3.1. Comparisons between Betaine-Treated and–Untreated Plants under FI and RDI (Experiment 1)
Table 1 illustrates that applying betaine as a foliar spray alters morpho-physiological and yield responses of maize under RDI. The effects of betaine concentrations (B) on maize crop growth and yield under irrigation conditions (I) displayed significant differences (
p ≤ 0.001, 0.01, and 0.05) for the main effect and the interaction effect except for plant height, which showed non-significant differences in B and IxB effects (
Table 1). There were no significant differences in plant height, leaf area fresh shoot, cob weight, and total fresh and dry weight among the various betaine concentrations under FI treatment. However, in the RDI treatments, all agronomic characteristics gradually increased as foliage-treated betaine concentrations increased. We detected significantly higher growth trait values (except fresh root weight) in plants subjected to 100 mM of betaine compared to 50 mM of betaine treatment and controls (no betaine treatment). Most leaves appeared healthy and green when foliage-treated with 100 mM of betaine under RDI compared to chitin-untreated plants, which were visually higher in leaf chlorosis than chitin-treated plants subjected to RDI (
Figure 1A). Therefore, adding betaine under RDI promotes maize plant growth and yield, and it can be used for the rapid monitoring and early detection of water stress injury in the seedling stage and screening of individual plant that exhibit tolerance to water stress.
There were significant differences in all photosynthesis and WUE values for the main effects, except for transpiration in B effects and net photosynthesis in I effects (
Table 2). Moreover, interaction effects did not significantly affect any of the measurements. There were no marked differences in transpiration after the 8 weeks under FI (0.80~0.96 µmol·m
−2·s
−1) and RDI (0.45~0.74 µmol·m
−2·s
−1) treatments compared to controls. However, all 100 mM betaine treatments displayed significantly higher net photosynthesis (16.18~17.01 mmol·m
−2·s
−1) values compared to controls (12.17 mmol·m
−2·s
−1). WUE
i under RDI with 50 mM of betaine treatment (46.21 mmol CO
2·mol
−1 H
2O) was significantly higher than in other treatments and controls, ranging from 28.16 to 15.78 mmol CO
2·mol
−1 H
2O. There were no marked differences in WUE
yield (2.85~4.16 kg·m
−3) and WUE
biomass (1.48~2.62 kg·mL) among the different betaine treatments under FI. Nevertheless, significantly higher WUE
yield (11.62 kg·m
−3) and WUE
biomass (6.06 kg·m
−3) values were detected in 100 mM betaine treatments under RDI compared to other treatments. In general, net photosynthesis and WUE values increased with increasing betaine concentrations and decreasing water application, except for WUEi in the 50 mM betaine treatment under RDI.
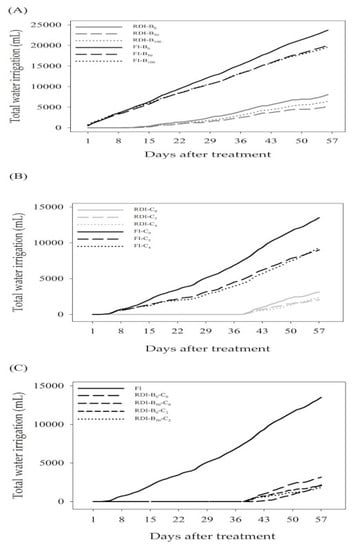
The total amount of water applied to maize from the first day (0 weeks) up to the 56th day (8 weeks) under FI was 19,600~23,700 mL. Compared to this amount, RDI received remarkably less irrigation water, ranging from 5200~8050 mL (
Figure S1). Total water irrigation decreased under RDI, indicating that maize suffered from water stress injury. Betaine was applied biweekly four times after planting, and because betaine application to RDI treated plants improved plant water status, it is reasonable to expect that this in turn led to favorable effects on the photosynthesis and WUE values. This implies that betaine treatment increased yield-related traits, photosynthesis, and WUE values under RDI and boosted water stress tolerance.
