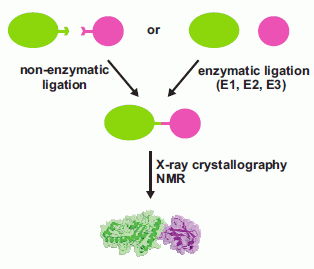
The Challenge of Producing Ubiquitinated Proteins for Structural Studies
Abstract
:1. Introduction

| Protein Name | Method: Non-Enzymatic | Method: Enzymatic | Native Isopeptide Bond 1 | Destabilizing Conditions | Stable to Reducing Agents | X-ray or NMR |
|---|---|---|---|---|---|---|
| Histone H2B | McGinty et al. [18] | yes (1) | 6 M guanidine hydrochloride | yes | no | |
| Chatterjee et al. [19] | no (2) | 6 M guanidine hydrochloride | no | no | ||
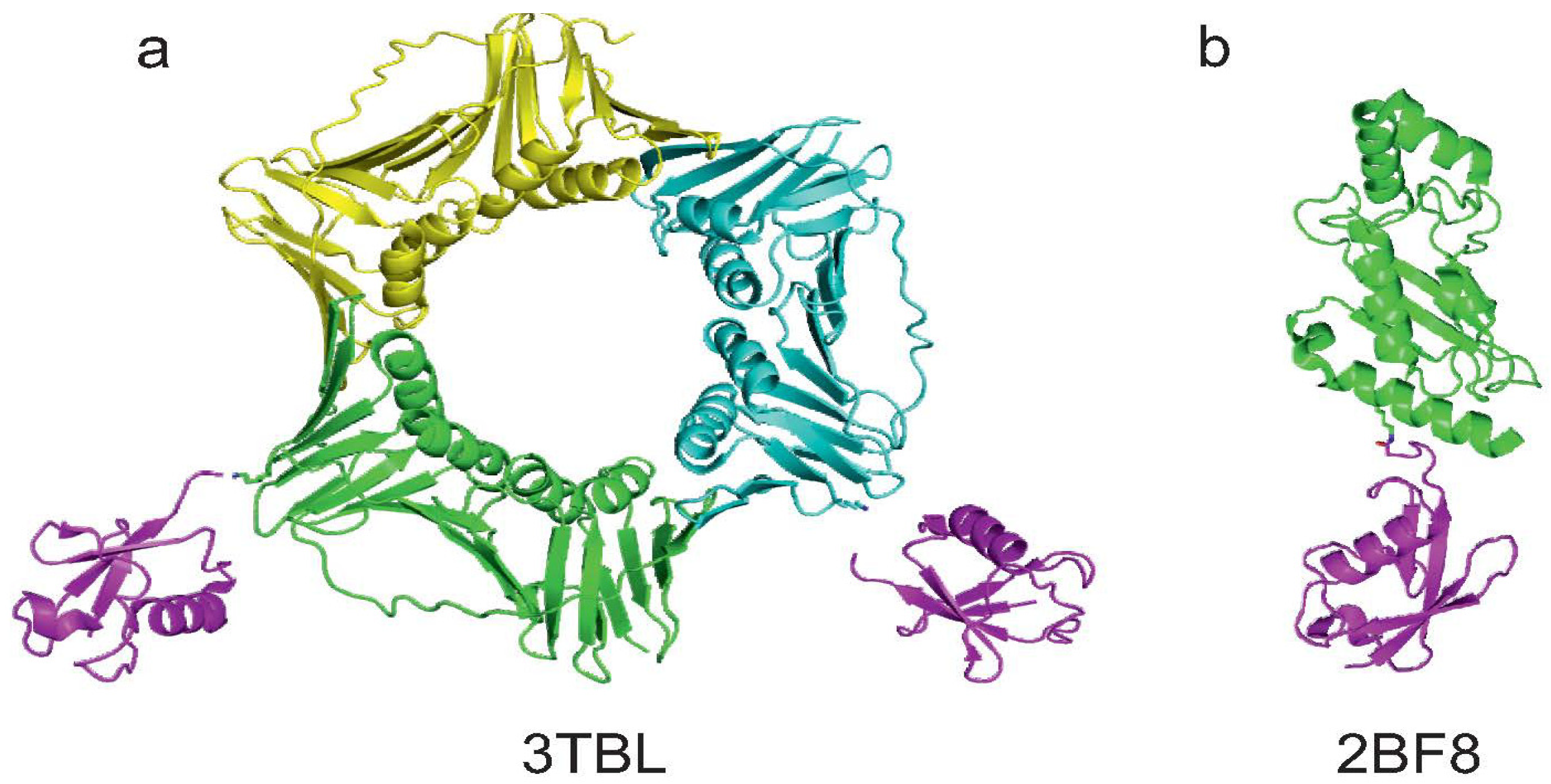
| PCNA | Chen et al. [20] | no (2) | no | no | no | |
| Freudenthal et al. [21] | no (no covalent bond) | no | yes | X-ray 3L10, 3L0W | ||
| Eger et al. [22] | no (3) | no | yes | no | ||
| Zhang et al. [23] | yes (1) | no | yes | X-ray 3TBL | ||
| α-synuclein | Hejjaoui et al. [24] | yes (1) | 6 M guanidine hydrochloride | yes | no | |
| Meier et al. [25] | no (2) | 6 M guanidine hydrochloride | no | no | ||
| calmodulin | Li et al. [26] | no (4) | no | yes | no | |
| SUMO | Virdee et al. [27] | yes (1) | no | yes | no | |
| Ras | Baker et al. [28] | no (5) | no | no | NMR | |
| Josephin | Faggiano et al. [29] | yes (1) | no | yes | NMR | |
| Rpn10 | Keren-Kaplan et al. [30] | yes (1) | no | yes | no | |
| Vps9 | Keren-Kaplan et al. [30] | yes (1) | no | yes | no |
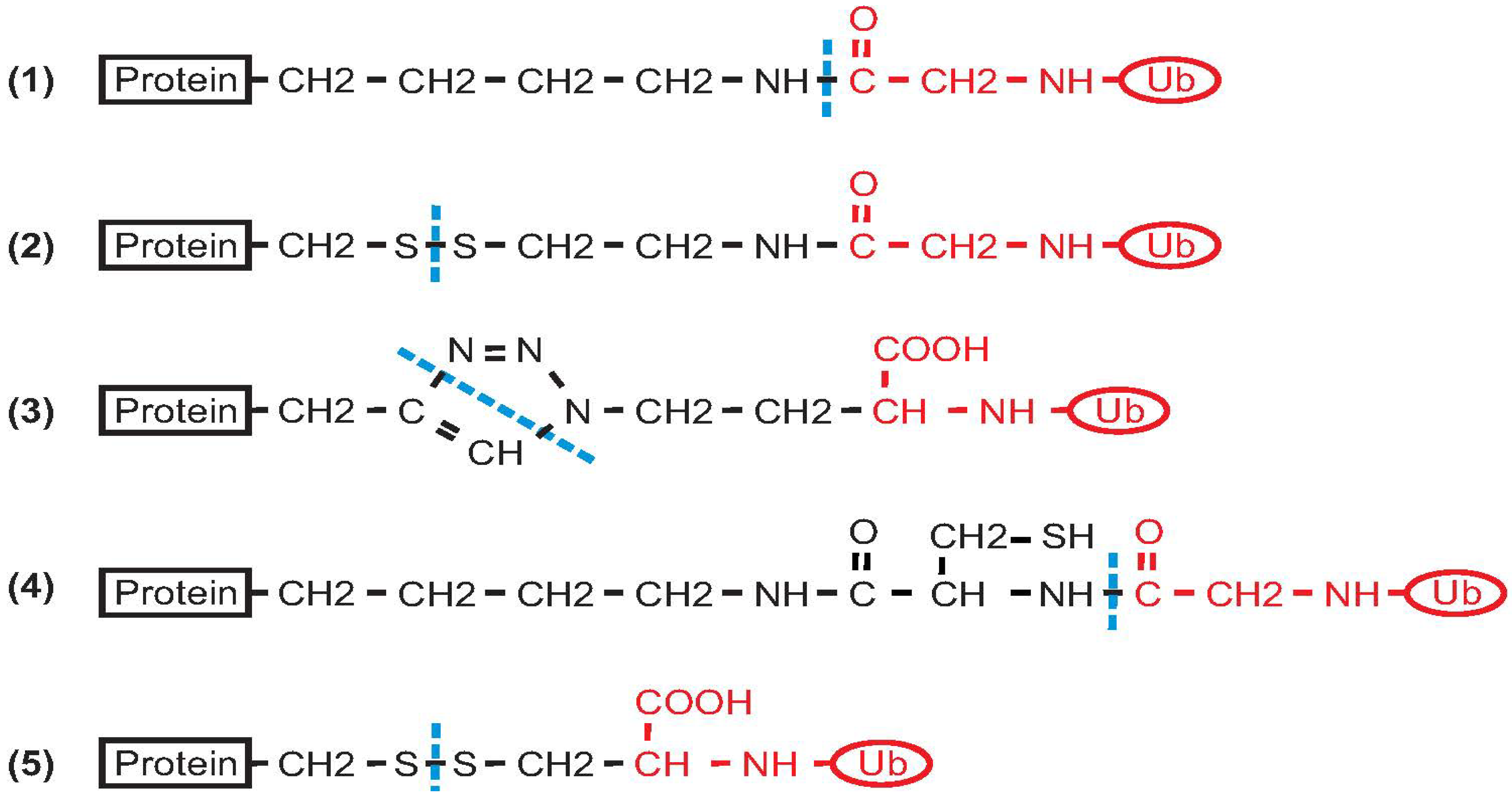
2. Non-Enzymatic Methods
2.1. Histone H2B
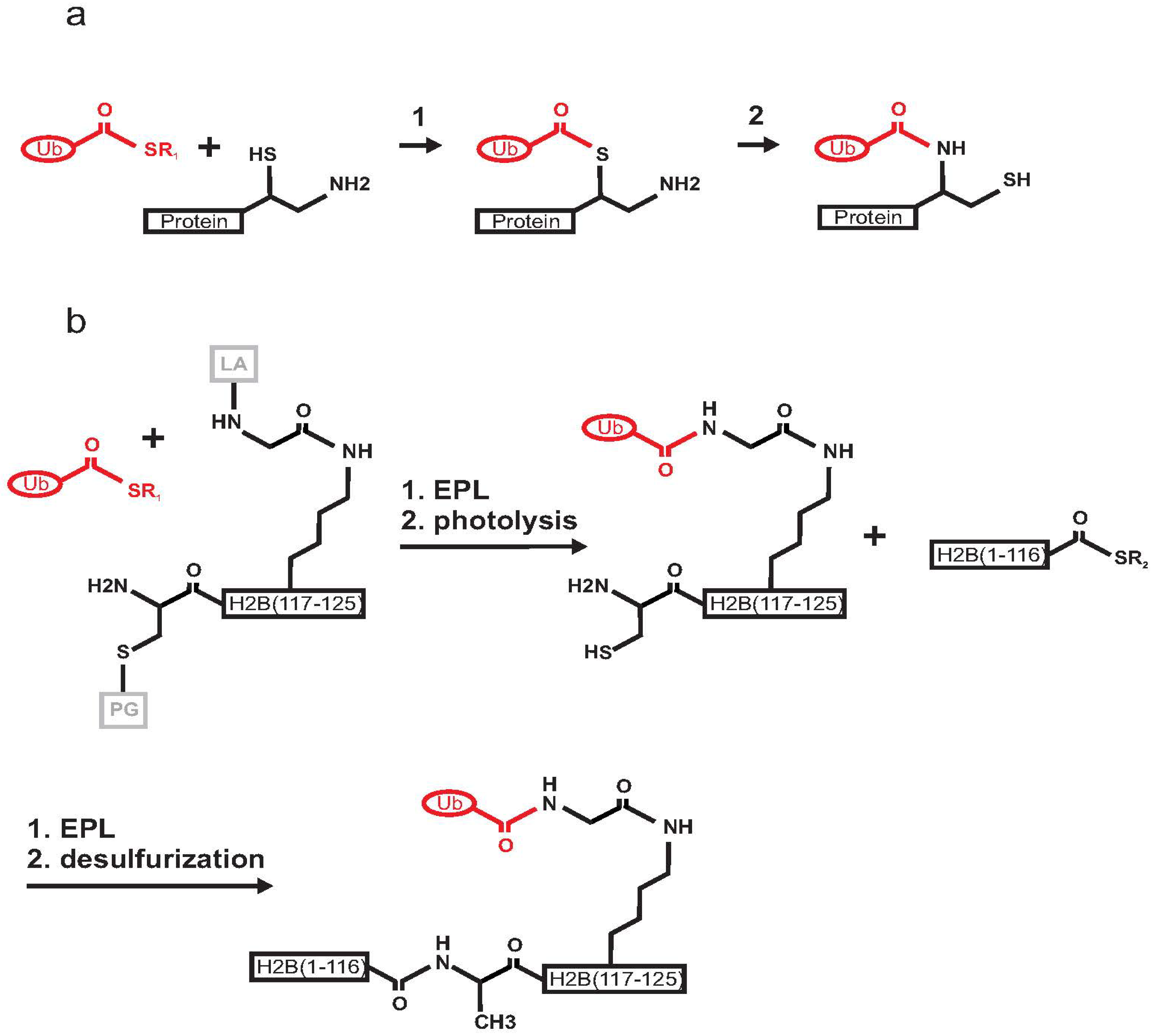
2.2. PCNA
2.3. α-Synuclein
2.4. Calmodulin
2.5. SUMO
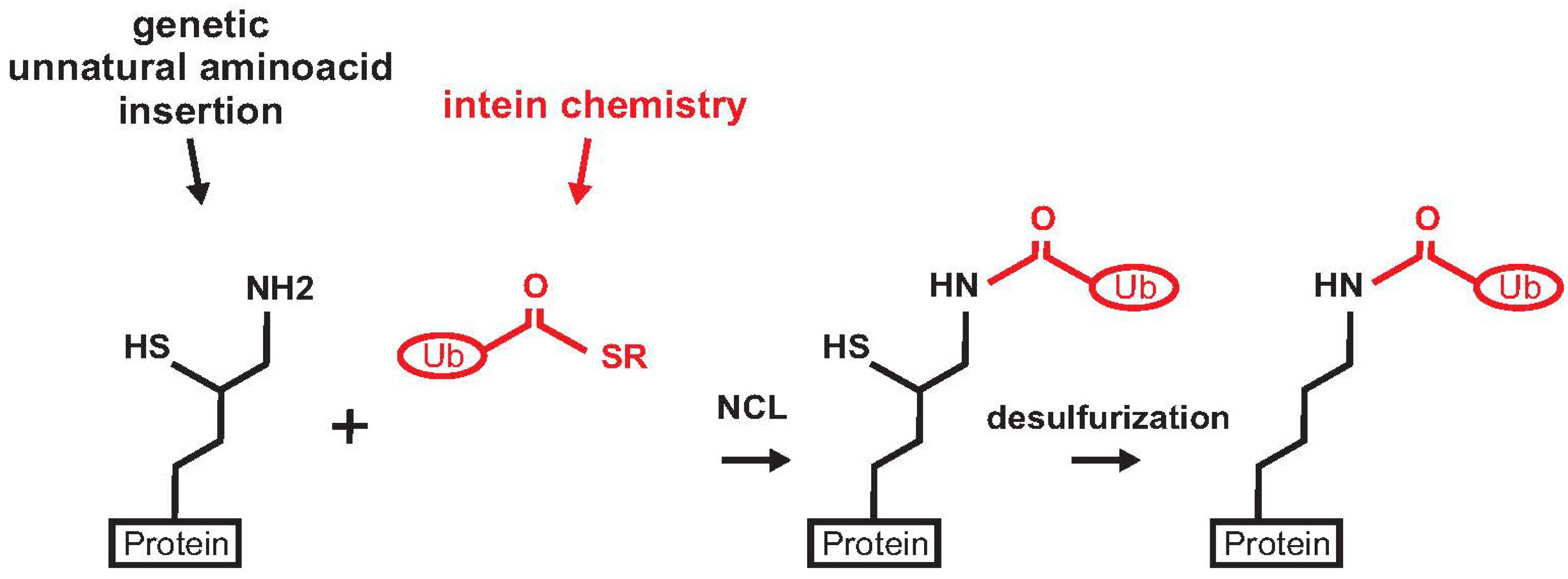
2.6. Ras
2.7. Production of Poly-Ubiquitinated Proteins
3. Enzymatic Methods
3.1. PCNA
3.2. Josephin
3.3. Rpn10 and Vps9
3.4. Sumoylation of E2-25K
