oostra, B.; Wu, S.K.; et al. Mutations in gdi1 are responsible for x-linked non-specific mental retardation. Nat. Genet. 1998, 19, 134–139. [Google Scholar] [CrossRef] [PubMed]
- D'Adamo, P.; Welzl, H.; Papadimitriou, S.; Raffaele di Barletta, M.; Tiveron, C.; Tatangelo, L.; Pozzi, L.; Chapman, P.F.; Knevett, S.G.; Ramsay, M.F.; et al. Deletion of the mental retardation gene gdi1 impairs associative memory and alters social behavior in mice. Hum. Mol. Genet. 2002, 11, 2567–2580. [Google Scholar] [CrossRef] [PubMed]
- D'Adamo, P.; Wolfer, D.P.; Kopp, C.; Tobler, I.; Toniolo, D.; Lipp, H.P. Mice deficient for the synaptic vesicle protein rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the rab3a regulator gdi1. Eur. J. Neurosci. 2004, 19, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Tsetsenis, T.; Younts, T.J.; Chiu, C.Q.; Kaeser, P.S.; Castillo, P.E.; Sudhof, T.C. Rab3b protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc. Natl. Acad. Sci. USA 2011, 108, 14300–14305. [Google Scholar] [CrossRef] [PubMed]
- Baskys, A.; Bayazitov, I.; Zhu, E.; Fang, L.; Wang, R. Rab-mediated endocytosis: Linking neurodegeneration, neuroprotection, and synaptic plasticity? Ann. N. Y. Acad. Sci. 2007, 1122, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Kim, J.; Paik, S.R.; Park, J.H.; Ahn, Y.S.; Chung, K.C. Induction of neuronal cell death by rab5a-dependent endocytosis of alpha-synuclein. J. Boil. Chem. 2001, 276, 27441–27448. [Google Scholar] [CrossRef] [PubMed]
- Otomo, A.; Hadano, S.; Okada, T.; Mizumura, H.; Kunita, R.; Nishijima, H.; Showguchi-Miyata, J.; Yanagisawa, Y.; Kohiki, E.; Suga, E.; et al. Als2, a novel guanine nucleotide exchange factor for the small gtpase rab5, is implicated in endosomal dynamics. Hum. Mol. Genet. 2003, 12, 1671–1687. [Google Scholar] [CrossRef] [PubMed]
- Kenna, K.P.; McLaughlin, R.L.; Byrne, S.; Elamin, M.; Heverin, M.; Kenny, E.M.; Cormican, P.; Morris, D.W.; Donaghy, C.G.; Bradley, D.G.; et al. Delineating the genetic heterogeneity of als using targeted high-throughput sequencing. J. Med. Genet. 2013, 50, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Severin, F.; Lommer, B.; Shevchenko, A.; Zerial, M. Huntingtin-hap40 complex is a novel rab5 effector that regulates early endosome motility and is up-regulated in huntington's disease. J. Cell Boil. 2006, 172, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Severin, F.; Hopfner, S.; Zerial, M. Regulation of endosome dynamics by rab5 and huntingtin-hap40 effector complex in physiological versus pathological conditions. Methods Enzymol. 2008, 438, 239–257. [Google Scholar] [PubMed]
- Li, X.; DiFiglia, M. The recycling endosome and its role in neurological disorders. Prog. Neurobiol. 2012, 97, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.; Tolun, A. Tbc1d24 truncating mutation resulting in severe neurodegeneration. J. Med. Genet. 2013, 50, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; Uytterhoeven, V.; Kuenen, S.; Wang, Y.C.; Slabbaert, J.R.; Swerts, J.; Kasprowicz, J.; Aerts, S.; Verstreken, P. Reduced synaptic vesicle protein degradation at lysosomes curbs tbc1d24/sky-induced neurodegeneration. J. Cell Boil. 2014, 207, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.E.; Wang, X.; Kumar, R.; Bhartur, S.G.; Navarre, J.; Woodrum, J.E.; Altschuler, Y.; Ray, G.S.; Goldenring, J.R. Association of rab25 and rab11a with the apical recycling system of polarized madin-darby canine kidney cells. Mol. Boil. Cell 1999, 10, 47–61. [Google Scholar] [CrossRef]
- Schlierf, B.; Fey, G.H.; Hauber, J.; Hocke, G.M.; Rosorius, O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp. Cell Res. 2000, 259, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, F.; Steinert, J.R. Rab11 as a modulator of synaptic transmission. Commun. Integr. Biol. 2013, 6, e26807. [Google Scholar] [CrossRef] [PubMed]
- Dumanchin, C.; Czech, C.; Campion, D.; Cuif, M.H.; Poyot, T.; Martin, C.; Charbonnier, F.; Goud, B.; Pradier, L.; Frebourg, T. Presenilins interact with rab11, a small gtpase involved in the regulation of vesicular transport. Hum. Mol. Genet. 1999, 8, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binotti, B.; Jahn, R.; Chua, J.J.E. Functions of Rab Proteins at Presynaptic Sites. Cells 2016, 5, 7. https://doi.org/10.3390/cells5010007
Binotti B, Jahn R, Chua JJE. Functions of Rab Proteins at Presynaptic Sites. Cells. 2016; 5(1):7. https://doi.org/10.3390/cells5010007
Chicago/Turabian StyleBinotti, Beyenech, Reinhard Jahn, and John Jia En Chua. 2016. "Functions of Rab Proteins at Presynaptic Sites" Cells 5, no. 1: 7. https://doi.org/10.3390/cells5010007