Deficit irrigation is correctly applied only thorough an understanding of the yield response to water. The capacity of plants to absorb nutrients is typically weak when anti-transpirants are sprayed onto leaves under water deficit conditions because of the resulting limited transpiration pull [
30]. Exogenous betaine significantly alleviates drought and salt stress-induced growth inhibition in rice [
31] and barley [
32]. Glycine betaine-treated leaves significantly increase the ability of the antioxidant defense system to resist abiotic stresses in different plant species [
33,
34,
35,
36]. Furthermore, plants can improve their capacity for osmotic adjustment by increasing glycine betaine accumulation under drought stress [
37]. The foliar application of glycine betaine under drought conditions regulates the turgor pressure of guard cells by increasing the bound water content in cells regulating stomatal opening and closing, thus increasing chlorophyll content, stomatal conductance, and photosystem-II efficiency and stabilizing total carotenoids in pepper [
38] and rice [
39]. Spraying 150 ppm glycine betaine on maize plants under drought stress improves chlorophyll content, plant height, and yield [
40]. When we applied 100 mM betaine under a soil water deficit, plants increased their transpiration capacity without affecting aboveground growth. It is not clear how betaine treatment improves the water status of leaves during water stress, but it might be that water uptake efficiency is improved or that water loss is retarded, or both. Betaine spraying could favorably activate leaf signaling to reduce water loss through transpiration, maintain a favorable level of transpiration, and facilitating the absorption of water and nutrients from soil while maintaining a higher level of photosynthesis. Reducing luxury transpiration and increasing net photosynthesis by applying betaine provides an opportunity for relieving the adverse effects of RDI on maize plants and improving their WUE when transpiration frequently exceeds water uptake. This contributes to reducing water loss and maintaining proper water consumption, thus saving agricultural water resources in dry-land areas.
3.2. Comparisons between Chitin-Treated and–Untreated Plants Subjected to FI and RDI (Experiment 2)
Table 3 and
Table 4 present the effects of chitin treatments on agronomic performance, photosynthesis, and WUE values of maize under FI and RDI. All measurements appeared to differ significantly in terms of chitin’s (C) main effect. Both plant height and leaf area showed significant differences in all effects (I, C, and I × C). Interestingly, under FI, there was an increasing trend in all measurements in all plants when chitin application increased from 0 to 4 g/kg. However, when 2 and 4 g/kg of chitin were applied to each pot under RDI, the plants exhibited significantly higher levels in all agronomic characteristics compared to the no-chitin treatment. The total amount of water applied under FI and RDI was 9000~13,500 mL and 2050~3150 mL, respectively. These observations demonstrated that plants were highly regulated by chitin and all measured traits drastically elevated; thus, treating maize with chitin can mitigate the effects of drought stress.
There were significant differences in photosynthesis and WUE values among chitin concentrations, with 2 and 4 g/kg chitin applications showing significantly higher photosynthesis and WUE values under all irrigation conditions, except transpiration under the FI condition, in comparison to controls. Interestingly, all chitin-treated plants displayed significantly higher (36.02~31.45) total chlorophyll (TC) content than chitin-untreated plants (17.27~10.72) in all irrigation treatments. We also assessed the effect of chitin treatment on plant growth under RDI.
Figure 1B depicts epinasty and senescence in the lower leaves of chitin-untreated plants under RDI after 8 weeks of stress; however, most leaves looked green and healthy under chitin applications and RDI conditions. Water stress had a harmful effect during RDI, the degree of chlorosis being related to a reduction in leaf TC content. These observations imply that chitin application might reduce or delay water stress, thereby allowing plants to survive and function during stress. This ability perhaps can be attributed to an avoidance of water stress, as indicated by the higher yield components, photosynthesis parameters, and WUE values in chitin-treated plants compared to chitin-untreated plants during RDI conditions. It is also possible that chitin has the capacity to absorb water, which in turn increases the water holding capacity of the soil and the water available for the plant to use, i.e., chitin increases soil moisture [
41].
The application of chitosan leads to increases in plant height, number of shoot branches, number of leaves, leaf area, biomass, and grain yield in maize [
42]. Moreover, chitosan treatment in white clover alleviates drought stress and increases the production of stress protective metabolites [
43]. Chitosan application under drought stress increases sweet basil growth [
44]. Treatment with chitin oligosaccharides increases the photosynthesis level of maize [
45]. Furthermore, water stress decreases pigment content and photosynthesis parameters in maize, but the application of chitosan and derivatives increases them [
46]. The application of a mixture of chitosan derivatives also induces a tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield [
47]. Chitin features prominent biochemical similarities in plant cell walls, including neutrally charged linear polysaccharide chains that provide mechanical, physical, and structural stability [
48].