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hicke, L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001, 2, 195–201. [Google Scholar]
- Komander, D. The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 2009, 37, 937–953. [Google Scholar] [CrossRef]
- Sadowski, M.; Suryadinata, R.; Tan, A.R.; Roesley, S.N.; Sarcevic, B. Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life 2012, 64, 136–142. [Google Scholar] [CrossRef]
- Hu, M.; Li, P.; Li, M.; Li, W.; Yao, T.; Wu, J.W.; Gu, W.; Cohen, R.E.; Shi, Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 2002, 111, 1041–1054. [Google Scholar] [CrossRef]
- Misaghi, S.; Galardy, P.J.; Meester, W.J.; Ovaa, H.; Ploegh, H.L.; Gaudet, R. Structure of the ubiquitin hydrolase UCH-L3 complexed with a suicide substrate. J. Biol. Chem. 2005, 280, 1512–1520. [Google Scholar]
- Eddins, M.J.; Carlile, C.M.; Gomez, K.M.; Pickart, C.M.; Wolberger, C. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nat. Struct. Mol. Biol. 2006, 13, 915–920. [Google Scholar] [CrossRef]
- Kamadurai, H.B.; Souphron, J.; Scott, D.C.; Duda, D.M.; Miller, D.J.; Stringer, D.; Piper, R.C.; Schulman, B.A. Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECT(NEDD4L) complex. Mol. Cell 2009, 36, 1095–1102. [Google Scholar] [CrossRef]
- Weeks, S.D.; Grasty, K.C.; Hernandez-Cuebas, L.; Loll, P.J. Crystal structure of a josephin-ubiquitin complex: Evolutionary restraints on ataxin-3 deubiquitinating activity. J. Biol. Chem. 2011, 286, 4555–4565. [Google Scholar] [CrossRef]
- Plechanovova, A.; Jaffray, E.G.; Tatham, M.H.; Naismith, J.H.; Hay, R.T. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 2012, 489, 115–120. [Google Scholar] [CrossRef]
- Haj-Yahya, M.; Fauvet, B.; Herman-Bachinsky, Y.; Hejjaoui, M.; Bavikar, S.N.; Karthikeyan, S.V.; Ciechanover, A.; Lashuel, H.A.; Brik, A. Synthetic polyubiquitinated alpha-synuclein reveals important insights into the roles of the ubiquitin chain in regulating its pathophysiology. Proc. Natl. Acad. Sci. USA 2013, 110, 17726–17731. [Google Scholar] [CrossRef]
- Hemantha, H.P.; Bavikar, S.N.; Herman-Bachinsky, Y.; Haj-Yahya, N.; Bondalapati, S.; Ciechanover, A.; Brik, A. Nonenzymatic polyubiquitination of expressed proteins. J. Am. Chem. Soc. 2014, 136, 2665–2673. [Google Scholar] [CrossRef]
- Pickart, C.M.; Raasi, S. Controlled synthesis of polyubiquitin chains. Methods Enzymol. 2005, 399, 21–36. [Google Scholar] [CrossRef]
- Strieter, E.R.; Korasick, D.A. Unraveling the complexity of ubiquitin signaling. ACS Chem. Biol. 2012, 7, 52–63. [Google Scholar] [CrossRef]
- Spasser, L.; Brik, A. Chemistry and biology of the ubiquitin signal. Angew. Chem. Int. Ed. Engl. 2012, 51, 6840–6862. [Google Scholar] [CrossRef]
- Weller, C.E.; Pilkerton, M.E.; Chatterjee, C. Chemical strategies to understand the language of ubiquitin signaling. Biopolymers 2013. [Google Scholar] [CrossRef]
- Hemantha, H.P.; Brik, A. Non-enzymatic synthesis of ubiquitin chains: Where chemistry makes a difference. Bioorg. Med. Chem. 2013, 21, 3411–3420. [Google Scholar] [CrossRef]
- Dixon, E.K.; Castaneda, C.A.; Kashyap, T.R.; Wang, Y.; Fushman, D. Nonenzymatic assembly of branched polyubiquitin chains for structural and biochemical studies. Bioorg. Med. Chem. 2013, 21, 3421–3429. [Google Scholar] [CrossRef]
- McGinty, R.K.; Kim, J.; Chatterjee, C.; Roeder, R.G.; Muir, T.W. Chemically ubiquitylated histone H2B stimulates hDot1L-mediated intranucleosomal methylation. Nature 2008, 453, 812–816. [Google Scholar] [CrossRef]