Total chlorophyll (TC) value has been widely examined in a number of plants to determine injury or tolerance to various environmental stresses, including drought, chilling, heat, and radiation [
49]. Typically, stressful conditions reduce TC levels, so TC values from chlorophyll meter readings can be used as a parameter of drought tolerance. The SPAD assesses TC contents and photosynthetic capacity [
50]. In this study, RDI affected maize yield components, photosynthesis parameters, and WUE values. Plants with higher SPAD, net photosynthesis, and WUE values also had higher cob and total dry weights. When SPAD values were >31 in the chitin-treated groups, it became useful for measuring WUE when developing indices for nondestructive chlorophyll estimation. This means that many hundreds of individual plants can be screened per day, providing many opportunities to discover individuals that manifest yield indicators and exhibit greater photosynthesis and WUE. However, no studies have been conducted on the effects of chitin treatments on yield, photosynthesis, and WUE values of maize under RDI. In addition, because of the low cost of chitin, improvements in these parameters under RDI conditions will reduce the cost per plant or per acre of new plantings.
3.3. Effects of Betaine and Chitin Treatments on Plant Physiology and Morphology under FI and RDI (Experiment 3)
Table 5 and
Table 6 summarize the impact of chitin and betaine on agronomic performance, photosynthesis, and WUE values of maize under FI and RDI conditions. All of the measurements appear to significantly differ in the main effect (I) and interaction effect (B × C), except for fresh root weight and transpiration. Thus, the agronomic traits of the plants responded differently to betaine and chitin treatments alone or mixtures under RDI. Compared to no chemical treatments, the application of betaine (50 mM) or chitin (2 g/kg) alone to plants under RDI had relatively higher growth, yield, photosynthesis parameters, and WUE values, except for plant height, fresh root weight, and transpiration. Notably, RDI combined with 2 g/kg of chitin produced significantly higher plant height, shoot fresh weight, cob weight, total fresh and dry weights, TC level, and net photosynthesis compared to the other treatments. The SPAD value (31.47) in leaves under 2 g/kg chitin treatment was significantly higher in RDI compared to other treatments, ranging from 26.23~10.72. Moreover, the application of 50 mM betaine with RDI also improved the ‘White Pearl’ maize yield and WUE as well compared to control.
Table 1 has shown that 50 and 100 mM betaine treatments under RDI resulted in relatively higher yield, photosynthesis, and WUE values compared to untreated plants. Therefore, RDI with WUE can be used in a nondestructive estimation of yield and biomass when screening for water stress tolerant plants.
Table 5 and
Table 6 reveal that treatment with a combination of betaine and chitin did not exhibit a synergistic optimum on yield, photosynthesis, and WUE values under RDI. However, using chitin alone on plants under RDI significantly increased yield-related traits compared to plants without chitin treatment and controls, and thus can be applied on a commercial scale for saving water without sacrificing yield. Compared to FI (13,503 mL), RDI plants received remarkably less irrigation water, ranging from 1800~3148 mL. Treatment with 2 g/kg chitin per plant in RDI conditions increases photosynthesis and WUE values in maize, which offers insight into the mechanism of its action for water saving advances in the future.
Figure 1C illustrates that chlorosis in most RDI and chitin-untreated plants was visibly greater than in chitin-treated plants subjected to RDI. Similarly, RDI control plants had significantly lower TC content along with the characteristic visual symptoms. In addition, there was a gradual inhibition in light-induced chlorophyll accumulation over the time of the RDI treatment (data not shown). Therefore, biostimulators can be applied to plants when SPAD values reach 31. Different biostimulators acted differently under RDI treatment; however, each biostimulator is not necessarily equally significant in protecting against water stress. The impacts of changing plant physiology and morphology on water stress tolerance and plant health were affected by betaine and chitin application.
The results can be applied for improving the water stress tolerance of maize plants, developing management practices for field cultivation, reducing energy consumption, and enhancing cultivation when water resources are limited. A better understanding of the growing characteristics of these plants would also aid in their effective cultivation on arid lands or in extreme climates. In addition, the cost-effectiveness of betaine (50 mM/plant) and chitin (2 g/kg soil) related to yields and reduction of water use would be 0.017 and 0.17 US dollars per plant, respectively. Increasing yield and WUE from different biostimulants under RDI provided plants with increased water stress tolerance, playing a key role in providing better adaptation to water stress. The effects of water stress on maize can be lessened by treatment with betaine and chitin because these chemicals may protect cell membranes from the adverse effects of water stress. Betaine and chitin act at a convergence point for integrating different signals, minimizing cell damage caused by water deficits, and improving the physiological and biochemical condition of plants, thus making plants more tolerant to aridity. Further transcriptomic and proteomic studies of water-stressed responsive genes and proteins need to be identified to provide better usage of betaine and chitin in water stress management.