- Chatterjee, C.; McGinty, R.K.; Fierz, B.; Muir, T.W. Disulphide-directed histone ubiquitylation reveals plasticity in hDot1L activation. Nat. Chem. Biol. 2010, 6, 267–269. [Google Scholar]
- Chen, J.; Ai, Y.; Wang, J.; Haracska, L.; Zhuang, Z. Chemically ubiquitylated PCNA as a probe for eukaryotic translesion DNA synthesis. Nat. Chem. Biol. 2010, 6, 270–272. [Google Scholar] [CrossRef]
- Freudenthal, B.D.; Gakhar, L.; Ramaswamy, S.; Washington, M.T. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nat. Struct. Mol. Biol. 2010, 17, 479–484. [Google Scholar] [CrossRef]
- Eger, S.; Castrec, B.; Hubscher, U.; Scheffner, M.; Rubini, M.; Marx, A. Generation of a mono-ubiquitinated PCNA mimic by click chemistry. Chembiochem 2011, 12, 2807–2812. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Lin, S.H.; Wang, X.; Wu, L.; Lee, E.Y.; Lee, M.Y. Structure of monoubiquitinated PCNA: Implications for DNA polymerase switching and okazaki fragment maturation. Cell Cycle 2012, 11, 2128–2136. [Google Scholar] [CrossRef]
- Hejjaoui, M.; Haj-Yahya, M.; Kumar, K.S.; Brik, A.; Lashuel, H.A. Towards elucidation of the role of ubiquitination in the pathogenesis of Parkinson’s disease with semisynthetic ubiquitinated alpha-synuclein. Angew. Chem. Int. Ed. Engl. 2011, 50, 405–409. [Google Scholar] [CrossRef]
- Meier, F.; Abeywardana, T.; Dhall, A.; Marotta, N.P.; Varkey, J.; Langen, R.; Chatterjee, C.; Pratt, M.R. Semisynthetic, site-specific ubiquitin modification of alpha-synuclein reveals differential effects on aggregation. J. Am. Chem. Soc. 2012, 134, 5468–5471. [Google Scholar] [CrossRef]
- Li, X.; Fekner, T.; Ottesen, J.J.; Chan, M.K. A pyrrolysine analogue for site-specific protein ubiquitination. Angew. Chem. Int. Ed. Engl. 2009, 48, 9184–9187. [Google Scholar] [CrossRef]
- Virdee, S.; Kapadnis, P.B.; Elliott, T.; Lang, K.; Madrzak, J.; Nguyen, D.P.; Riechmann, L.; Chin, J.W. Traceless and site-specific ubiquitination of recombinant proteins. J. Am. Chem. Soc. 2011, 133, 10708–10711. [Google Scholar] [CrossRef]
- Baker, R.; Lewis, S.M.; Sasaki, A.T.; Wilkerson, E.M.; Locasale, J.W.; Cantley, L.C.; Kuhlman, B.; Dohlman, H.G.; Campbell, S.L. Site-specific monoubiquitination activates ras by impeding gtpase-activating protein function. Nat. Struct. Mol. Biol. 2013, 20, 46–52. [Google Scholar]
- Faggiano, S.; Menon, R.P.; Kelly, G.P.; McCormick, J.; Todi, S.V.; Scaglione, K.M.; Paulson, H.L.; Pastore, A. Enzymatic production of mono-ubiquitinated proteins for structural studies: The example of the Josephin domain of ataxin-3. FEBS Open Bio. 2013, 3, 453–458. [Google Scholar] [CrossRef]
- Keren-Kaplan, T.; Prag, G. Purification and crystallization of mono-ubiquitylated ubiquitin receptor Rpn10. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1120–1123. [Google Scholar] [CrossRef]
- Weake, V.M.; Workman, J.L. Histone ubiquitination: Triggering gene activity. Mol. Cell 2008, 29, 653–663. [Google Scholar] [CrossRef]
- Du, H.N. Transcription, DNA damage and beyond: The roles of histone ubiquitination and deubiquitination. Curr. Protein Pept. Sci. 2012, 13, 447–466. [Google Scholar] [CrossRef]
- McGinty, R.K.; Chatterjee, C.; Muir, T.W. Semisynthesis of ubiquitylated proteins. Methods Enzymol. 2009, 462, 225–243. [Google Scholar] [CrossRef]
- Muralidharan, V.; Muir, T.W. Protein ligation: An enabling technology for the biophysical analysis of proteins. Nat. Methods 2006, 3, 429–438. [Google Scholar] [CrossRef]
- McGinty, R.K.; Kohn, M.; Chatterjee, C.; Chiang, K.P.; Pratt, M.R.; Muir, T.W. Structure-activity analysis of semisynthetic nucleosomes: Mechanistic insights into the stimulation of Dot1L by ubiquitylated histone H2B. ACS Chem. Biol. 2009, 4, 958–968. [Google Scholar] [CrossRef]
- Fierz, B.; Chatterjee, C.; McGinty, R.K.; Bar-Dagan, M.; Raleigh, D.P.; Muir, T.W. Histone H2B ubiquitylation disrupts local and higher-order chromatin compaction. Nat. Chem. Biol. 2011, 7, 113–119. [Google Scholar] [CrossRef]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar]
- Hoege, C.; Pfander, B.; Moldovan, G.L.; Pyrowolakis, G.; Jentsch, S. Rad6-dependent DNA repair is linked to modification of PCNA by ubiquitin and sumo. Nature 2002, 419, 135–141. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujiwara, H.; Nonaka, T.; Wakabayashi, K.; Takahashi, H.; Lee, V.M.; Trojanowski, J.Q.; Mann, D.; Iwatsubo, T. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J. Biol. Chem. 2002, 277, 49071–49076. [Google Scholar] [CrossRef]
- Abeywardana, T.; Lin, Y.H.; Rott, R.; Engelender, S.; Pratt, M.R. Site-specific differences in proteasome-dependent degradation of monoubiquitinated alpha-synuclein. Chem. Biol. 2013, 20, 1207–1213. [Google Scholar] [CrossRef]
- Laub, M.; Jennissen, H.P. Synthesis and decay of calmodulin-ubiquitin conjugates in cell-free extracts of various rabbit tissues. Biochim. Biophys. Acta. 1998, 1357, 173–191. [Google Scholar] [CrossRef]
- Laub, M.; Steppuhn, J.A.; Bluggel, M.; Immler, D.; Meyer, H.E.; Jennissen, H.P. Modulation of calmodulin function by ubiquitin-calmodulin ligase and identification of the responsible ubiquitylation site in vertebrate calmodulin. Eur. J. Biochem. 1998, 255, 422–431. [Google Scholar]
- Virdee, S.; Ye, Y.; Nguyen, D.P.; Komander, D.; Chin, J.W. Engineered diubiquitin synthesis reveals Lys29-isopeptide specificity of an OTU deubiquitinase. Nat. Chem. Biol. 2010, 6, 750–757. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J. The dark side of ras: Regulation of apoptosis. Oncogene 2003, 22, 8999–9006. [Google Scholar] [CrossRef]
- Baker, R.; Wilkerson, E.M.; Sumita, K.; Isom, D.G.; Sasaki, A.T.; Dohlman, H.G.; Campbell, S.L. Differences in the regulation of K-Ras and H-Ras isoforms by monoubiquitination. J. Biol. Chem. 2013, 288, 36856–36862. [Google Scholar]
- Zhang, S.; Chea, J.; Meng, X.; Zhou, Y.; Lee, E.Y.; Lee, M.Y. PCNA is ubiquitinated by RNF8. Cell Cycle 2008, 7, 3399–3404. [Google Scholar] [CrossRef]
- Paulson, H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 2012, 103, 437–449. [Google Scholar] [CrossRef]
- Burnett, B.; Li, F.; Pittman, R.N. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum. Mol. Genet. 2003, 12, 3195–3205. [Google Scholar] [CrossRef]
- Nicastro, G.; Menon, R.P.; Masino, L.; Knowles, P.P.; McDonald, N.Q.; Pastore, A. The solution structure of the Josephin domain of ataxin-3: Structural determinants for molecular recognition. Proc. Natl. Acad. Sci. USA 2005, 102, 10493–10498. [Google Scholar]
- Winborn, B.J.; Travis, S.M.; Todi, S.V.; Scaglione, K.M.; Xu, P.; Williams, A.J.; Cohen, R.E.; Peng, J.; Paulson, H.L. The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J. Biol. Chem. 2008, 283, 26436–26443. [Google Scholar] [CrossRef]
- Todi, S.V.; Winborn, B.J.; Scaglione, K.M.; Blount, J.R.; Travis, S.M.; Paulson, H.L. Ubiquitination directly enhances activity of the deubiquitinating enzyme ataxin-3. EMBO J. 2009, 28, 372–382. [Google Scholar] [CrossRef]
- Todi, S.V.; Scaglione, K.M.; Blount, J.R.; Basrur, V.; Conlon, K.P.; Pastore, A.; Elenitoba-Johnson, K.; Paulson, H.L. Activity and cellular functions of the deubiquitinating enzyme and polyglutamine disease protein ataxin-3 are regulated by ubiquitination at Lysine 117. J. Biol. Chem. 2010, 285, 39303–39313. [Google Scholar] [CrossRef]
- Tsou, W.L.; Burr, A.A.; Ouyang, M.; Blount, J.R.; Scaglione, K.M.; Todi, S.V. Ubiquitination regulates the neuroprotective function of the deubiquitinase ataxin-3 in vivo. J. Biol. Chem. 2013, 288, 34460–34469. [Google Scholar]
- Pichler, A.; Knipscheer, P.; Oberhofer, E.; van Dijk, W.J.; Korner, R.; Olsen, J.V.; Jentsch, S.; Melchior, F.; Sixma, T.K. SUMO modification of the ubiquitin-conjugating enzyme E2–25K. Nat. Struct. Mol. Biol. 2005, 12, 264–269. [Google Scholar] [CrossRef]
- Keren-Kaplan, T.; Attali, I.; Motamedchaboki, K.; Davis, B.A.; Tanner, N.; Reshef, Y.; Laudon, E.; Kolot, M.; Levin-Kravets, O.; Kleifeld, O.; et al. Synthetic biology approach to reconstituting the ubiquitylation cascade in bacteria. EMBO J. 2012, 31, 378–390. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faggiano, S.; Pastore, A. The Challenge of Producing Ubiquitinated Proteins for Structural Studies. Cells 2014, 3, 639-656. https://doi.org/10.3390/cells3020639
Faggiano S, Pastore A. The Challenge of Producing Ubiquitinated Proteins for Structural Studies. Cells. 2014; 3(2):639-656. https://doi.org/10.3390/cells3020639
Chicago/Turabian StyleFaggiano, Serena, and Annalisa Pastore. 2014. "The Challenge of Producing Ubiquitinated Proteins for Structural Studies" Cells 3, no. 2: 639-656. https://doi.org/10.3390/cells3020639